Abstract
Objective
Peripheral arterial catheterization is a common invasive procedure performed in critically ill children. However, the benefits of using ultrasound guidance for this procedure in critically ill children, especially when utilized by inexperienced trainees, are unclear. Our aim was to evaluate whether the use of ultrasound guidance for the placement of radial arterial lines reduced time and improved success when compared to the palpation method, and also to determine patient and trainee variables that influence procedure outcomes. Finally, we evaluated whether adoption of ultrasound guidance among trainees comes at the expense of learning landmark-based methods.
Design
Prospective observational cohort
Setting
University affiliated PICU
Patients
208 procedures performed by 45 trainees in 192 unique patients (ages of 1 month – 20 years) were observed.
Measurements and Main Results
The main outcome measures were time and number of attempts required for the procedure. Compared to palpation method, ultrasound guidance was associated with reduced procedure time (8.1 ± 5.2 minutes compared to 16.5 ± 8.8 minutes, p < 0.001), reduced number of attempts (3.1 ± 2.6 attempts compared to 6.9 ± 4.2 attempts, p < 0.001), and improved first attempt success rate (28% compared to 11%, p = 0.001) even after adjusting for key confounders in multivariate random effects models. The factors most likely to interfere with peripheral arterial catheterization are patient age, patient systolic blood pressure, patient BMI, degree of fluid overload, and trainee months in fellowship. The use of ultrasound guidance mitigates the influence of each of these factors. We found no evidence that the adoption of ultrasound guidance by trainees is associated with reduced proficiency in landmark-based methods.
Conclusions
The use of ultrasound guidance by trainees for radial artery catheterization in critically ill children is associated with improved outcomes compared with the palpation method.
Keywords: Ultrasound, radial artery, catheter, palpation, PICU, pediatric
Introduction
Peripheral arterial catheterization is a common invasive procedure performed in critically ill children that enables continuous hemodynamic monitoring and facilitates blood sampling. Insertion of peripheral artery catheters using the standard palpation method is associated with a low first attempt success rate when utilized by trainees and can be challenging in certain circumstances thereby delaying patient care(1, 2). Accumulating evidence in adults has demonstrated that the use of ultrasound guidance for peripheral artery catheterization improves the first attempt success rate while reducing both the overall procedure time and the incidence of complications(3–9). However, the benefits of ultrasound guidance for the placement of peripheral arterial catheters in pediatric patients are less clear, especially when utilized by inexperienced trainees(2, 10, 11). Further, the use of ultrasound to guide arterial line placement has never been evaluated specifically in critically ill pediatric patient populations. Finally, the clinical scenarios and patient characteristics that most lend themselves to the use of ultrasound guidance have not been described.
In this study, we report on our experience with peripheral arterial catheterizations performed in pediatric patients by critical care trainees as an ultrasound training program was being implemented. We evaluated the effectiveness of ultrasound guidance compared to the standard palpation method for the placement of peripheral arterial catheters, and also identified patient and trainee characteristics that are associated with procedural success and other outcomes.
Material and Methods
Study Population and Procedures
This was a prospective observational cohort study of radial artery catheters inserted for clinical indications, which occurred in a pediatric medical surgical intensive care unit at a single urban children’s hospital during the period from January 2009 to January 2015. A convenience sample of consecutive procedures was observed during those times when a study investigator was on clinical service. Outcomes were observed and recorded by bedside nurses who were blind to the aims of the study. This study was approved by the Boston Children’s Hospital IRB.
Beginning in 2012, all trainees received brief didactic and hands on training sessions on the use of ultrasound guidance for the placement of central venous and peripheral arterial lines, in addition to the training in landmark based procedures that had been standard prior to this date. The technique employed to attempt radial artery cannula insertion (ultrasound guidance vs. palpation method) was determined by the trainee, and not dictated by the study protocol.
With minor variations, the following procedures were employed for placement of arterial catheters. The skin at and around the insertion site was cleaned and disinfected. The wrist was extended over a gauze roll and the hand and forearm was taped to a board to maintain wrist extension. In the ultrasound method, a portable ultrasound device (Sonosite Micromaxx; Sonosite, Bothwell, WA) with a linear 13-6 MHz transducer (Sonosite L25e) was used to either localize and topically mark the position of the radial artery with a surgical pen (9/82 or 11%) or to visually guide the advancing needle to the target (73/82 or 89%). In the palpation method, the position of the artery was identified by palpation, and the cannula was directed by continued or intermittent palpation of the arterial pulsation. For both techniques, the identified artery was cannulated using a Seldinger technique as follows: a conventional peripheral IV catheter (Jelco, Smiths Medical, Dublin, OH) was used to transfix the radial artery, the stylet needle was removed, the cannula was withdrawn slightly until pulsatile blood return was noted, a spring guide wire (0.018″, Arrow, Teleflex, Morrisville, NC) was introduced, the cannula inserted over the guide wire into the radial artery, and the guide wire was withdrawn.
Evaluation of Procedure Success and Other Measures
The following outcome data were recorded: 1) time taken by the first operator at the chosen first cannulation site. The start time occurred when the skin was first punctured by the cannulating needle. The end time occurred when pulsatile blood flow was noted from the radial artery cannula or when the procedure failed (see #4 below), 2) number of attempts, defined as insertion of the needle through the skin at the first cannulation site, 3) first attempt success, defined as successful cannulation of the radial artery with the first insertion of the cannulating needle, 4) procedure failure, which occurred if the first operator aborted the procedure altogether, switched to another site, switched to another technique, or turned over the procedure to another operator, and 5) hematoma formation, defined as a visible or palpable swelling around the cannulation site following the procedure.
The following patient variables were recorded at the time of the procedure: age, sex, race/ethnicity charted in the electronic medical record, weight, height, non-invasive oscillometric blood pressure, mechanical ventilation requirement (including invasive and non-invasive modes of ventilation), vasopressor infusion requirement (defined as dopamine infusion ≥ 10 mcg/kg/min, and/or epinephrine or norepinephrine infusion ≥ 0.05 mcg/kg/min), sedative infusion in use, and the presence of wrist flexion contractures. Data related to the Pediatric Index of Mortality 3 (PIM3) was abstracted from the electronic medical record according to the developers’ coding rules(12).
Weight-for-age and BMI-for-age percentiles and z-scores were based on normative data from the World Health Organization child growth standards(13). Blood pressure percentiles and z-scores for children ages 2 years and older were based on National Heart Lung and Blood Institute normative data for children and adolescents(14). Blood pressure percentiles and z-scores for children under age 2 years was based on normative data from Voors et al(15). The PIM3 scores and probability of death were calculated according the developer’s instructions(12). The degree of fluid overload was determined using published methods (16) according to the following formula: fluid overload percent percent = (mL fluid in − mL fluid out from the day of PICU admission)/PICU admission weight in kg * 100.
The following trainee variables were also recorded: number of years post medical school graduation at the time the observed procedure was performed, number of months in pediatric critical care fellowship at the time the observed procedure was performed, and the self reported number of arterial line procedures logged by the trainee prior to the observed procedure (derived from a pre-existing database unrelated to this study in which trainees log procedures for the purpose of evaluating training progress).
Statistical Analysis
Comparisons were made between procedures that were performed using the palpation method and procedures that were performed using ultrasound guidance. Univariate and multivariate linear regression models were used to investigate the association between continuous outcome variables (time and number of attempts) and predictor variables. Univariate and multivariate logistic regression models were used to investigate the association between binary outcome variables (first attempt success, failed procedures, and hematoma formation) and predictor variables. Because each trainee recorded multiple procedures over time, a random effect for trainees was added to the models to account for this by-trainee variation. For the linear models, each trainee was allowed a different slope and intercept. Certain models included an interaction term in order to investigate how the association between explanatory variables and outcome measures changed depending on whether arterial lines were placed with the palpation method or the ultrasound method.
Covariates for multivariable models were chosen based on a purposeful selection algorithm with a significance threshold of 0.25 and a change in coefficient threshold of 20% (17). The following covariates were included in the multivariable models: patient age in years, patient weight for age, patient systolic blood pressure percentile, patient pediatric index of mortality 3 (PIM3) probability of death, fluid overload percent, and trainee months in fellowship at the time of the procedure. Due to the non-linear relationship between BMI-for-age Z-score and continuous outcome variables, a quadratic term was included for this covariate. A two-sided p-value < 0.05 was considered significant. All statistical analyses were performed with Stata version 13.1 (StataCorp, College Station, TX).
Results
A total of 208 radial artery catheterizations performed by 45 trainees in 192 unique patients were observed over the course of the six year study period, with 126 arterial lines placed using the palpation method and 82 placed using ultrasound guidance (6.7% of the total number of patients with arterial lines during the study period). Patient and trainee characteristics were not significantly different between the palpation group and the ultrasound group (Table 1). The mean Pediatric Index of Mortality 3 (PIM3) score for patients in this study (6.8 ± 7.9%, mean ± SD) was higher and statistically different than either of the following two groups for whom PIM3 data was available during the study period: a) all intensive care unit admissions (PIM3, 1.8 ± 6.4%; n = 1449; p < 0.001) or b) patients who were mechanically ventilated within the first hour of admission to the intensive care unit (PIM3, 3.84 ± 10.36; n = 449; p < 0.001).
Table 1.
Characteristics of Study Cohort
| Variables | Total | Palpation | Ultrasound | p-value * |
|---|---|---|---|---|
| Observed procedures, n (% total) | 208 (100) | 126 (60.6) | 82 (39.4) | n/a |
|
| ||||
| Patient Variables | ||||
|
| ||||
| Age, mean years (± SD) | 5.8 (5.3) | 5.7 (5.5) | 5.9 (4.9) | 0.782 |
|
| ||||
| Male, n (%) | 113 (54.3) | 73 (57.9) | 40 (48.8) | 0.203 |
|
| ||||
| Ethnicity, n (%) | ||||
| African American | 35 (16.8) | 22 (17.5) | 13 (15.9) | 0.764 |
| Caucasian | 100 (48.1) | 61 (48.4) | 39 (47.5) | 0.904 |
| Hispanic | 51 (24.5) | 30 (23.8) | 21 (25.6) | 0.769 |
| Other | 22 (10.6) | 13 (10.3) | 9 (11.0) | 0.881 |
|
| ||||
| Anthropometrics ♯ | ||||
| Weight-for-age, %ile, mean (± SD) | 42.1 (26.3) | 39.9 (27.1) | 45.6 (24.9) | 0.125 |
| BMI, %ile, mean (± SD) | 47.8 (26.5) | 43.9 (25.7) | 52.8 (27.0) | 0.053 |
|
| ||||
| Blood pressure § | ||||
| Systolic pressure, %ile, mean (± SD) | 23.2 (22.5) | 22.5 (22.0) | 24.4 (23.3) | 0.541 |
| Mean pressure, %ile, mean (± SD) | 14.1 (10.9) | 13.6 (10.6) | 14.8 (11.4) | 0.439 |
|
| ||||
| Fluid overload percent ∫ | 6.7 (4.4) | 6.5 (4.5) | 7.0 (4.3) | 0.786 |
|
| ||||
| PIM3 probability, mean (± SD) † | 6.8 (7.9) | 6.7 (7.9) | 6.9 (7.7) | 0.810 |
|
| ||||
| Mechanical ventilation, n (%) ‡ | 190 (91.3) | 113 (89.7) | 77 (93.9) | 0.326 |
|
| ||||
| Vasopressor, n (%) Σ | 141 (67.8) | 83 (65.9) | 58 (70.7) | 0.544 |
|
| ||||
| Sedation infusion, n (%) | 186 (89.4) | 111 (88.1) | 75 (91.5) | 0.497 |
|
| ||||
| Wrist flexion contractures, n (%) | 54 (26.0) | 32 (25.4) | 22 (26.8) | 0.872 |
|
| ||||
| Trainee Variables | ||||
|
| ||||
| Trainees, n (%) | 45 (100) | 37 (82.2) | 22 (48.9) | n/a |
|
| ||||
| Post graduate years, mean (± SD) ° | 5.5 (1.1) | 5.6 (1.1) | 5.4 (1.2) | 0.332 |
|
| ||||
| Months in fellowship, mean (± SD) | 17.4 (9.1) | 17.3 (9.5) | 17.7 (8.5) | 0.792 |
|
| ||||
| Prior procedures, mean (± SD) ♮ | 30.5 (16.9) | 29.45 (17.5) | 32.2 (15.9) | 0.274 |
Student’s t-test or Pearson’s chi squared test for continuous or categorical variables, respectively
World Health Organization child growth standards (WHO, 2006)
Non-invasive BP at procedure start; %tiles derived from NHLBI, 2005 and Voors et al,1977
(Fluid intake - fluid output (mL) from PICU admission)/admit weight (kg) * 100
Pediatric Index of Mortality 3 (Straney et al, 2013)
Invasive and non-invasive modes of ventilation
Dopamine infusion ≥ 10 mcg/kg/min, and/or epinephrine or norepinephrine infusion ≥ 0.05 mcg/kg/min
Years since medical school graduation
Self-reported number of arterial lines logged prior to date of observed procedure
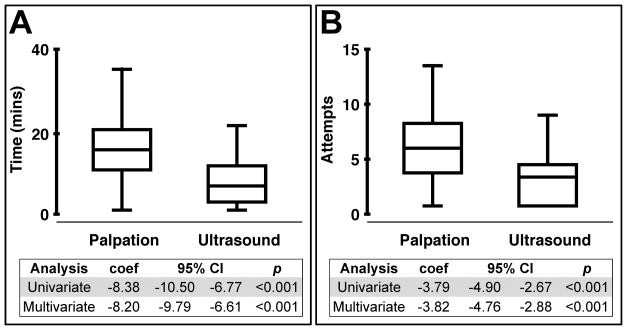
The mean procedure time was greater in the palpation group (16.5 ± 8.8 minutes) compared to the ultrasound group (8.1 ± 5.2 mins, p < 0.001), and the mean number of attempts was also greater in the palpation group (6.9 ± 4.2 attempts) compared to the ultrasound group (3.1 ± 2.6 attempts, p < 0.001) (Figure 1). These findings remained significant even after adjusting for variables which we found influenced procedural success, namely patient age, patient systolic blood pressure, patient BMI, and trainee months in fellowship (Supplemental Table 1 and Supplemental Table 2).
Figure 1. Ultrasound guidance improves the efficiency of peripheral artery catheterization.
Radial artery catheterizations performed using ultrasound guidance (n= 82) were compared to procedures using the palpation method (n=126). Time (A) and number of attempts (B) to success were the outcome measures. The linear regression coefficients, 95% CIs, and p-values are shown. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, fluid overload percent, and trainee months in fellowship.
We found that the odds of achieving successful catheterization on the first attempt were greater in the ultrasound group (23/82 or 28%) compared to the palpation group (14/126 or 11%, multivariate analysis, OR = 3.99, p = 0.001) while the odds of procedure failure were lower in the ultrasound group (3/82 or 4%) compared to the palpation group (18/126 or 14%, multivariate analysis, OR 0.27, p = 0.032) even after adjusting for variables known to influence procedural success (Table 2). Further, we found that the odds of hematoma formation were lower in the ultrasound group (13/82 or 16%) compared to the palpation group (34/126 or 27%, multivariate analysis, OR = 0.62, p = 0.001) even after adjusting for variables that influence procedural success (Table 2). While we recorded other complications, namely infection and impaired perfusion, this study was not adequately powered to analyze these very low probability events (data not shown).
Table 2.
Logistic regression comparing ultrasound guidance to the palpation method for peripheral arterial catheterization.
| Outcomes | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||
| First attempt success | 3.12 | 1.49 | 6.51 | 0.002 | 4.48 | 1.87 | 10.75 | 0.001 |
| Hematoma | 0.47 | 0.29 | 0.89 | 0.025 | 0.62 | 0.13 | 0.96 | 0.041 |
| Failed procedure | 0.23 | 0.06 | 0.80 | 0.021 | 0.27 | 0.04 | 0.86 | 0.032 |
Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, and trainee months in fellowship (palpation method, n = 126; ultrasound guidance, n = 82).
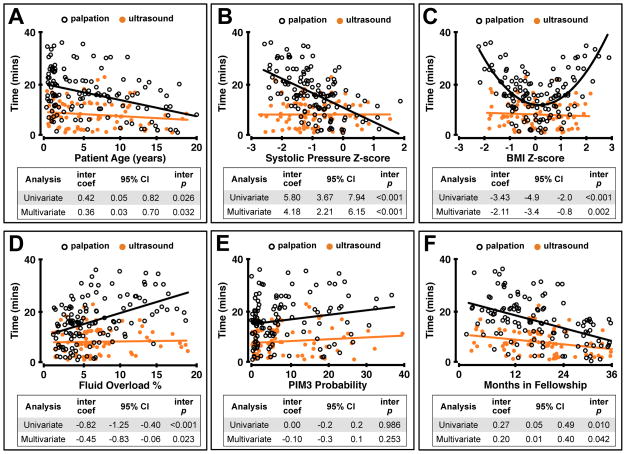
Patient and trainee variables that most consistently interfered with procedural success included patient age, patient systolic blood pressure at the time of the procedure, patient BMI, fluid overload percent, and trainee months in fellowship (Supplemental Table 1 and Supplemental Table 2). Ultrasound guidance mitigated the influence of each of these covariates, using either time or number of attempts as an outcome variable (Figure 2 and Supplemental Figure 1). Patients receiving the greatest benefit from ultrasound guidance included those with blood pressures approximately 1 standard deviation below the population mean for age (Figure 2 and Supplemental Figure 2), those with BMI approximately 0.5 standard deviations above or below the population mean for age (Figure 2 and Supplemental Figure 2), those with fluid overload greater than approximately 5%, (Figure 2 and Supplemental Figure 2), and older patients (Figure 2). We further compared third year trainees who used the palpation method to first year trainees who used ultrasound, and found that the first year trainees had significantly improved outcomes (first year ultrasound 7.8 ± 3.8 mins; third year palpation 12.5 ± 6.4 mins, p = 0.024). By contrast, third year trainees who used palpation had significantly better outcomes compared to first year trainees who used palpation (first year palpation, 20.7 ± 7.8 mins; third year palpation 12.5 ± 6.4 mins, p < 0.001).
Figure 2. Ultrasound guidance mitigates patient and trainee variables that interfere with peripheral arterial catheterization.
The effects of patient age (A), patient systolic blood pressure (B), patient BMI (C), degree of fluid overload (D), PIM3 probability (E), and trainee months in fellowship (F) on time to successful radial artery cannulation were compared between procedures performed using ultrasound guidance (n = 82) and those performed using the palpation method (n = 126). Interaction coefficients, 95% CIs, and interaction p-values represent the comparison between ultrasound guided and palpation guided procedures. BMI for age Z-score was replaced with a quadratic term in the regression models due to the non-linear relationship with time. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship.
Finally, we looked for evidence supporting the hypothesis that the adoption of ultrasound by trainees comes at the expense of proficiency in landmark based methods(18). Evaluating only palpation-guided procedures, we found no significant differences in outcomes between trainees who exclusively utilized the palpation method (n= 23 trainees, n = 65 procedures, 16.7 ± 9.6 minutes) compared to trainees who were capable of utilizing either ultrasound or palpation (n = 22 trainees, n = 61 procedures, 16.4 ± 7.8 minutes, p = 0.915) (Supplemental Figure 3). These findings remained significant even after adjusting for variables that we found influenced procedural success, namely patient age, patient systolic blood pressure, patient BMI, fluid overload percent, and trainee months in fellowship. Next, we asked whether implementing an ultrasound curriculum was associated with decreased proficiency with the palpation method. We found no significant differences in outcomes for palpation-guided procedures performed before (n = 76, 17.3 ± 8.8 minutes) and after (n = 50, 16.3 ± 8.8 minutes, p = 0.426) curriculum implementation, even after adjusting for confounders (Supplemental Figure 3).
Discussion
Here we report on the first observational study of peripheral arterial catheter insertion performed by trainees in a cohort of critically ill pediatric patients. We demonstrate that the use of ultrasound guidance for radial artery catheterization is associated with significant differences compared to the palpation method in the following outcome measures: a) shortened procedure time, b) reduced number of attempts, c) improved odds of first attempt success, and d) reduced odds of hematoma formation. Further, we demonstrate that patient age, patient systolic blood pressure, patient BMI, degree of fluid overload, and trainee months in fellowship are the factors that most consistently interfere with peripheral arterial catheterization. For each of these factors, the use of ultrasound guidance significantly mitigates the influence of these covariates. This study is unique because it demonstrates for the first time that the use of ultrasound guidance by relatively inexperienced trainees for the insertion of radial artery catheters in pediatric patients is associated with significantly improved outcomes compared to the palpation method. Further, this study is the first to evaluate the use of ultrasound guidance specifically in critically ill pediatric patients, and to identify clinical situations that are most likely to benefit from the use of ultrasound guidance.
Several studies in adults have demonstrated that use of ultrasound guidance for peripheral arterial cannula placement is superior to the palpation method(3–5). However, the data regarding the superiority of ultrasound guidance in pediatric patients, especially when utilized by trainees, has been more difficult to interpret(2, 10, 11). In fact, the largest previous study(2) of peripheral arterial catheter insertion performed mainly by anesthesia trainees in a cohort of pediatric patients concluded that ultrasound guidance did not improve the speed or success of the procedure. However, our analysis of patient factors that influence the success of arterial catheterization suggests ultrasound guidance is less likely to improve outcomes for patients who are close to the population mean for BMI and blood pressure or for older patients. While complete anthropometric data is not reported in Ganesh et al(2), the sample means for patient weight, systolic blood pressure and age appear unlikely to fall within the ranges that, according to our results, would significantly favor the use of ultrasound guidance.
In contrast to previous studies, we found no evidence that the adoption of ultrasound guidance by trainees is associated with reduced proficiency in landmark-based methods(18). This may be because the two methods share a common requisite skill set, and becoming proficient in the landmark-based approach flows naturally from first learning ultrasound-based techniques.
This study has several limitations. First, neither patients nor trainees were prospectively randomized by procedure type, raising the possibility that unmeasured patient or trainee variables biased the results. It is possible that the trainees who chose to use ultrasound were simply more adept at procedures than those trainees who chose to use the palpation method. We do not favor this possibility because trainees who utilized both ultrasound and palpation were no better at the palpation method than those trainees who exclusively utilized the palpation method (Supplemental Figure 3). Nonetheless, it is not possible for us to rule out that differences among operators with respect to procedural expertise influenced the results. Notably, our study design does have an advantage in that trainees presumably selected the catheterization method with which they were most proficient, and so were not artificially handicapped by a randomization process. Our patient population focused on critically ill children, most of whom were hypotensive at the time of arterial catheterization. This may exaggerate the benefits of ultrasound guidance compared to the palpation method, and thus our results cannot be readily extrapolated to other patient populations. In addition, our data regarding how the use of ultrasound influences proficiency with the palpation method should be interpreted with some caution as the regression model was not adjusted to account for independent levels of proficiency with each of the two techniques. It is also important to note that this study specifically evaluated the time from skin puncture to blood return, and did not evaluate how the use of ultrasound might influence overall setup and preparation time. A final limitation of this study is that the observers who recorded outcome measures were not blind to the method used.
Conclusion
We demonstrate that the use of ultrasound guidance by trainees for radial artery catheterization in critically ill children is associated with improved outcomes compared with the palpation method. The shorter times to successful arterial catheterization in critically ill children is a particularly notable finding as the placement of peripheral arterial lines is often a time critical procedure necessary to generate hemodynamic and other data that helps to guide patient resuscitation in the PICU.
Supplementary Material
The effects of patient age (A), patient systolic blood pressure (B), patient BMI (C), degree of fluid overload (D), PIM3 probability (E), and trainee months in fellowship (F) on number of attempts to successful radial artery cannulation were compared between procedures performed using ultrasound guidance (n = 82) and those performed using the palpation method (n = 126). Interaction coefficients, 95% CIs, and interaction p-values represent the comparison between ultrasound guided and palpation guided procedures. BMI for age Z-score was replaced with a quadratic term in the regression models due to the non-linear relationship with time. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship
Sample data for systolic blood pressure (A), BMI (B), and degree of fluid overload (C) were binned, and multivariate linear regression p-values were determined for individual bins with respect to time. The negative log of the p-value (y-axis, ) was then plotted against z-scores to determine the thresholds where ultrasound guidance is associated with a significant improvement in time. The significance threshold of 0.05 is noted with a dotted line – values above the line are more significant and values below the line are not significant. Multivariate models were adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship.
A subset of trainees only utilized the palpation method (Single, n = 23 trainees), while another subset of trainees used both ultrasound guidance and the palpation method (Dual, n = 22 trainees). Examining only procedures that were performed with the palpation method, there was no significant differences in Time (A) or Attempts (B) between Single procedure trainees (n = 65 procedures) and Dual procedure trainees (n = 61 procedures). There was no significant differences in Time (C) or Attempts (D) between palpation based procedures performed before (n = 76 procedures and after (n = 50 procedures) implementation of ultrasound curriculum. The linear regression coefficients, 95% CIs, and p-values are shown. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship.
Acknowledgments
This work was performed at Boston Children’s Hospital
Funding Sources:
This work was supported by the following sources: NIH T32 HD040128 (DBK) and NIH K12 HD047349 (DBK). The sponsors of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
The authors thank the following: Meredith van der Velden, David Zurakowski, Nilesh Mehta, Michael McManus, Enid Martinez, Lisa DelSignore, Robert Graham, Carolyn Stickney, Robert Truog and Daniel Kohane for helpful comments; the nursing staff in the Boston Children’s Hospital Medical and Surgical Intensive Care Unit for help in recording outcome measures, and Chonel Petti and Gretchen Sampadian for providing data related to historical PIM3 data and trainee procedure logs.
Footnotes
Reprints will not be ordered
References
- 1.Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6:199–204. doi: 10.1186/cc1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganesh A, Kaye R, Cahill AM, et al. Evaluation of ultrasound-guided radial artery cannulation in children. Pediatr Crit Care Med. 2009;10:45–48. doi: 10.1097/PCC.0b013e31819368ca. [DOI] [PubMed] [Google Scholar]
- 3.Tang L, Wang F, Li Y, et al. Ultrasound Guidance for Radial Artery Catheterization: An Updated Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2014;9:e111527. doi: 10.1371/journal.pone.0111527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu W-J, Tie H-T, Liu J-C, et al. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. 2014;18:1–7. doi: 10.1186/cc13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiloh AL. Ultrasound-Guided Catheterization of the Radial Artery. Chest. 2011;139:524. doi: 10.1378/chest.10-0919. [DOI] [PubMed] [Google Scholar]
- 6.Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Critical Care Medicine. 2003;31:481–484. doi: 10.1097/01.CCM.0000050452.17304.2F. [DOI] [PubMed] [Google Scholar]
- 7.Shiver S, Blaivas M, Lyon M. A Prospective Comparison of Ultrasound-guided and Blindly Placed Radial Arterial Catheters. Academic Emergency Medicine. 2006;13:1275–1279. doi: 10.1197/j.aem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.HANSEN MA, JUHL-OLSEN P, THORN S, et al. Ultrasonography-guided radial artery catheterization is superior compared with the traditional palpation technique: a prospective, randomized, blinded, crossover study. Acta Anaesthesiologica Scandinavica. 2014;58:446–452. doi: 10.1111/aas.12299. [DOI] [PubMed] [Google Scholar]
- 9.Bobbia X, Grandpierre RG, Claret P-G, et al. Ultrasound guidance for radial arterial puncture: a randomized controlled trial. Am J Emerg Med. 2013;31:810–815. doi: 10.1016/j.ajem.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Ishii S, Shime N, Shibasaki M, et al. Ultrasound-Guided Radial Artery Catheterization in Infants and Small Children*. Pediatr Crit Care Med. 2013;14:471–473. doi: 10.1097/PCC.0b013e31828a8657. [DOI] [PubMed] [Google Scholar]
- 11.Schwemmer U, Arzet HA, Trautner H, et al. Ultrasound-guided arterial cannulation in infants improves success rate. European Journal of Anaesthesiology. 2006;23:476–480. doi: 10.1017/S0265021506000275. [DOI] [PubMed] [Google Scholar]
- 12.Straney L, Clements A, Parslow RC, et al. Paediatric Index of Mortality 3. Pediatr Crit Care Med. 2013;14:673–681. doi: 10.1097/PCC.0b013e31829760cf. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. World Health Organization; Geneva: 2006. [Google Scholar]
- 14.National Heart, Lung, and Blood Institute. Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. 2005. pp. 1–60. [PubMed] [Google Scholar]
- 15.Voors AW, Webber LS, Frerichs RR, et al. Body height and body mass as determinants of basal blood pressure in children--The Bogalusa Heart Study. American Journal of Epidemiology. 1977;106:101–108. doi: 10.1093/oxfordjournals.aje.a112439. [DOI] [PubMed] [Google Scholar]
- 16.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children*. Pediatr Crit Care Med. 2012;13:253–258. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- 17.Dunkler D, Plischke M, Leffondré K, et al. Augmented Backward Elimination: A Pragmatic and Purposeful Way to Develop Statistical Models. PLoS ONE. 2014;9:e113677. doi: 10.1371/journal.pone.0113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maizel J, Guyomarch L, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18:R36. doi: 10.1186/cc13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effects of patient age (A), patient systolic blood pressure (B), patient BMI (C), degree of fluid overload (D), PIM3 probability (E), and trainee months in fellowship (F) on number of attempts to successful radial artery cannulation were compared between procedures performed using ultrasound guidance (n = 82) and those performed using the palpation method (n = 126). Interaction coefficients, 95% CIs, and interaction p-values represent the comparison between ultrasound guided and palpation guided procedures. BMI for age Z-score was replaced with a quadratic term in the regression models due to the non-linear relationship with time. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship
Sample data for systolic blood pressure (A), BMI (B), and degree of fluid overload (C) were binned, and multivariate linear regression p-values were determined for individual bins with respect to time. The negative log of the p-value (y-axis, ) was then plotted against z-scores to determine the thresholds where ultrasound guidance is associated with a significant improvement in time. The significance threshold of 0.05 is noted with a dotted line – values above the line are more significant and values below the line are not significant. Multivariate models were adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship.
A subset of trainees only utilized the palpation method (Single, n = 23 trainees), while another subset of trainees used both ultrasound guidance and the palpation method (Dual, n = 22 trainees). Examining only procedures that were performed with the palpation method, there was no significant differences in Time (A) or Attempts (B) between Single procedure trainees (n = 65 procedures) and Dual procedure trainees (n = 61 procedures). There was no significant differences in Time (C) or Attempts (D) between palpation based procedures performed before (n = 76 procedures and after (n = 50 procedures) implementation of ultrasound curriculum. The linear regression coefficients, 95% CIs, and p-values are shown. Multivariate models included random effects to account for by-trainee variation, and were also adjusted for subject age, subject BMI, subject systolic blood pressure, subject PIM3 score, degree of fluid overload, and trainee months in fellowship.