Abstract
Female sex workers (FSW) have a high prevalence of substance use and HIV, but the impact of substance use on HIV treatment engagement is not well established. We evaluated the association between alcohol and marijuana use and sub-optimal HIV treatment engagement outcomes among HIV-infected FSW in Lilongwe, Malawi. We enroled FSW using venue-based recruitment into a cross-sectional evaluation assessing substance use and HIV treatment engagement. Seropositive FSW, identified through HIV rapid testing, received rapid CD4 count and viral load testing. We used Poisson regression with robust variance estimates to ascertain associations of alcohol and marijuana use with sub-optimal HIV treatment outcomes: (1) lack of ART use among previously diagnosed, ART-eligible FSW and (2) viral nonsuppression among FSW on ART. Of previously diagnosed, ART-eligible FSW (n = 96), 29% were not using ART. Patterns of hazardous drinking were identified in 30%, harmful drinking in 10%, and alcohol dependence in 12%. ART-eligible FSW with harmful drinking or alcohol dependency were 1.9 (95% CI: 1.0, 3.8) times as likely to not use ART compared to FSW without harmful or dependent drinking. Among those on ART, 14% were virally nonsuppressed. The prevalence ratio for viral nonsuppression was 2.0 (95% CI: 0.6, 6.5) for harmful drinkers and alcohol-dependent FSW. Over 30% of ART-eligible FSW reported using marijuana. Marijuana-using FSW were 1.9 (95% CI: 0.8, 4.6) times as likely to not use ART compared to FSW who were not using marijuana. Given the high prevalence of alcohol use and its association with lack of ART use, ART uptake and alcohol reduction strategies should be tailored for alcohol-using FSW in Malawi.
Keywords: FSW, alcohol, marijuana, antiretroviral therapy, viral suppression
Introduction
Substance use is prevalent among female sex workers (FSW) globally (Abdool Karim et al., 2010; El-Bassel, Witte, Wada, Gilbert, & Wallace, 2001; Shakarishvili et al., 2005; Strathdee et al., 2010; Tegang et al., 2010; Wechsberg et al., 2009; Wechsberg, Luseno, Lam, Parry, & Morojele, 2006). FSW use substances to facilitate soliciting clients and to cope with stigma and stress related to sex work (Abdool Karim et al., 2010; Chersich et al., 2007; de Graaf, Vanwesenbeeck, van Zessen, Straver, & Visser, 1995; El-Bassel et al., 2001; Gupta, Raj, Decker, Reed, & Silverman, 2009). Substance-using FSW have low condom usage, commonly experience sexual abuse, and are at high risk for HIV acquisition (Agha & Chulu Nchima, 2004; Greenberg et al., 2009; Wechsberg et al., 2006; Wechsberg, Luseno, & Lam, 2005).
The global HIV prevalence among FSW is 12%, with a prevalence of 37% among FSW in Sub-Saharan Africa (Baral et al., 2012). The HIV prevalence among Malawian FSW is one of the highest globally – approximately 70% live with HIV (Lancaster et al., 2016). To improve health outcomes and reduce ongoing transmission risk, HIV-infected FSW must use antiretroviral therapy (ART) and become virally suppressed. But engagement in HIV treatment appears to be a particular challenge among FSW in Sub-Saharan Africa (Chersich et al., 2013; Mountain et al., 2014; WHO UNAIDS UNICEF, 2010).
For FSW, one potential barrier to engagement in HIV treatment is substance use. Heavy use of alcohol and/or marijuana, the most commonly used substances in Malawi, severely affects cognitive functions, such as decision-making and memory (Azar, Springer, Meyer, & Altice, 2010; Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Curran, Brignell, Fletcher, Middleton, & Henry, 2002; Korthuis et al., 2012). Substance users with impairment of these cognitive skills may experience difficulty managing their HIV treatment (Azar et al., 2010; Chitwood, McBride, French, & Comerford, 1999; Korthuis et al., 2012; Sohler et al., 2007; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003). Hence, the impact of substance use on engagement in HIV treatment among FSW must be understood.
For this study, we examined the association between alcohol and marijuana use and sub-optimal HIV treatment engagement among HIV-infected FSW in Lilongwe, Malawi.
Methods
Study design
In this study, we analyzed data from a cross-sectional study of FSW in Lilongwe, Malawi, who were recruited using venue-based sampling in 2011. Detailed study method has been described previously (Lancaster et al., 2016). Briefly, consented FSW received a behavioral survey seeking detailed information on alcohol and marijuana use, and HIV testing and treatment engagement. CD4 measurements and plasma HIV-RNA levels were obtained from all HIV-seropositive FSW.
Substance use assessment
We measured alcohol use using the World Health Organization’s (WHO) Alcohol Use Identification Test (AUDIT) (Allen, Litten, Fertig, & Babor, 1997; Saunders, Aasland, Babor, de la Fuente, & Grant, 1993; World Health Organization (WHO), 2000), an internationally validated screening tool that measures alcohol-use behaviors and alcohol-use disorder symptoms (Parry et al., 2005; Woolf-King & Maisto, 2011). An AUDIT score of 0–6 was considered indicative of abstinence or nonhazardous drinking, 7–15 of hazardous drinking, 16–19 of harmful drinking, and ≥20 of alcohol dependency (World Health Organization (WHO), 2000).
Marijuana use was assessed by reports of lifetime marijuana and number of days using marijuana within the past 30 days. We defined current marijuana use as FSW who reported using marijuana at least one day within the prior 30 days.
HIV treatment engagement assessments
We asked the date and results of their most recent HIV test to determine new versus previous diagnosis. We defined a new HIV diagnosis as FSW who were seropositive, based on HIV rapid testing, and self-reported being HIV negative at their most recent HIV test, previously tested but did not receive their results, or never tested previously.
We assessed reported current ART use among all previously diagnosed, ART-eligible FSW. ART eligibility was defined as those reporting current ART use, a CD4 ≤500 cells/mm3 following the Malawi national guidelines, currently pregnant or breastfeeding, or any pregnancy after implementation of Option B+ policy (World Health Organization, 2010) in July 2011. FSW who answered no to the question “Are you currently on ART?” were classified as not using ART.
We measured HIV-1 RNA concentration for viral suppression. FSW were classified as nonsuppressed with an HIV-1 RNA >5000 copies/mL, the WHO’s recommended threshold when using fingerstick dried blood spots (Arredondo et al., 2012; Rutstein et al., 2014; World Health Organization, 2012).
Covariates
Based on the literature, we identified a parsimonious set of covariates: age, education, marital status, housing, gravidity, financial dependents, probable depression measured by the Patient Health Questionnaire-9 (Cholera et al., 2014; Monahan et al., 2009; Pence et al., 2012), and treated for an STI in the prior 12 months, years in sex work, location for soliciting clients, weekly number of clients, condom use during vaginal sex with clients in prior 7 days, client ever demanded not using a condom for vaginal sex, and alcohol use prior to last vaginal sex with client.
Statistical analysis
We conducted separate analyses for the associations of alcohol and marijuana use with two primary treatment engagement outcomes: (1) lack of ART use among those previously diagnosed and ART-eligible at the time of survey and (2) viral nonsuppression among those on ART. Poisson regression with robust variance estimates was used to estimate bivariable and, when sample size permitted, multivariable prevalence ratios (PRs) with 95% confidence intervals (CIs) (Barros & Hirakata, 2003; Zou, 2004).
We assessed potential confounders and retained them in the final adjusted multivariable models if removal resulted in a >10% change in estimate. Interactions were only considered for variables of interest that met positivity assumptions and were considered to have public health relevance; they were retained in final adjusted multivariable models if they reached statistical significance at alpha = 0.10. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Among the 200 enrolled FSW, 111 (56%) were previously diagnosed and confirmed HIV infected. Of those previously diagnosed, 96 (86%) were considered eligible for ART, with the median age of 26 years (IQR: 23–30).
Among those previously diagnosed and ART eligible, over half (58%) were living at a bar or bottle shop (Table 1). Almost all had a previous pregnancy (92%). The median time exchanging sex for money was 3 years (IQR: 1–6) and the number of clients per week was 20 (IQR: 10–35).
Table 1.
Characteristics and substance use of previously diagnosed, ART-eligible FSW in Lilongwe, Malawi, July–September 2014 (N = 96).
| n | (%) | |
|---|---|---|
| Age (years) | ||
| 18–24 | 37 | (39) |
| 25–29 | 32 | (34) |
| ≥30 | 27 | (28) |
| Education | ||
| Never attended or only primary school | 66 | (69) |
| Any secondary or more school | 30 | (31) |
| Marital status | ||
| Never married | 10 | (10) |
| Married or co-habitating | 3 | (3) |
| Separated, divorced, or widowed | 83 | (87) |
| Housing | ||
| Private house | 13 | (14) |
| Bar or bottle shop | 56 | (58) |
| Guesthouse or hotel | 27 | (28) |
| Number of pregnancies | ||
| 0 | 8 | (8) |
| ≥1 | 88 | (92) |
| Number of financial dependents | ||
| 0 | 4 | (4) |
| ≥1 | 92 | (96) |
| Depression | ||
| No probable depression | 87 | (91) |
| Probable depression | 9 | (9) |
| Treated for an STI in prior 12 months | ||
| No | 72 | (75) |
| Yes | 24 | (25) |
| Duration of sex work (years) | 11 | (11) |
| <1.0 | 15 | (16) |
| 1.0–1.9 | 17 | (18) |
| 2.0–2.9 | 53 | (55) |
| ≥3.0 | ||
| Location for soliciting clients | ||
| Bar or bottle shop | 11 | (11) |
| Other | 85 | (89) |
| Number of clients per weeka | ||
| <10 | 19 | (20) |
| 10–19 | 22 | (23) |
| 20–29 | 27 | (28) |
| ≥30 | 27 | (28) |
| Condom use with client in prior 7 days | ||
| Inconsistent | 22 | (23) |
| Consistent | 74 | (77) |
| Ever had a client demand not using a condom during vaginal sex | ||
| No | 40 | (42) |
| Yes | 56 | (58) |
| Alcohol use prior to last vaginal sex with client | ||
| No | 68 | (71) |
| Yes | 28 | (29) |
| Substance use | ||
| Alcohol use (AUDIT) | ||
| Nonhazardous drinking (score 0–6) | 46 | (48) |
| Hazardous drinking (score 7–15) | 29 | (30) |
| Harmful drinking (score 16–19) | 10 | (10) |
| Alcohol dependent (score ≥ 20) | 11 | (12) |
| Marijuana use | ||
| No current marijuana use | 76 | (79) |
| Current marijuana useb | 20 | (21) |
Note: AUDIT = Alcohol Use Disorder Identification Test.
Missing data due to not knowing or refused to answer: number of clients in past 7 days: n = 1.
Current use defined as reported use in past 30 days.
Approximately 30% (n = 28) reported using alcohol prior to their last vaginal sex act with a client (Table 1). Overall, over half (52%; n = 50) had AUDIT scores ≥7. Nearly one-third had patterns of hazardous drinking. Over 20% were heavily consuming alcohol. Twenty one percent of all ART-eligible FSW reported current marijuana use.
Twenty-five percent were not using ART. FSW not using ART had a lower median CD4 count (391, IQR: 261–474) and higher median viral load (86,345, IQR: 26,319–337,689) when compared to FSW who were using ART (Figure 1).
Figure 1.
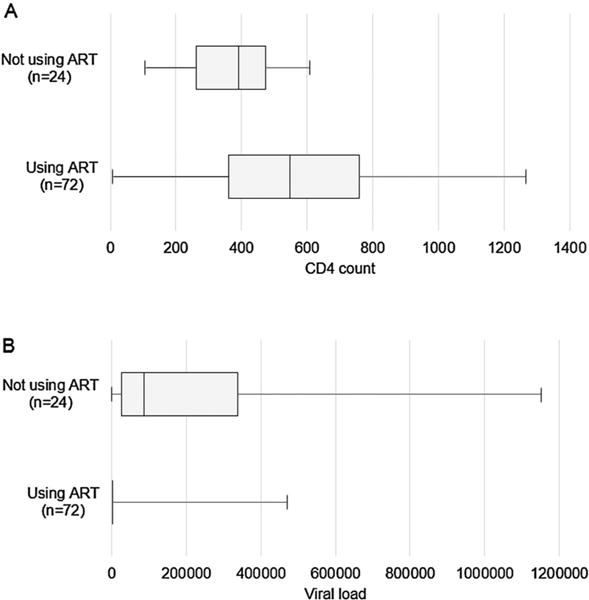
CD4 count (a) and viral loads (b) at the time of cross-sectional survey by reported ART use among ART-eligible HIV-infected FSW (n = 96); box plots showing median (line), interquartile range (IQR; box), minimum (lower whiskers), and maximum (upper whiskers). ART = antiretroviral therapy
The prevalence of ART nonuse was 38% among those who were harmful drinkers or alcohol dependent and 21% among those who were not harmful or dependent drinkers (Table 2). In adjusted multivariable analyses, FSW who were harmful drinkers or alcohol dependent were 1.9 (95% CI: 1.0, 3.8) times as likely to not use ART compared to FSW who were not harmful or dependent drinkers.
Table 2.
Prevalence and association of alcohol and marijuana use with sub-optimal HIV treatment engagement (not using ART and virally nonsuppressed) among HIV-infected FSW in Lilongwe, Malawi.
| Reported ART use (N = 96)
|
Not using ART
|
Viral suppression (N = 72)
|
Virally nonsuppressed
|
|||||
|---|---|---|---|---|---|---|---|---|
| Not using ART (n = 24) | Using ART (n = 72) | Unadjusted PR (95% CI) | Adjusted PR (95% CI) | Virally nonsuppressed (n = 10) | Virally suppressed (n = 62) | Unadjusted PR (95% CI) | Adjusted PR (95% CI) | |
| Alcohol use (AUDIT) | ||||||||
| Nonharmful/alcohol dependence (score ≤15) | 16 (21) | 59 (79) | 1.0 | 1.0 | 7 (12) | 52 (88) | 1.0 | 1.0 |
| Harmful/alcohol dependence (score ≥16) | 8 (38) | 13 (62) | 1.8 (0.9, 3.6) | 1.9 (1.0, 3.8)b | 3 (23) | 10 (77) | 2.0 (0.6, 6.5) | 1.71 (0.5, 5.5)c |
| Marijuana use | ||||||||
| No current marijuana use | 17 (23) | 59 (78) | 1.0 | 1.0 | 9 (15) | 50 (85) | 1.0 | 1.00 |
| Current marijuana usea | 7 (35) | 13 (65) | 1.5 (0.8, 3.2) | 1.9 (0.8, 4.6)c | 1 (8) | 12 (92) | – | – |
Note: ART = antiretroviral therapy; PR = prevalence ratio; CI = confidence interval; AUDIT = Alcohol Use Disorder Identification Test.
Current use defined as reported use in past 30 days.
Adjusted for number of clients per week, housing, and duration in sex work (years).
Adjusted for treatment for an STI in the prior 12 months.
Among the FSW using ART, 14% were nonsuppressed. Among ART users who were harmful drinkers or alcohol dependent, the prevalence of viral nonsuppression was 23%, compared to a viral nonsuppression prevalence of 12% among ART users who were not harmful drinkers or alcohol dependent. In adjusted multivariable analyses, FSW who were harmful drinkers or alcohol dependent were 1.71 (95% CI: 0.5, 5.5) times as likely to be virally nonsuppressed compared to FSW who were not harmful or dependent drinkers.
The prevalence of ART nonuse was 35% among current marijuana users. FSW who were currently using marijuana were 1.9 (95% CI: 0.8, 4.6) times as likely to not use ART compared to FSW who were not currently using marijuana.
Discussion
We found that harmful drinking or alcohol dependence is associated with sub-optimal engagement in HIV treatment among HIV-infected FSW. FSW who were harmful drinkers or alcohol dependent were more likely to not use ART. Among the proportion of FSW on ART, increased alcohol use was also associated with viral non-suppression. Current marijuana use was uncommon but was associated with not using ART among FSW in our study.
The high level of ART use is likely a result of scaled-up ART coverage in Malawi. ART coverage has expanded in Malawi due public and private partnerships to implement Option B+ beginning in July 2011 and earlier initiation of ART (CD4 count ≤500 cells/mm3) in April 2014 (Malawi Ministry of Health, 2014). For the ART-eligible FSW who were previously diagnosed but not using ART, we were unable to determine the timing for ART eligibility. Our study began shortly after the expanded ART guidelines were implemented in Malawi. FSW we identified as ART-eligible may have previously been ineligible to initiate ART due to a high CD4 count (Lancaster et al., 2015).
The adverse influence of alcohol use on ART use has been well documented (Chander, Lau, & Moore, 2006; Kalichman et al., 2014; Palepu, Horton, Tibbetts, Meli, & Samet, 2004). Alcohol impairs memory, organizational skills, judgment, and other cognitive abilities, likely resulting in delayed ART initiation or treatment interruptions (Azar et al., 2010; Bolla et al., 2002; Curran et al., 2002; Kalichman et al., 2014; Korthuis et al., 2012). Health-care providers may withhold providing ART to heavy alcohol users, despite eligibility, because individuals perceived to be alcohol users are viewed as incapable of competently using ART (Hahn & Samet, 2010). There are beliefs that ART medications should not be mixed with alcohol, and therefore should be not be used when consuming alcohol (Kalichman et al., 2009; Sankar, Wunderlich, Neufeld, & Luborsky, 2007). The mechanisms underlying the relationship between alcohol and ART use are unknown among ART-eligible FSW in Malawi.
This study is one of the first to examine alcohol use and viral suppression among FSW in Sub-Saharan Africa. Alcohol use among HIV-infected persons in Sub-Saharan Africa predicts viral nonsuppression. Alcohol may have a direct effect on viral nonsuppression by increasing HIV viral replication (Bagasra et al., 1996; Bagasra, Kajdacsy-Balla, Lischner, & Pomerantz, 1993; Liu, Zha, Nishitani, Chen, & Zack, 2003).
The observed prevalence of heavy alcohol use may be driven by FSW living at alcohol-serving venues (Abdool Karim et al., 2010; Chersich et al., 2007; de Graaf et al., 1995; El-Bassel et al., 2001; Gupta et al., 2009). In similar studies, FSW who worked at alcohol-serving venues were more likely to consume alcohol or binge drink when compared to FSW not working in alcohol-serving venues (Abdool Karim et al., 2010; Agha & Chulu Nchima, 2004; Chersich et al., 2007).
Marijuana use was less common than alcohol use, which was unexpected given reported high prevalence of marijuana use among FSW in the region (Parry et al., 2009; Wechsberg et al., 2009). The pathways of both alcohol and marijuana use affecting HIV treatment are analogous (Azar et al., 2010; Bolla et al., 2002; Curran et al., 2002; Korthuis et al., 2012; Palepu et al., 2004). Heavy or severe marijuana use does negatively affect ART adherence and viral suppression (Bonn-Miller, Oser, Bucossi, & Trafton, 2014; Tucker et al., 2003). More thorough examinations of the frequency, potency, duration, and dependent symptoms of marijuana use and longitudinal assessments of viral load among FSW would provide insight on the impact of heavy marijuana use, along with concurrent alcohol use, on HIV treatment outcomes.
Although this study contributes to the currently limited understanding of the effects of substance use on HIV treatment engagement among FSW in Sub-Saharan Africa, the size of our sample is small and does not represent all HIV-infected FSW in Malawi. Our modest sample size resulted in decreased estimate precision, limited power to detect small differences, and decreased ability to control confounding in multivariable analyses.
Among HIV-infected FSW in Malawi, harmful or dependent alcohol use is common and associated with lack of ART use. To improve HIV treatment engagement, targeted interventions for HIV-infected FSW should prioritize the reduction of alcohol use, along with other substances. ART uptake strategies are critically needed for alcohol-using, ART-eligible FSW to induce viral suppression, improve health outcomes, and reduce transmission.
Acknowledgments
We gratefully acknowledge the outreach team for their dedication, interviewing skills, knowledge, and commitment to this work. We are also grateful to the study participants who courageously shared their time, thoughts, and stories to this research. All participants provided written informed consent and the study was approved by the Institutional Review Board at the University of North Carolina and the Malawi Ministry of Health and Population National Health Sciences Research Committee.
Funding
This work was supported by the NIH Research Training Grant (R25 TW009340) funded by the Fogarty International Center, the NIH Office of the Director Office of AIDS Research, ORWH, NCI, and NHLBI, the NIAID T32 training grant (T32 AI0700), the UNC Center for AIDS Research, an NIH funded program (P30 AI50410), and the National Institutes of Health (KL2 TR001109).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Group, C. T Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha S, Chulu Nchima M. Life-circumstances, working conditions and HIV risk among street and nightclub-based sex workers in Lusaka, Zambia. Culture, Health & Sexuality. 2004;6(4):283–299. doi: 10.1080/13691050410001680474. [DOI] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcoholism: Clinical and Experimental Research. 1997;21(4):613–619. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9194913. [PubMed] [Google Scholar]
- Arredondo M, Garrido C, Parkin N, Zahonero N, Bertagnolio S, Soriano V, de Mendoza C. Comparison of HIV-1 RNA measurements obtained by using plasma and dried blood spots in the automated Abbott real-time viral load assay. Journal of Clinical Microbiology. 2012;50(3):569–572. doi: 10.1128/jcm.00418-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence. 2010;112(3):178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: Potential immunopathogenic mechanisms. Journal of Infectious Diseases. 1996;173(3):550–558. doi: 10.1093/infdis/173.3.550. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8627016. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. Journal of Infectious Diseases. 1993;167(4):789–797. doi: 10.1093/infdis/167.4.789. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8450242. [DOI] [PubMed] [Google Scholar]
- Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, Kerrigan D. Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. The Lancet Infectious Diseases. 2012;12(7):538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Medical Research Methodology. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. Journal of Behavioral Medicine. 2014;37(1):1–10. doi: 10.1007/s10865-012-9458-5. [DOI] [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;43(4):411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chersich MF, Luchters SM, Malonza IM, Mwarogo P, King’ola N, Temmerman M. Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. International Journal of STD & AIDS. 2007;18(11):764–769. doi: 10.1258/095646207782212342. [DOI] [PubMed] [Google Scholar]
- Chersich MF, Luchters S, Ntaganira I, Gerbase A, Lo YR, Scorgie F, Steen R. Priority interventions to reduce HIV transmission in sex work settings in sub-Saharan Africa and delivery of these services. Journal of the International AIDS Society. 2013;16:17980. doi: 10.7448/ias.16.1.17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood DD, McBride DC, French MT, Comerford M. Health care need and utilization: A preliminary comparison of injection drug users, other illicit drug users, and nonusers. Substance Use & Misuse. 1999;34(4–5):727–746. doi: 10.3109/10826089909037240. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10210102. [DOI] [PubMed] [Google Scholar]
- Cholera R, Gaynes BN, Pence BW, Bassett J, Qangule N, Macphail C, Miller WC. Validity of the patient health questionnaire-9 to screen for depression in a high-HIV burden primary healthcare clinic in Johannesburg, South Africa. Journal of Affective Disorders. 2014;167:160–166. doi: 10.1016/j.jad.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-Tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164(1):61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Witte SS, Wada T, Gilbert L, Wallace J. Correlates of partner violence among female street-based sex workers: Substance abuse, history of childhood abuse, and HIV risks. AIDS Patient Care STDS. 2001;15(1):41–51. doi: 10.1089/108729101460092. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Vanwesenbeeck I, van Zessen G, Straver CJ, Visser JH. Alcohol and drug use in heterosexual and homosexual prostitution, and its relation to protection behaviour. AIDS Care. 1995;7(1):35–48. doi: 10.1080/09540129550126948. [DOI] [PubMed] [Google Scholar]
- Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Affairs. 2009;28(6):1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- Gupta J, Raj A, Decker MR, Reed E, Silverman JG. HIV vulnerabilities of sex-trafficked Indian women and girls. International Journal of Gynecology & Obstetrics. 2009;107(1):30–34. doi: 10.1016/j.ijgo.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Samet JH. Alcohol and HIV disease progression: Weighing the evidence. Current HIV/AIDS Reports. 2010;7(4):226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Amaral CM, White D, Swetsze C, Pope H, Kalichman MO, Eaton L. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care and STDs. 2009;23(6):449–454. doi: 10.1089/apc.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Grebler T, Amaral CM, McNerney M, White D, Kalichman MO, Eaton L. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. International Journal of Behavioral Medicine. 2014;21(5):811–820. doi: 10.1007/s12529-013-9353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthuis PT, Fiellin DA, McGinnis KA, Skanderson M, Justice AC, Gordon AJ, Kraemer KL. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;61(2):171–178. doi: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KE, Lungu T, Hosseinipour MC, Chadwick K, Dibb Z, Go VF, Miller WC. Closing the gap: Integrating mobile HIV testing and point-of-care CD4 testing for timely identi fication of HIV-infected and ART-eligible venue-based female sex workers in Lilongwe, Malawi. Paper presented at the 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention Vancouver; Canada. 2015. [Google Scholar]
- Lancaster KE, Powers KA, Lungu T, Mmodzi P, Hosseinipour MC, Chadwick K, Miller WC. The HIV care continuum among female sex Workers: A key population in Lilongwe, Malawi. PLoS One. 2016;11(1):e0147662. doi: 10.1371/journal.pone.0147662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zha J, Nishitani J, Chen H, Zack JA. HIV-1 infection in Peripheral Blood Lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307(1):37–44. doi: 10.1016/s0042-6822(02)00031-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12667812. [DOI] [PubMed] [Google Scholar]
- Malawi Ministry of Health. Clinical Management of HIV in children and adults. 2014 Retrieved from https://aidsfree.usaid.gov/sites/default/files/tx_malawi_2014.pdf.
- Monahan PO, Shacham E, Reece M, Kroenke K, Ong’or WO, Omollo O, Ojwang C. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. Journal of General Internal Medicine. 2009;24(2):189–197. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain E, Mishra S, Vickerman P, Pickles M, Gilks C, Boily MC. Antiretroviral therapy uptake, attrition, adherence and outcomes among HIV-infected female sex Workers: A systematic review and meta-analysis. PLoS One. 2014;9(9):e105645. doi: 10.1371/journal.pone.0105645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: The impact of substance abuse treatment. Addiction. 2004;99(3):361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Parry CDH, Dewing S, Petersen P, Carney T, Needle R, Kroeger K, Treger L. Rapid assessment of HIV risk behavior in drug using sex Workers in three cities in South Africa. AIDS and Behavior. 2009;13(5):849–859. doi: 10.1007/s10461-008-9367-3. [DOI] [PubMed] [Google Scholar]
- Parry CDH, Plüddemann A, Steyn K, Bradshaw D, Norman R, Laubscher R. Alcohol use in South Africa: Findings from the first demographic and health survey. Journal of Studies on Alcohol. 2005;66(1):91–97. doi: 10.15288/jsa.2005.66.91. [DOI] [PubMed] [Google Scholar]
- Pence BW, Gaynes BN, Atashili J, O’Donnell JK, Tayong G, Kats D, Ndumbe PM. Validity of an interviewer-administered patient health questionnaire-9 to screen for depression in HIV-infected patients in Cameroon. Journal of affective disorders. 2012;143(1):208–213. doi: 10.1016/j.jad.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein SE, Kamwendo D, Lugali L, Thengolose I, Tegha G, Fiscus SA, Mataya R. Measures of viral load using Abbott RealTime HIV-1 assay on venous and finger-stick dried blood spots from provider-collected specimens in Malawian district hospitals. Journal of Clinical Virology. 2014;60(4):392–398. doi: 10.1016/j.jcv.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar A, Wunderlich T, Neufeld S, Luborsky M. Sero-positive African Americans’ beliefs about alcohol and their impact on anti-retroviral adherence. AIDS and Behavior. 2007;11(2):195–203. doi: 10.1007/s10461-006-9144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shakarishvili A, Dubovskaya L, Zohrabyan L, St Lawrence J, Aral S, Dugasheva L, Ryan C. Sex work, drug use, HIV infection, and spread of sexually transmitted infections in Moscow, Russian Federation. The Lancet. 2005;366(9479):57–60. doi: 10.1016/S0140-6736(05)66828-6. [DOI] [PubMed] [Google Scholar]
- Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni ML, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care and STDs. 2007;21(S1):S-68–S-76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, Hankins CA. HIV and risk environment for injecting drug users: The past, present, and future. The Lancet. 2010;376(9737):268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegang SP, Abdallah S, Emukule G, Luchters S, Kingola N, Barasa M, Mwarogo P. Concurrent sexual and substance-use risk behaviours among female sex workers in Kenya’s Coast Province: Findings from a behavioural monitoring survey. SAHARA J. 2010;7(4):10–16. doi: 10.1080/17290376.2010.9724972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. The American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK, Lam W. Violence against substance-abusing South African sex workers: Intersection with culture and HIV risk. AIDS Care. 2005;17(S1):55–64. doi: 10.1080/09540120500120419. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Luseno WK, Lam WK, Parry CD, Morojele NK. Substance use, sexual risk, and violence: HIV prevention intervention with sex workers in Pretoria. AIDS and Behavior. 2006;10(2):131–137. doi: 10.1007/s10461-005-9036-8. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Wu LT, Zule WA, Parry CD, Browne FA, Luseno WK, Gentry A. Substance abuse, treatment needs and access among female sex workers and non-sex workers in Pretoria, South Africa. Substance Abuse Treatment, Prevention, and Policy. 2009;4:11. doi: 10.1186/1747-597x-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO UNAIDS UNICEF. (Progress report 2009).Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 Retrieved from http://www.who.int/hiv/pub/2010progressreport/report/en/index.html.
- Woolf-King SE, Maisto SA. Alcohol use and high-risk sexual behavior in sub-Saharan Africa: A narrative review. Archives of Sexual Behavior. 2011;40(1):17–42. doi: 10.1007/s10508-009-9516-4. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International guide for monitoring alcohol consumption and related harm. Geneva: Author; 2000. [Google Scholar]
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants. Geneva: Author; 2010. [PubMed] [Google Scholar]
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva: Author; 2012. [Google Scholar]
- Zou G. A modified Poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]


