Abstract
Purpose
Retroillumination photography analysis (RPA) is an objective tool for assessment of the number and distribution of guttae in eyes affected with Fuchs Corneal Dystrophy (FCD). Current protocols include manual processing of images; here we assess validity and interrater reliability of automated analysis across various levels of FCD severity.
Methods
Retroillumination photographs of 97 FCD-affected corneas were acquired and total counts of guttae previously summated manually. For each cornea, a single image was loaded into ImageJ software. We reduced color variability and subtracted background noise. Reflection of light from each gutta was identified as a local area of maximum intensity and counted automatically. Noise tolerance level was titrated for each cornea by examining a small region of each image with automated overlay to ensure appropriate coverage of individual guttae. We tested interrater reliability of automated counts of guttae across a spectrum of clinical and educational experience.
Results
A set of 97 retroillumination photographs were analyzed. Clinical severity as measured by a modified Krachmer scale ranged from a severity level of 1 to 5 in the set of analyzed corneas. Automated counts by an ophthalmologist correlated strongly with Krachmer grading (R2=0.79) and manual counts (R2=0.88). Intraclass correlation coefficient demonstrated strong correlation, at 0.924 (95% CI, 0.870- 0.958) among cases analyzed by three students, and 0.869 (95% CI, 0.797- 0.918) among cases for which images was analyzed by an ophthalmologist and two students.
Conclusions
Automated RPA allows for grading of FCD severity with high resolution across a spectrum of disease severity.
Keywords: Fuchs Corneal Dystrophy, retroillumination photography, cornea, imaging
INTRODUCTION
Fuchs Corneal Dystrophy (FCD) is a progressive, hereditary condition affecting approximately 4% of individuals over 40 years of age and a leading indication for corneal transplantation in the United States. It is characterized by the formation of guttae, excrescences of Descemet membrane that begin in middle age and increase in number over time.
Objective methods frequently utilized to determine disease severity include pachymetry to track development of corneal thickening or specular microscopy to identify loss of endothelial cell density. However, each method is limited by differences in baseline measurements, both among individuals and among populations. In clinical settings, a one-to-five scale is frequently used, often in modified form, but interrater reliability is limited and discrete numbering prevents determination of detailed levels of progression.
We have previously utilized retroillumination photography analysis (RPA) to demonstrate formation of new guttae and progression over time as well as to identify distinct rates of progression associated with specific genotypes.3,4,5,6 In this method, individual guttae are summated for each image and the distribution of guttae assessed. Increased severity, as determined by the number of guttae, reflects not only a widening distribution of guttae, but also increased central corneal density of excrescences.
Despite its successful use in individuals, families, and an island population, historical utility of this technique has been limited by the resource-intensive nature of manual counting, as corneas with advanced disease may demonstrate over 10,000 guttae. Here, we introduce an automated method for RPA which is rapidly conducted, highly correlates with both manual and Krachmer grading of guttae, and demonstrates high interrater reliability.
METHODS
Selection
We analyzed 97 consecutive retroillumination images acquired from corneas with clinically significant Fuchs dystrophy (1+ grading or higher). Eyes with history of intraocular surgery or inflammation and were excluded. All patients were examined by an anterior segment specialist (A.O.E or J.D.G) and assigned Krachmer grading. The Krachmer scale ranges from 1 to 5 and is defined by the number and distribution of guttae; the presence of more than 12 central guttae in a cornea is defined as 1+, larger diameters of confluent guttae range from 2+ (central 2mm) to 4+ (more than 5mm), and the presence of edema is designated as 5+.2 In cases felt to be on the border of two levels, a range was given (e.g., “2-3+) and was documented as the average score (e.g., “2.5”). In our modified approach, trace numbers of guttae (5-12 central guttae) is considered 0.5 and previous corneal transplant is considered at 6+.
The study protocol was approved by the Joint Committee on Clinical Investigation at Johns Hopkins University School of Medicine and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed written consent was obtained from all participants after explanation of the nature and potential consequences of the study.
Image acquisition
Retroillumination photographs were acquired as previously described.4 A minimum of two images were acquired from each side of the cornea. The number of guttae, visible as punctate opacities overlying a red reflex, were previously manually counted for each image. 1
Processing
One image from each patient's cornea was loaded onto Image J software (National Institutes of Health, Bethesda, Maryland, USA). Each image was converted to 8 bit grayscale. To allow adequate visualization of guttae in upcoming steps, the subtract background function was employed with Rolling Ball Radius of 15.0 and images were inverted. The find maxima function was utilized in each image to identify local maximum signals. At this point, an image overlay exists with a single yellow dot over each gutta.
In the Find Maxima function, points may be previewed and noise tolerance is adaptable for each image. This number, averaging approximately 4.6 but ranging from 1 to 11, was titrated for each image until each gutta was grossly covered by approximately one dot. To ensure an appropriate balance of noise reduction, a section of cornea was examined in close-up to identify individual guttae and the noise reduction level finely titrated to ensure appropriate coverage. The image was then zoomed out to provide a final view to ensure a reasonable adjustment. If two consecutive levels of noise reduction were felt to best approximate optimal coverage, with one slightly overestimating and the other slightly underestimating, the two counts were averaged. If two consecutive images were considered as above but one count was affected by peripheral artifact (e.g., recognition of an irregular pupillary margin as indicative of high density of guttae), the image with less artifact was selected. Total number of guttae were then documented for each image. Images from this process are visualized in Figure 1.
Figure 1.
Sample processing of retroillumination photography images of Fuchs corneal dystrophy. After the original image was uploaded to Image J Software (far left), subsequent steps included conversion to 8 bit-grayscale (left), subtraction of background noise to better visualize guttae (center), application of Find maxima function to overlay each gutta with a point (right), and refinement by magnifying a selected area to ensure an appropriate ratio of points to guttae (far right).
Data were entered into Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) and SPSS (IBM Corporation) was utilized for statistical analyses. Correlation with both Krachmer grading and manual counts were assessed by using the mean number of guttae counted for each image by three reviewers.
Validity analyses
Each image was analyzed by three reviewers. The mean number of guttae across reviewers was calculated for each image. Pearson correlation coefficient was then calculated between mean automated count and manual counts of guttae. To explore whether automated counts were distributed in rank according to Krachmer grading, the Kruskal-Wallis Test was conducted across the range of scores of 1 to 5.
Interrater reliability
To assess whether the automated method could be replicated reliably, we sought to determine interrater reliability of automated summation conducted by an ophthalmologist experienced in retroillumination photography analysis, and both medical and undergraduate students. Intraclass correlation coefficient was calculated using a two-way random effect model, which controls for rater effects.
To assess whether examiners rated the quality of images similarly, we additionally explored interrater reliability of image quality assessment, administered on a 1-to-10 scale.
RESULTS
A total of 97 corneal images were analyzed from 51 individuals, 36 females and 15 males. Average Krachmer grading was 2.70, with range from 1+ to 5+ severity. 1+ severity is defined as minimal disease with at least 12 central guttae and 5+ severity is defined as extensive stromal or epithelial edema. Scores from 2+ to 4+ are based on diameter of guttae with increasing size.
Three reviewers across a spectrum of educational levels rated each image. Of 97 images, 38 were rated by three students ranging from an undergraduate to fourth-year medical student. A total of 59 images were analyzed by an anterior segment specialist with experience conducting manual RPA analysis, a medical student, and an undergraduate student.
The mean number of guttae counted by three examiners for each image in corneas at each clinical level of severity (Krachmer grading in parentheses) was 465.9 (1+), 1337.8 (2+), 2172.4 (3+), 5590.9 (4+), and 8957 (5+).
Validity of automated grading
The total range for manual counts ranged from 32 to 10,832 guttae per image, and for automated counts from 61 to 11,707.5 guttae per image.
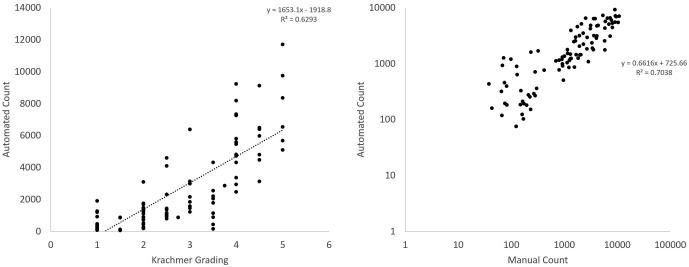
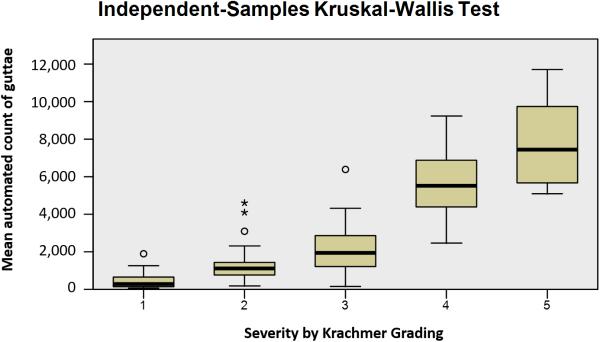
A plot of mean automated count across reviewers as compared to Krachmer grading and manual counts is displayed in Figure 2. Automated counts correlated strongly with manual counting (R2=0.7038). Kruskal-Wallis test of automated counts across 5 levels of Krachmer grading with step-wise comparison reveals distinct distributions of the numbers of guttae across levels (p<0.001), demonstrated in Figure 3.
Figure 2.
Comparison of mean automated count of guttae with Krachmer score and manual counts. Krachmer Grading versus mean automated count of guttae using retroillumination photography analysis (left) shows strong correlation (R2= 0.6293). Automated counts of guttae were also strongly correlated with manual counting (right, R2=0.7038).
Figure 3.
Box Plot Analysis using Kruskal-Wallis Test of automated counts across varying Krachmer scores revealing distinct mean distribution of guttae across each score. Differences between number of guttae for each Krachmer score were significant (p<0.001).
Interrater reliability
Two-way random effects models were produced to assess intraclass correlation coefficient (ICC) for 36 images assessed by three students and for 59 images assessed by a cornea specialist, medical student, and undergraduate student. The ICC was calculated using a consistency definition, assessing reliability, with average measures using a two-way random effects model and demonstrated strong correlation, at 0.924 (95% CI, 0.870- 0.958) among cases analyzed by three students and 0.869 (95% CI, 0.797- 0.918) among cases for which images was analyzed by an ophthalmologist and two students.
DISCUSSION
In this study, we demonstrate the use of automated RPA to provide objective assessment of FCD severity, utilizing a method that is efficient and leverages readily available imaging technology. Its results correlate strongly with both manual counting and clinical grading utilizing the Krachmer scale.
The use of RPA is particularly advantageous over a subjective clinical scale for high levels of severity, where two points of progression years apart may result in the development of thousands of guttae while maintaining a similar clinical grading score of 4+ or 5+. 1 It is notable, therefore, that these cases require the greatest human resources; manual summation of an image with over 10,000 guttae generally requires days of effort. Automation of this process, in contrast, allows measurements of severity to take place within seconds to minutes.
Given that multiple steps are involved, we sought to explore whether measurements could take place across a variety of educational levels, from students to physicians. The results suggest that, even in the absence of clinical experience with Fuchs dystrophy, students were able to follow instructions successfully.
Clinical experience, however, may guide accuracy of measurements. The relationship between Krachmer grading and number of guttae by RPA is familiar to those with previous manual grading experience. Standardized images may be helpful as a guide for new graders, as individuals who have not previously counted guttae manually may be less familiar with the number of guttae expected in each image, and outliers may be more likely. Results in this study suggest such an impact. Further studies with a larger number of graders may help to clarify this point.
Several approaches currently offer quantitative estimates of severity of Fuchs dystrophy, including ultrasound pachymetry, Scheimpflug analyses of corneal volume, and specular microscopy analyses of corneal endothelial cell density. However, baseline corneal thickness or endothelial cell density varies from person to person; in contrast, RPA begins with a common numerical figure of 0, a key strength of this approach.
While the described protocol offers one way to automate summation of guttae, further refinement will be helpful to minimize steps and maximize accuracy. Imaging depends on an adequate red reflex, and techniques to optimize the sharpness of guttae in each image will likely build on the accuracy of the current technique. While some peripheral artifact (identified points that are not true guttae at the edge of each image) may exist and is illustrated in Figure 1, its presence allows the development of an adjustment factor to subtract such extraneous counts from the total. Further refinement may additionally address any skewing that occurs at the extremes of severity; for images with 1+ severity, any artefactual addition to the number of total guttae may affect counts by an order of magnitude, while at extremely high levels of severity, the adjustment of settings in ImageJ for noise reduction could affect total counts by over one thousand guttae with a single click. The total area or diameter of confluent guttae in each image could be utilized as an indicator for severity in approaching such refinements.
In summary, we demonstrate the utility of an objective technique to quantify severity of Fuchs dystrophy in an efficient, automated fashion that correlates well with manual counting and subjective clinical grading of disease.
Acknowledgments
Financial support: NIH R01 EY016835 (JDG), NIH K12 EY015025-10 (AOE)
Footnotes
The authors have no financial conflicts of interest to declare.
References
- 1.Eghrari AO, Garrett BS, Mumtaz AA, Edalati AE, Meadows DN, McGlumphy EJ, Iliff BW, Gottsch JD. Retroillumination Photography Analysis Enhances Clinical Definition of Severe Fuchs Corneal Dystrophy. Cornea. 2015;34:1623–6. doi: 10.1097/ICO.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krachmer JH, Purcell JJ, Jr, Young CW. Corneal endothelial dystrophy: a study of 64 families. Arch Ophthalmol. 1978;96:2036–2039. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 3.Gottsch JD, Sundin OH, Rencs EV, et al. Analysis and documentation of progression of Fuchs corneal dystrophy with retroillumination photography. Cornea. 2006;25:485–489. doi: 10.1097/01.ico.0000178726.11693.14. [DOI] [PubMed] [Google Scholar]
- 4.Meadows DN, Eghrari AO, Riazuddin SA, et al. Progression of Fuchs corneal dystrophy in a family linked to the FCD1 locus. Invest Ophthalmol Vis Sci. 2009;50:5662–5666. doi: 10.1167/iovs.09-3568. [DOI] [PubMed] [Google Scholar]
- 5.McGlumphy EJ, Yeo WS, Riazuddin SA, et al. Age-severity relationships in families linked to FCD2 with retroillumination photography. Invest Ophthalmol Vis Sci. 2010;51:6298–6302. doi: 10.1167/iovs.10-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eghrari AO, McGlumphy EJ, Iliff BW, et al. Prevalence and severity of fuchs corneal dystrophy in Tangier Island. Am J Ophthalmol. 2012;153:1067–1072. doi: 10.1016/j.ajo.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]