Abstract
Retention in early HIV care has been associated with decreased mortality and improved viral suppression, however the consequences of poor retention in early care in Brazil remain unknown. We assessed the effect of poor retention on mortality in a Brazilian HIV-infected clinical cohort. The analysis included ART-naïve, HIV-infected adults linked to care at the Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz between 2000 and 2010, who did not become pregnant nor participate in a clinical trial during the first two years in care (early care). Poor retention in early care was defined as less than 3 out of 4 six-month intervals with a CD4 or HIV-1 RNA laboratory result during early care. Cox proportional hazards models were used to identify factors associated with mortality, and Kaplan-Meier plots were used to describe the survival probability for participants with poor retention versus good retention. Among 1054 participants with a median (interquartile range) follow-up time of 4.2 years (2.6, 6.3), 20% had poor retention in early care and 8% died. Poor early retention [adjusted hazard ratio (aHR) 3.09; 95% CI 1.65–5.79], AIDS defining illness (aHR 1.95; 95% CI 1.20–3.18), lower education (aHR 2.33; 95% CI 1.45–3.75) were associated with increased mortality risk. Our findings highlight the importance of adopting strategies to improve retention in early HIV care.
Keywords: retention, HIV, survival analysis, cohort studies, urban population
Introduction
Brazil’s policy of universal access to antiretroviral therapy (ART) has been credited with improving survival among HIV-infected persons (Marins et al., 2003) and avoiding generalization of the AIDS epidemic (Greco & Simao, 2007). Access to HIV testing and ART have been fundamental to the success of the Brazilian HIV/AIDS program; however, there are disparities in clinical outcomes. In particular, using intravenous drugs (Malta et al., 2009; Tancredi & Waldman, 2014a, 2014b), being of self-reported black race (Tancredi & Waldman, 2014a) and having lower education (Tancredi & Waldman, 2014a, 2014b) have been associated with increased mortality and faster progression to AIDS.
In high-income settings, retention during the first 1–2 years of HIV care has been shown to improve survival (Giordano et al., 2007; Giordano, Hartman, Gifford, Backus, & Morgan, 2009), but there is limited information on the effects of retention on mortality from low- and middle-income countries in Latin America (Fox & Rosen, 2015). We previously reported that early ART initiation, older age and higher educational level were associated with good retention in early care (Silva et al., 2016); however, the effect of retention in early care on mortality among HIV-infected persons in our setting remains unknown. We aimed to assess whether poor retention in early HIV care is associated with increased mortality in an urban, Brazilian, clinical HIV cohort.
Methods
The Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI) is a national reference center for HIV/AIDS care, research and training. Since 1998, INI has maintained a clinical cohort of HIV-infected adults in care (Grinsztejn et al., 2009). All cohort procedures were approved by the INI Institutional Review Board. This analysis used de-identified health information, and was exempt by the Institutional Review Board of the University of California, Los Angeles.
For the present analysis, ART-naïve, HIV-infected persons ≥18 years old initiating care at INI between January 1, 2000 and June 30, 2010 were eligible for inclusion. Participants must have been linked to care (defined as the first outpatient CD4+ T lymphocyte count [CD4] or HIV-1 RNA measurement or ART initiation date) within 6 months after their first clinic visit and have survived 2 years post-linkage (early care) to be included in the analysis. Individuals who became pregnant during early care (N=35) or ever participated in a clinical trial (N=492) were excluded because of unique laboratory monitoring requirements (please see Silva et al., 2015 for further details).
Follow-up began at the end of early care (i.e., 2 years after linkage to care). End of follow-up was defined as the date of death, and, for those not known to have died, as 1 year after the date of last clinical contact (last CD4, HIV-1 RNA or clinic visit) with a maximum censor date of December 31, 2013. Loss to follow-up (LTFU) was defined for participants with a censor date before December 31, 2012. Mortality data was exhaustively verified up to December 31, 2013 using participants’ medical charts, outreach to participants’ personal contacts and by linkage with the State of Rio de Janeiro Mortality database using a previously validated algorithm (Pacheco et al., 2008).
The Brazilian Ministry of Health recommendations of laboratory monitoring every 6 months (Brazilian Ministry of Health, 2013) guided our definitions for linkage and retention. Poor retention in early care was defined as the presence of an outpatient CD4 or HIV-1 RNA measurement in less than 3 out of 4 six-month intervals during early care, as described previously (Silva et al., 2016). Socio-demographic and clinical factors were extracted from the clinical cohort database. Year of linkage was used as a dichotomous variable because a prior study of this cohort (Silva et al., 2016) demonstrated two periods from 2000–2002 and from 2003–2011 when the prevalence of good retention was significantly different, increasing from 54–61 to 77–89 %, respectively.
Kaplan-Meier plots were used to describe the survival probability for participants with poor versus good retention in early care. Factors associated with mortality were identified using Cox proportional hazards regression models. Factors with an unadjusted p-value ≤0.25 were included in the initial adjusted model, and kept in the final model when factor adjusted p-value was ≤0.05 or when factor removal changed the effect size of another factor in >10% (change-in-estimate approaches, Greenland & Pearce, 2015). Additionally, factors known to be associated with mortality through prior studies were kept in the final model (i.e. ART initiation). Moreover, sex and age were included in the adjusted models and maintained a priori. The proportional hazards assumption was tested using Schoenfeld residuals. Schoenfeld residuals were significant for our exposure of interest (i.e. poor retention in early care), suggesting time-varying effects on mortality which were resolved with the inclusion of an interaction term between year of linkage (as defined above) and poor retention in early care. Analyses were performed using R Statistical Software.
Results
The study population consisted of 1054 participants with a median (interquartile range [IQR]) follow-up time of 4.2 years (2.6, 6.3), LTFU rate of 1.6 per 100 person-years (PY) and mortality rate of 1.8 per 100 PY. Participants with good retention in early care (80%) had a median follow-up time of 4.3 years (2.8, 6.1), LTFU rate of 0.9 per 100 PY and mortality rate of 1.4 per 100 PY. Participants with poor retention in early care (20%) had a median follow-up time of 3.8 (1.4, 7.8), LTFU rate of 4.7 per 100 PY and mortality rate of 3.2 per 100 PY.
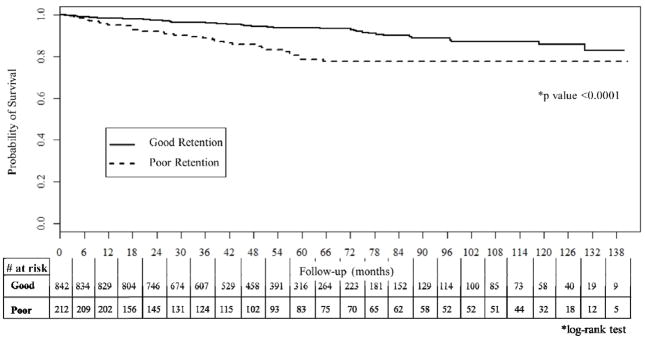
In total, 89 (8%) participants died after completing early care. Compared to those who survived, participants who died were older and had higher frequencies of poor retention in early care (37% vs 19%, p <0.001), AIDS defining illness (63% vs 40%, p <0.001) and ≤8 years of education (72% vs 46%, p<0.001) (Table 1). Participants with poor retention in early care had significantly worse survival than those with good early retention (log-rank p<0.0001; Figure 1).
Table 1.
Descriptive statistics and Cox proportional hazards models for predicting mortality among HIV-infected persons receiving care at INI, 2002–2013
| Study population (n=1054) | Alive (n=965) | Dead (n=89) | p value‡ | Unadjusted Hazard Ratio | p value | Adjusted Hazard Ratio⋄ | p value | |
|---|---|---|---|---|---|---|---|---|
| Sex (male)a | 758 (72) | 692 (72) | 66 (74) | 0.71 | 1.20 [0.75, 1.93] | 0.45 | 1.43 [0.87, 2.33] | 0.15 |
| Race (non-white)b | 478 (45) | 432 (45) | 46 (52) | 0.25 | 1.16 [0.95, 1.43] | 0.14 | ||
| Median Age (IQR)c | 35 (28, 42) | 35 (28, 42) | 36 (30, 45) | 0.15 | 1.47 [0.97, 2.23] | 0.07 | 1.21 [0.99, 1.50] | 0.06 |
| Education (≤8 years)d | 511 (48) | 447 (46) | 64 (72) | < 0.001 | 2.65 [1.73, 4.36] | <0.0001 | 2.33 [1.45, 3.75] | 0.0005 |
| Transmission Routee | 0.95 | |||||||
| Heterosexual | 585 (56) | 535 (55) | 50 (56) | Ref | Ref | |||
| MSM | 412 (39) | 379 (39) | 33 (37) | 1.02 [0.66, 1.59] | 0.92 | |||
| Other | 14 (1) | 51 (5) | 6 (7) | 1.40 [0.60, 3.26] | 0.44 | |||
| ART initationf (>3 months post-linkage) | 555 (53) | 515 (53) | 40 (45) | 0.16 | 0.80 [0.52, 1.22] | 0.3 | 1.17 [0.73, 1.89] | 0.52 |
| Nadir CD4 Count (<200 cells/mm3)g | 437 (41) | 394 (41) | 43 (48) | 0.21 | 1.19 [0.78, 1.81] | 0.41 | ||
| AIDS Defining Diseaseh | 445 (42) | 389 (40) | 56 (63) | <0.001 | 2.21 [1.43, 3.40] | 0.0003 | 1.95 [1.20 3.18] | 0.007 |
| Depressioni | 180 (17) | 166 (17) | 14 (16) | 0.84 | 0.87 [0.49, 1.54] | 0.63 | ||
| Metabolic Diseasej | 549 (52) | 495 (51) | 54 (61) | 0.11 | 1.37[0.90, 2.10] | 0.14 | ||
| Year of linkage ≤ 2003 | 220 (21) | 180 (19) | 40 (45) | < 0.001 | 1.73 [1.10, 2.73] | 0.02 | 1.72 [0.97, 3.05] | 0.06 |
| Poor retention in early care | 212 (20) | 179 (19) | 33 (37) | < 0.001 | 2.42 [1.57, 3.73] | <0.0001 | 3.09 [1.65, 5.79] | 0.0004 |
Chi-squared test for categorical factors and Kruskal-Wallis test for continuous asymmetric factors
Results also adjusted by an interaction term between year of linkage ≤2003 and poor retention in early care (adjusted hazard ratio = 0.46 [0.19, 1.11]; p value = 0.11)
Biological sex at birth
Categorized as white or non-white
Age = date of care initiation – date of birth, adjusted and unadjusted HR per 10 year increase
Categorized as >8 years and ≤8 years
Hierarchically categorized into intravenous drug use (IDU, regardless of sexual exposure), men who have sex with men (MSM), heterosexual (including bisexual women), other, and unknown. Women with unknown HIV transmission route (n=45) were categorized as heterosexual. Vertical transmission (n=2), work accident (n=3), and transfusion (n=6), intravenous drug use (n=14) and unknown (n=34) were included in the “other” transmission group.
Determined by first recorded ART initiation date
Nadir CD4 < 200 cells/mm3 before the start of follow-up (i.e end of early care period) + 6 months. 5 participants with missing values categorized as CD4 ≥ 200 cells/mm3.
Diagnosis of an AIDS defining disease, per the Centers for Disease Control and Prevention 1993 criteria, before the start of follow-up (i.e. end of early care period) + 6 months.
Clinical diagnosis before the start of follow-up (i.e. end of early care period) + 6 months.
Diagnosis before the start of follow-up (i.e. end of early care period) + 6 months with ≥1 of the following: diabetes (fasting blood glucose ≥126mg/dL, random blood glucose ≥200 mg/dL or hemoglobin A1c >6.5%), dyslipidemia (LDL >159 mg/dL or HDL <40 mg/dL), hypercholesterolemia (total cholesterol >239mg/dL), hypertriglyceridemia (triglycerides >199mg/dL) or hypertension (diastolic blood pressure >100mmHg)
Figure 1.
Kaplan-Meier survival curve of good versus poor retention in early HIV care at INI, 2002–2013
In the final adjusted model, poor retention in early care had the highest association with mortality (adjusted hazard ratio [aHR] 3.09; 95% confidence interval [95% CI] 1.65, 5.79). Having an AIDS defining illness (aHR 1.95; 95% CI 1.20, 3.18), ≤8 years of education (aHR 2.33; 95% CI 1.45, 3.75) were also associated with increased mortality risk.
Discussion
In this study, poor retention in early HIV care was strongly associated with mortality, highlighting the potential value of monitoring and improving retention in early care. Our results emphasize the need for interventions to improve clinical outcomes among HIV-infected persons with AIDS defining illnesses and lower education in Brazil.
Increased mortality risk among HIV-infected persons with poor retention in early HIV care may be explained by missed opportunities for effective management of their HIV infection. Our measure of retention was based on laboratory results, which served as proxies for clinic visits as patients require a prescription from their HIV care provider in order to have tests performed. Thus, poor retention implied inadequate HIV monitoring though not necessarily worse management of HIV disease. However, worse retention in HIV care has been associated with worse adherence to ART (Giordano et al., 2007), and it is plausible that patients with poor retention also had worse management of HIV disease. In addition, unmeasured factors, such as unmet housing needs, may have played a role in retention (Tobias et al., 2007) as well as mortality risk (Buchanan, Kee, Sadowski, & Garcia, 2009). Available data for this analysis did not include unmet needs, which should be addressed in future studies.
We previously reported that poor retention in early care was associated with younger age and ≤8 years of education (Silva et al., 2016). In the present analysis, lower education was associated with increased mortality risk. Importantly, lower education has also been associated with virologic failure in this cohort (Cardoso et al., 2014), which likely contributes to our findings. Lower education may be an indicator of lower socio-economic status (Szwarcwald, Souza-Junior, & Damacena, 2010), which has also been associated with increased mortality risk (Burkey et al., 2014). We also observed an approximately two-fold increased mortality risk among persons with AIDS defining illnesses, consistent with prior results from our cohort that showed that AIDS defining illnesses increased risk of AIDS-related and non-AIDS related deaths (Grinsztejn et al., 2013) [REF]. Presence of AIDS defining illness at baseline implies late entry, and prior studies have shown that late entry to care in Brazil stems from many factors and prevails even in well-developed urban settings with access to HIV testing and care (Grangeiro, Escuder, & Pereira, 2012) REF]. Our findings thus corroborate and highlight the importance of early entry into care (Grangeiro, Escuder, Menezes, Alencar, & Ayres de Castilho, 2011) and early ART initiation (Group et al., 2015) in improving survival.
Our analysis is not without limitations. First, although poor retention has been previously associated with poor ART adherence (Giordano et al., 2007), we were unable to adjust for ART adherence. However, as ART adherence and its immunologic impact are on the causal pathway to AIDS-related mortality, similar survival analyses have not adjusted for these factors (Giordano et al., 2007). We were also unable to adjust for substance abuse, which has been shown to correlate with both retention in HIV care (Giordano et al., 2009) and mortality (Malta et al., 2009), and should be addressed in future research. We did not include causes of death because our data was incomplete for 2012 and 2013. Further studies are necessary to investigate the possible link between good and poor retention in early HIV care with mortality from specific causes. Finally, considering this a non-probabilistic sample, our results may not be generalizable to other HIV-infected cohorts or populations.
Conclusion
This analysis demonstrated associations between poor retention in early care, lower education and history of an AIDS diagnosis with increased risk of mortality in an urban Brazilian cohort of HIV-infected persons with universal access to ART. Monitoring retention in early care and adopting strategies to improve retention in early HIV care may to lead to improved health outcomes.
Acknowledgments
We would like to thank the participants enrolled in the INI HIV/AIDS cohort, as well as the hard working and dedicated staff at INI. This work was supported by the National Institutes of Health under Grant R25 MH087222 to JLC, and under Grant K23 AI110532. This work was supported in part by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). PML and BG acknowledge funding from the National Council of Technological and Scientific Development (CNPq) and the Research Funding Agency of the State of Rio de Janeiro (FAPERJ).
Footnotes
Conflicts of Interest: None
Contributor Information
Paula M. Luz, Email: luzpaulamendes@gmail.com.
Jordan E. Lake, Email: jlake@mednet.ucla.edu.
Sandra W. Cardoso, Email: dra.wagner@gmail.com.
Sayonara Ribeiro, Email: rocha.sayonara@gmail.com.
Ronaldo I. Moreira, Email: ronaldo.ismerio@gmail.com.
Jesse L. Clark, Email: JLClark@mednet.ucla.edu.
Valdilea G. Veloso, Email: valdilea.veloso@gmail.com.
Beatriz Grinsztejn, Email: beatriz.grinsztejn@gmail.com.
Raquel B. De Boni, Email: raqueldeboni@gmail.com.
References
- Brazilian Ministry of Health. Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. 2013 Retrieved from http://www.aids.gov.br/pcdt on Sept 1. 2014.
- Buchanan D, Kee R, Sadowski LS, Garcia D. The health impact of supportive housing for HIV-positive homeless patients: a randomized controlled trial. Am J Public Health. 2009;99(Suppl 3):S675–680. doi: 10.2105/AJPH.2008.137810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey MD, Weiser SD, Fehmie D, Alamo-Talisuna S, Sunday P, Nannyunja J, … Chang LW. Socioeconomic determinants of mortality in HIV: evidence from a clinical cohort in Uganda. J Acquir Immune Defic Syndr. 2014;66(1):41–47. doi: 10.1097/QAI.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SW, Luz PM, Velasque L, Torres T, Coelho L, Freedberg KA, … Grinsztejn B. Effectiveness of first-line antiretroviral therapy in the IPEC cohort, Rio de Janeiro, Brazil. AIDS Res Ther. 2014;11:29. doi: 10.1186/1742-6405-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, … Morgan RO. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- Grangeiro A, Escuder MM, Menezes PR, Alencar R, Ayres de Castilho E. Late entry into HIV care: estimated impact on AIDS mortality rates in Brazil, 2003–2006. PLoS One. 2011;6(1):e14585. doi: 10.1371/journal.pone.0014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangeiro A, Escuder MM, Pereira JC. Late entry into HIV care: lessons from Brazil, 2003 to 2006. BMC Infect Dis. 2012;12:99. doi: 10.1186/1471-2334-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco DB, Simao M. Brazilian policy of universal access to AIDS treatment: sustainability challenges and perspectives. AIDS. 2007;21(Suppl 4):S37–45. doi: 10.1097/01.aids.0000279705.24428.a3. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. 2015;36:89–108. doi: 10.1146/annurev-publhealth-031914-122559. [DOI] [PubMed] [Google Scholar]
- Grinsztejn B, Luz PM, Pacheco AG, Santos DV, Velasque L, Moreira RI, … Veloso VG. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One. 2013;8(4):e59768. doi: 10.1371/journal.pone.0059768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsztejn B, Veloso VG, Friedman RK, Moreira RI, Luz PM, Campos DP, … Moore RD. Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS. 2009;23(16):2107–2114. doi: 10.1097/QAD.0b013e32832ec494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, … Neaton JD. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malta M, Bastos FI, da Silva CM, Pereira GF, Lucena FF, Fonseca MG, Strathdee SA. Differential survival benefit of universal HAART access in Brazil: a nation-wide comparison of injecting drug users versus men who have sex with men. J Acquir Immune Defic Syndr. 2009;52(5):629–635. doi: 10.1097/QAI.0b013e3181b31b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marins JR, Jamal LF, Chen SY, Barros MB, Hudes ES, Barbosa AA, … Hearst N. Dramatic improvement in survival among adult Brazilian AIDS patients. AIDS. 2003;17(11):1675–1682. doi: 10.1097/01.aids.0000072649.21517.80. [DOI] [PubMed] [Google Scholar]
- Pacheco AG, Saraceni V, Tuboi SH, Moulton LH, Chaisson RE, Cavalcante SC, … Harrison LH. Validation of a hierarchical deterministic record-linkage algorithm using data from 2 different cohorts of human immunodeficiency virus-infected persons and mortality databases in Brazil. Am J Epidemiol. 2008;168(11):1326–1332. doi: 10.1093/aje/kwn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DS, De Boni RB, Lake JE, Cardoso SW, Ribeiro S, Moreira RI, … Luz PM. Retention in Early Care at an HIV Outpatient Clinic in Rio de Janeiro, Brazil, 2000–2013. AIDS Behav. 2016;20(5):1039–1048. doi: 10.1007/s10461-015-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarcwald CL, Souza-Junior PR, Damacena GN. Socioeconomic inequalities in the use of outpatient services in Brazil according to health care need: evidence from the World Health Survey. BMC Health Serv Res. 2010;10:217. doi: 10.1186/1472-6963-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi MV, Waldman EA. Predictors of progression to AIDS after HIV infection diagnosis in the pre- and post-HAART eras in a Brazilian AIDS-free cohort. Trans R Soc Trop Med Hyg. 2014a;108(7):408–414. doi: 10.1093/trstmh/tru078. [DOI] [PubMed] [Google Scholar]
- Tancredi MV, Waldman EA. Survival of AIDS patients in Sao Paulo-Brazil in the pre- and post-HAART eras: a cohort study. BMC Infect Dis. 2014b;14:599. doi: 10.1186/s12879-014-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, … Drainoni ML. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21(6):426–434. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]