Abstract
Despite significant clinical advancements, cancer remains a leading cause of mortality throughout the world due largely to the process of metastasis and the dissemination of cancer cells from their primary tumor of origin to distant secondary sites. The clinical burden imposed by metastasis is further compounded by a paucity of information regarding the factors that mediate metastatic progression. Linear chromosomes are capped by structures known as telomeres, which dictate cellular lifespan in humans by shortening progressively during successive cell divisions. Although telomere shortening occurs in nearly all somatic cells, telomeres may be elongated via two seemingly disjoint pathways: (i) telomerase-mediated extension, and (ii) homologous recombination-based alternative lengthening of telomeres (ALT). Both telomerase and ALT are activated in various human cancers, with more recent evidence implicating both pathways as potential mediators of metastasis. Here we review the known roles of telomere homeostasis in metastasis and posit a mechanism whereby metastatic activity is determined by a dynamic fluctuation between ALT and telomerase, as opposed to the mere activation of a generic telomere elongation program. Additionally, the pleiotropic nature of the telomere processing machinery makes it an attractive therapeutic target for metastasis, and as such, we also explore the therapeutic implications of our proposed mechanism.
Keywords: Alternative lengthening of telomeres, DNA damage, DNA repair, Epithelial-mesenchymal transition, Metastasis, Signal Transduction, Telomeres, Telomere Homeostasis
1. Introduction
When considered as a single disease, cancer is one of the leading causes of global mortality, with an estimated 14.9 million new cases and 8.2 million deaths attributable to cancer each year [1]. The incidence of many cancers is increasing in both developed and developing nations due in part to the prevalence of risk factors (e.g., tobacco and obesity) in an expanding and increasingly aging population [2]. Metastasis, while comprising only a fraction of this growing clinical burden, is responsible for the overwhelming majority of cancer mortality. Indeed, although the rates of diagnosing metastatic disease are typically low in many cancers (<10–30 percent; [3–5]), approximately 90 percent of cancer-related deaths are attributable to metastasis [6]. The underlying lethality of metastasis reflects its molecular complexity, which has greatly limited the success of therapies targeting this process in both overt disease and adjuvant settings [7–9]. Thus, there remains a significant unmet need for novel therapeutic approaches to target metastasis.
Metastasis is most accurately thought of as a cascade of systemic and cellular events undertaken by a subset of cells within the primary tumor [10, 11]. Generally speaking, metastatic cells become liberated from well-vascularized, angiogenic primary tumors and undergo intravasation to gain access to the circulation, where they persist in the blood, lymph, or bone marrow. Upon reaching their target tissue, disseminated cells extravasate and initiate growth of pre-angiogenic “micrometastases” before fully colonizing the metastatic niche upon reinstatement of angiogenesis [10]. The classical view of metastasis as the terminal stage of cancer progression suggests that a subpopulation of primary tumor cells progressively acquire genetic alterations necessary for their dissemination and colonization, and that these cells remain rare until clonally expanded within secondary organs [12]. However, recent evidence indicates that the capacity of tumor cells to metastasize is present in the earliest stages of primary tumor development [13, 14] and that these variant cells are often genetically divergent from their primary tumor counterparts and from one another [15–18]. In many respects, metastases may be considered as discrete entities from their primary tumors of origin due in part to their acquisition of genomic alterations during dissemination and distant organ colonization, suggesting that distinct regulatory pathways are operant during metastasis versus those active in primary tumor development [19].
Telomeres have long been implicated in driving tumorigenesis, yet emerging evidence indicates that the established concept whereby telomeres and their homeostatic machinery serve solely as cellular “immortalizers” may be drastically oversimplified. Indeed, telomeres and telomeric proteins subserve diverse functions in many of the stages that define the metastatic cascade. Herein we examine the varying roles that telomeres play in driving the dissemination and interaction of cancer cells with the metastatic microenvironment. We also discuss the therapeutic potential of targeting telomeres as a novel means to alleviate metastatic disease.
2. Metastasis at the cellular level
The metastatic cascade is defined by the following sequence of events: (i) primary tumor angiogenesis; (ii) cancer cell migration away from the primary tumor and intravasation into the tumor vascular supply; (iii) cancer cell survival within the circulation; (iv) extravasation of circulating tumor cells at secondary organs; and (v) proliferation of disseminated tumor cells (DTCs) at these secondary sites [19]. Each of these stages is spatially and temporally regulated by a host of cancer cell-intrinsic and -extrinsic (microenvironmental) signaling inputs (Fig. 1). The initial dissemination of cancer cells is reliant upon the development of a tumor blood supply, a process known as angiogenesis. Neovascularization involves both the intussusception of the tumor into the surrounding vasculature and the recruitment of endothelial cells and other vascular precursors required to form new vessels [20]. This process is driven largely by the secretion of vascular endothelial growth factor (VEGF) and angiopoietin (Ang) family members by cancer or stromal cells, and by the endothelium of preexisting vessels [21, 22]. The spread of cancer cells is further restricted by a complex network of extracellular matrix (ECM) and proteoglycan-rich basement membrane. This network is readily remodeled by secreted matrix metalloproteinases (MMPs) in response to mechanical forces or chemical stimuli, including inflammatory cytokines and reactive oxygen species (ROS) [23]. In addition, MMPs have been implicated in regulating cell growth, thus disrupting the normal balance between proliferative and cytostatic signals. For instance, extracellular proteases release latent epidermal growth factor (EGF), which subsequently signals through its receptor (EGFR) and downstream effectors, phosphatidylinositide 3-kinase (PI3K), AKT, and the mitogen-activated protein kinases (MAPK) ERK1/2. Collectively, these signals coalesce to activate proliferative programs, as well as propagate MMP production [24, 25]. MMPs 2, 9, and 14 also proteolytically activate latent transforming growth factor-β (TGF-β), which in turn stimulates cancer cell invasion and epithelial-mesenchymal transition (EMT) in carcinomas [26–29]. EMT is a process whereby epithelial cells shed their native polarity and adhesive properties and adopt the migratory and invasive features of mesenchymal stem cells [29]. There is substantial evidence that EMT is essential in cancer cell dissemination, as EMT promotes invasion via alterations in the expression of cell adhesion proteins (e.g., epithelial cadherin (E-cad) and β1 integrin) [30–32]. Intravasation is mediated by many of the same factors that control angiogenesis and cell migration, including MMPs 1, 2, and 3, TGF-β, and VEGF and angiopoietin family members, as well as the EGF-related peptide, epiregulin (EREG) [33–35].
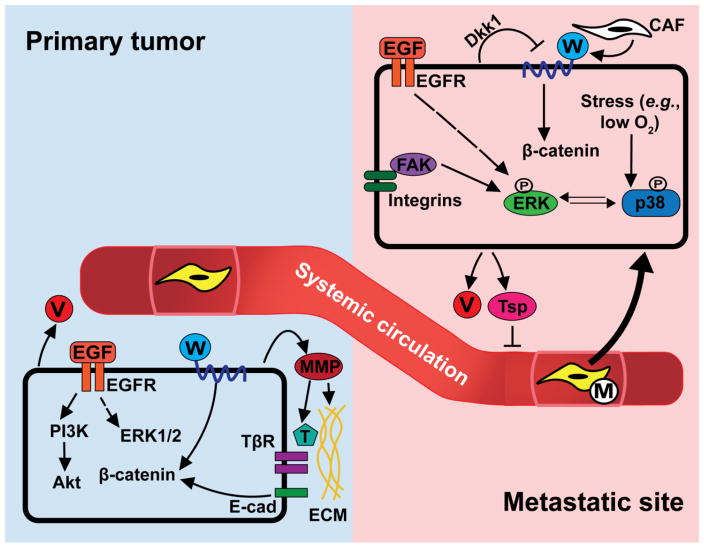
Fig. 1.
Overview of the metastatic cascade. The ability of carcinoma cells to disseminate is dependent on the presence of a vascular supply and the cellular responses to multiple signaling inputs. Cancer cells secrete pro-angiogenic factors, such as vascular endothelial growth factor (VEGF; denoted by V), that facilitate tumor invasion of existing vasculature and recruit endothelial cells for neovascularization. These cells similarly secrete matrix metalloproteinases (MMPs), which remodel the surrounding extracellular matrix (ECM). MMPs also cleave and activate latent signaling molecules, including transforming growth factor-β (TGF-β; denoted by T). TGF-β binds and activates its receptors (TβR) to promote cancer cell migration, intravasation, and epithelial-mesenchymal transition (EMT). ECM proteins and secreted growth factors, including epidermal growth factor (EGF) and Wnt (denoted by W), activate convergent signaling pathways that further stimulate cancer cell growth and invasiveness and impart these cells with mesenchymal properties. Once in the circulation, disseminated tumor cells (DTCs) persist in isolation, in small clusters, or in association with macrophages (denoted M) until they reach distant organs that are amenable to DTC outgrowth. Metastatic outgrowth is determined in part by the relative activities of the protein kinases ERK1/2 and p38 MAPK. ERK1/2 are activated downstream of EGF receptor (EGFR) and integrin stimulation, while p38 MAPK is activated in response to environmental stressors, such as hypoxia. In addition, DTCs secrete pro- (VEGF) and anti-angiogenic (thrombospondin-1; Tsp) factors that control vascular supply and tumor growth. DTC growth at metastatic sites is further influenced by cells of the surrounding tissues, including cancer-associated fibroblasts (CAFs) that release Wnt and other factors into the microenvironmental milieu. In response, DTCs negatively regulate Wnt signaling in an autocrine manner. Dkk1, Dickkopf-related protein 1; E-cad, epithelial cadherin; FAK, focal adhesion kinase; PI3K, phosphatidylinositide 3-kinase.
While persisting in isolation or as small clusters within the vasculature, circulating tumor cells (CTCs) must activate pro-survival programs, as well as escape immune detection [33]. AKT serves as the master regulator of CTC survival, doing so by suppressing apoptotic signals, and by blocking anoikis. These survival signals largely derive from inputs initiated by EGFR and VCAM-1 (vascular cell adhesion molecule 1) present on the surface of DTCs [36–38]. Interestingly, the survival of DTCs within secondary organs requires them to cooperate with cells in the newly colonized tissue, including stromal and resident immune cells [39]. Crosstalk between these malignant and normal cell types forms the so-called metastatic niche; however, not all cellular niches are permissive to DTC outgrowth. Indeed, some DTCs are maintained as single cells in a quiescent or senescent state within their niche, while others exist as small “micrometastases” whose growth is controlled by pro- and anti-angiogenic signals, and by the activation status of resident immune responses [40]. Collectively, these two phenomena define the stage of metastasis known as dormancy.
On a molecular level, the transition from dormancy and overt metastasis may reflect an intricate balance between the activation of proliferative and stress response pathways in DTCs, with proliferation being driven largely by ERK1/2 and stress responses by p38 MAPK [41]. The activation of ERK1/2 in dormant DTCs is mediated by EGFR and focal adhesion kinase (FAK), which functions synergistically with the Wnt and Notch pathways [42–44]. In contrast, p38 MAPK is responsive to microenvironmental stressors, such as oxidative stress and inflammation, and induces an endoplasmic reticulum stress response while simultaneously inhibiting ERK1/2 activation [40, 45–48]. Dormant states are also maintained by stromally-derived signals, such as bone morphogenetic proteins (BMPs), which diminish Wnt signaling in a p53-dependent manner [49, 50]. In addition to their role in metastatic dormancy, canonical Wnt-dependent and -independent activation of β-catenin fulfill pivotal functions along the entire metastatic cascade (Fig. 1; [51]). For instance, β-catenin is a critical mediator of both cell proliferation via Wnt, and of cell adhesion via E-cad and other cell adhesion molecules [52, 53]. Thus, the balance between proliferative and adhesive inputs that activate β-catenin influences cancer cell migration, intravasation, and invasion [54–56]. Furthermore, Wnt signaling stimulates EMT and regulates both cellular and immune-mediated micrometastatic dormancy [57, 58]. As we discuss in detail below, these links between Wnt/β-catenin signaling and metastasis are particularly provocative because Wnt/β-catenin are known to regulate the expression of multiple telomeric proteins [59–61].
3. Telomeres: Dynamic structures with dynamic functions
Telomeres function as determinants of cellular age and replicative potential [62]. Consequently, aberrant telomere length imparts cells with replicative immortality, one of the hallmarks of cancer [63]. Indeed, abnormal telomere elongation is found in nearly all human cancers, with the vast majority of these exhibiting activation of the reverse transcriptase telomerase, while the remaining cases accomplish this task via the recombination-based Alternative Lengthening of Telomeres (ALT; [64–66]). In humans, somatic cells ordinarily undergo a finite number of cell divisions before entering senescence and ultimately undergoing apoptosis, thereby protecting them from accumulating pathologic genomic abnormalities [67]. In contrast, rapidly dividing cancer cells experience pronounced telomere attrition, which cells interpret as DNA double-strand breaks (DSBs). In the absence of functional mediators of the DNA damage response (DDR), such as p53, these cells bypass replicative senescence and enter a phase of widespread genomic instability known as crisis, which is characterized by the emergence of chromosome end-to-end fusions [68]. Reactivation of telomere maintenance programs occurs in a subset of cancer cells once their critical telomere length is reached. This endows these cells with expanded replicative potential and enables their transmission of abnormal chromosomal structures (i.e., amplifications, deletions, translocations, inversions) that arise as a result of iterative chromosomal breakage-fusion-bridge (BFB) cycles [69–71]. Interestingly, recent evidence indicates that telomerase and other telomeric proteins directly modulate many of the key effectors of metastatic progression (see below).
Structurally, telomeres are nucleoprotein complexes, and as such, both DNA and protein elements are important regulators of telomere length and stability. In humans, telomeres are composed of tandem (TTAGGG)n repeats with an obligate 3’ single-stranded overhang on the lagging strand [72]. The 3’ overhang invades the telomeric DNA duplex, forming a duplex DNA loop (T-loop) with a terminal triplex structure (D-loop) that protects telomeres from recombination, fusion, and eliciting a DDR [73, 74]. T-loop formation is facilitated by the proteins Telomeric Repeat-binding Factor 1 (TRF1), Telomeric Repeat-binding Factor 2 (TRF2), and Protection of Telomeres 1 (POT1), which in turn are members of the larger shelterin complex that serves to protect telomeres from the DDR and coordinate telomere length homeostasis [75, 76]. TRF1 and TRF2 control T-loop formation by binding to telomeric DNA either as homodimers or oligomers that are capable of bridging distant intratelomeric loci [77, 78]. Other components of the shelterin complex include TRF1-Interacting Nuclear Protein 2 (TIN2), Repressor/Activator Protein 1 (RAP1), and TPP1. Once bound to double-stranded (TRF1 and TRF2) or single-stranded (POT1) telomeric repeat sequences, TRF1 and TRF2 assemble the remaining shelterin proteins to yield functional telomere protection units [76, 79].
Shelterin proteins perform diverse functions at telomeres, including shielding telomeres from degradation, recombination, and DDR activation, as well as controlling telomere length in conjunction with telomerase or ALT-associated factors [76]. Activation of DDRs occurs in response to telomere shortening, thereby reducing the degree of telomere protection by shelterin complexes [80]. Diminished protection of telomeres exposes telomeric double-stranded DNA (dsDNA) and single-stranded (ssDNA) 3’ overhangs, which are sensed as DNA damage foci by the PI3K-related kinases Ataxia Telangiectasia Mutated (ATM; [81]) and Ataxia Telangiectasia and Rad3-related (ATR; [82]). ATM and ATR phosphorylate the cell cycle-specific checkpoint kinases, Chk1 and Chk2, whose activation blocks cell cycle progression and the transmission of damaged DNA [76]. The structural specificities of ATM and ATR are underscored by the fact that these proteins are impeded from activating DDRs at telomeres by TRF2 (i.e., ATM) and POT1 (i.e., ATR), whose DNA binding specificities mirror those of their corresponding kinase [83]. Chk1 and Chk2 further cooperate with ATM and ATR to activate p53, which inhibits cell cycle progression through induction of the cyclin-dependent kinase inhibitor p21 [84]. Conversely, the absence of functional p53 perpetuates telomere dysfunction and simultaneously prevents replicative senescence, thereby driving the evolution of cancer cells harboring significant chromosomal instability secondary to telomere crisis [85, 86].
Repairing DNA DSBs in mammalian cells is accomplished by one of two recombination-based mechanisms: (i) non-homologous end joining (NHEJ), or (ii) homologous recombination (HR). Telomere shortening, concurrent with the activation of the DDR and NHEJ or HR repair pathways, yields chromosome end-to-end fusions [68]. By shielding chromosome ends from being recognized as DSBs, shelterin proteins play an essential role in maintaining genomic integrity. ALT is also a HR-mediated process that is characterized by genomic instability in the presence of an intact DDR [87, 88]. Indeed, telomere elongation via ALT occurs by sister chromatid exchange (T-SCE), resulting in the appearance of heterogeneous telomeres within individual cells that are typically considerably longer than those found in terminally differentiated cells [89, 90]. Moreover, uncontrolled telomere recombination can induce HR within T-loops, resulting in the production of extrachromosomal DNA circles (C-circles) that serve as markers of ALT activity [91]. As with generalized recombination-based DSB repair, ALT is mediated by the MRN complex (MRE11-RAD50-NBS1; [92]), which may be recruited to telomeres as part of the ALT-associated Promyelocytic leukemia (PML) Body (APB; [93]). PML is a functionally promiscuous protein that can assemble numerous multimeric protein complexes in response to both environmental and cell cycle-dependent cues [94]. The prevailing model suggests that critically short telomeres are recognized and spatially organized within APBs by PML, which also recruits MRN complexes to perform T-SCE [93].
By repressing DNA damage signaling and homology-directed repair at telomeres, shelterin proteins, particularly TRF1, TRF2, and POT1, exert regulatory control over ALT activity. Indeed, changes in expression [95–97] or modification state [98] of these proteins alter T-SCE and APB formation. Because the DDR is intact in cells performing ALT, these cells must bypass replicative senescence and apoptosis. Similar to their telomerase-positive counterparts, ALT-positive cells often carry mutations in TP53, the gene encoding p53 [99]. Thus, although DDR kinases (i.e., ATM and ATR) can become activated in response to telomere shortening and uncapping, the absence of functional p53 prevents the acquisition of a senescent phenotype, resulting in ALT activation and cell proliferation [100]. p53 dependence provides a unifying mechanism for telomere maintenance via telomerase and ALT; however, the functional diversity of telomerase manifests important differences between these two pathways that may prove to be essential in driving human disease, including the acquisition of metastatic phenotypes.
4. Telomerase: More than a telomere machine
Telomerase is an RNA-dependent DNA polymerase that is composed of two moieties: (i) a RNA component (TR) that serves as a template for telomeric DNA replication, and (ii) a protein component (TERT) that is responsible for polymerase activity. The TERT protein contains four domains, and regions of the N- and C-terminal domains are sufficient to induce cell immortalization, even in the absence of catalytic activity [101]. Indeed, alternative splice variants of TERT lacking its catalytic domain continue to promote cell growth, independently of telomere length [102]. Moreover, Khattar et al [103] determined that TERT drives cancer cell proliferation in part by binding to and enhancing the chromatin localization of the RNA polymerase III subunit, RPC32, leading to elevated tRNA expression and increased protein synthesis in cancers of the skin, lung, and liver. These findings indicate that the phenotypic effects of telomerase may stem in part from non-telomeric functions of telomerase, which are termed “extratelomeric” and include its ability to regulate gene expression, modulate intracellular signaling, and transduce microenvironmental stimuli [104]. In the context of cancer, telomerase is not only sufficient to bestow cells with tumorigenic properties, but it is also uniquely poised to regulate multiple processes that have been implicated in metastatic progression (Fig. 2).
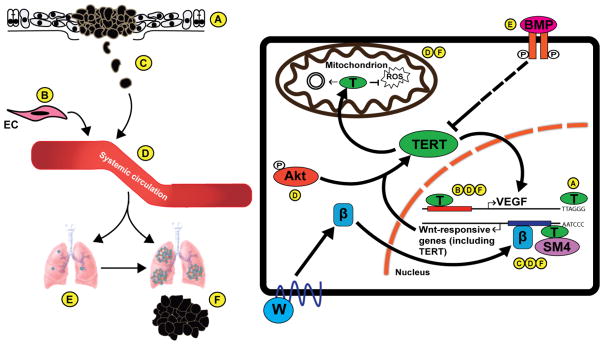
Fig. 2.
Extratelomeric functions of TERT coupled to cancer cell dissemination and tumor formation. (Left Panel) Schematic representation of the metastatic cascade, encompassing (A) primary tumor formation, (B) endothelial cell (EC) recruitment and angiogenesis, (C) cancer cell migration and invasion into surrounding tissue, (D) cancer cell intravasation and survival within the system circulation, and (E) extravasation and colonization of distant organs either as single cells/micrometastases or (F) overt metastatic lesions. (Right Panel) Depicts the pathways regulated by telomerase TERT (denoted by T) that influence specific stages of the metastatic cascade. Canonical TERT activity is responsible for amplifying telomeric repeat DNA, an event that is essential for malignant transformation, and for tumor growth and development (A). In addition to its role in telomere elongation, TERT also controls gene expression by acting as a transcription factor. In doing so, TERT targets the expression of vascular endothelial growth factor (VEGF), as well as genes governed by the Wnt:β-catenin axis (Wnt denoted by W; β-catenin by β). VEGF orchestrates multiple stages of the metastatic cascade, particularly tumor angiogenesis (B), intravasation (D), and proliferation of disseminated cells at distant sites (F). Wnt signaling promotes EMT-mediated cancer cell migration (C) and intravasation (D). Additionally, Wnt signaling regulates the proliferation and outgrowth of DTCs in part by governing various features of the metastatic niche (F). Activation of Wnt-responsive genes, which includes VEGF, by TERT is dependent on its association with the chromatin remodeler SMARCA4 (SM4). In turn, TERT expression is reciprocally regulated by β-catenin-dependent transcription. TERT function and localization are controlled by both stimulatory (e.g., AKT) and inhibitory (e.g., bone morphogenetic proteins, BMPs) signals present in the metastatic microenvironment. AKT is an important regulator of cancer cell dissemination and survival in circulation (D), doing so by preventing the death of CTCs. In contrast, BMPs inhibit DTC outgrowth and maintain these cells as subclinical micrometastases (E). Under conditions of oxidative stress, TERT may translocate to mitochondria and regulate the expression of mitochondrial genes (Mitochondrion, left arrow) related to glucose metabolism and energy production; it also mediates an antioxidant response to reactive oxygen species (ROS; Mitochondrion, right bar). ROS are ordinarily present as a defense mechanism against disseminated tumor cells both in the circulation (D), and in the metastatic niche (F). Thus, expression of TERT in these cell may provide them with a selective advantage in colonizing metastatic sites.
Investigations into the effects of TERT abundance on global patterns of gene expression have revealed a cohort of genes whose transcription appears to be directly controlled by TERT, most notably EGFR and VEGF [105, 106]. In addition to the role of VEGF in angiogenesis, EGF and VEGF signaling coalesce to promote cancer cell proliferation [107]. In vitro and in vivo studies have confirmed a link between TERT and growth factor signaling, such that genetic depletion of EGFR in TERT-overexpressing human mammary epithelial cells resulted in decreased cell proliferation [105]. Likewise, genetic and pharmacologic inhibition of telomere maintenance decreased VEGF expression and abrogated angiogenesis in vivo [108, 109]. Along these lines, TERT functions as a downstream effector of VEGF signaling [110], thus creating a positive feedback loop that can promote cancer cell intravasation and sustain tumor angiogenesis.
The most well-characterized transcriptional effects of TERT revolve around the Wnt/β-catenin pathway. Indeed, when localized to the promoters of Wnt-dependent genes, TERT physically interacts with SMARCA4, a SWI/SNF-related chromatin remodeling protein that induces the expression of MMP-7, VEGF, and the mitogen-responsive transcription factor c-Myc [59, 111]. In turn, TERT expression is reciprocally controlled by β-catenin [112]. In addition, telomerase activity is greatly enhanced by phosphorylation of TERT by AKT [113], as is its nuclear localization [114]. Thus, AKT may influence both the canonical and extratelomeric functions of telomerase. Indeed, upon gaining entry into the nucleus, TERT may bind directly to nuclear factor-κB (NF-κB), thereby enhancing the expression of NF-κB-dependent targets, particularly MMPs [115, 116]. NF-κB-dependent MMP expression likely contributes to changes in adhesive properties and increased invasiveness seen in cancer cells treated with telomerase template antagonists [117, 118]. Additionally, NF-κB acts synergistically with TGF-β to promote EMT and cancer cell dissemination [119, 120], while TERT enhances EMT stimulated by TGF-β by interacting directly with ZEB1, which suppresses E-cad expression [121, 122]. Taken together, these findings demonstrate that TERT functions at the center of an intricate transcriptional network that controls multiple regulators of metastasis.
TERT plays a crucial part in mediating the interactions of DTCs with their surrounding microenvironments. For instance, telomerase activity is increased in response to inflammatory cytokines (e.g., interleukin-6), which also facilitate tumorigenesis, invasion, and NF-κB-dependent activation of tumor-associated macrophages [115, 123]. Of note, TERT expression has been shown to be suppressed by BMPs, which are prevalent at metastatic sites and negatively regulate DTC outgrowth. Thus, downregulation of TERT via BMP signaling may serve as a mechanism for establishing the dormant niche [124, 125]. Mitochondrial function is also reliant on both expression and subcellular localization of TERT, whose association with mitochondria protects cells from oxidative stress [126]. As such, TERT activation may serve to stimulate mitochondrial biogenesis concomitant with increased glucose uptake and utilization [127, 128]. This holds functional significance, as reactive oxygen species (ROS) within primary tumor microenvironments can induce DNA mutations that instill cancer cells with a growth advantage [129], while systemic ROS production serves as a defense against cancer cell dissemination [130]. Thus, one mechanism underlying telomerase-driven metastasis may be a spatiotemporally regulated response to oxidative stress.
As telomere protectors, shelterin proteins dictate the accessibility of telomerase to its substrate. Predictably, each shelterin protein functions as a negative regulator of telomerase-mediated telomere extension [76]. POT1 and TPP1 play specific and well-studied roles in this process. For instance, POT1 and telomerase both bind to telomeric ssDNA and are, in effect, competitive inhibitors of one another [131]. TPP1 is primarily responsible for recruiting and activating telomerase at shortened telomeres [132]. As such, defects in either of these proteins may preclude telomerase-mediated telomere elongation and shift the cellular TERT reserve toward performing extratelomeric functions. As a separate consideration, some shelterin components function within the same extratelomeric regulatory networks as TERT. Indeed, TRF2 expression is regulated by Wnt signaling [61] and excess TRF2 promotes angiogenesis by binding directly to the promoter of the platelet-derived growth factor receptor-β (PDGFRβ; [133]). Moreover, TIN2 is localized to telomeres via its interaction with TPP1 and abolishing this interaction targets TIN2 to mitochondria where it regulates energy metabolism and oxidative stress response [134]. Hence, telomeres may be considered cellular control centers for metastasis, doing so by integrating information regarding replicative potential with programs that guide each step of the metastatic cascade.
5. Telomere homeostasis: Determinant or consequence of metastatic progression?
Associations between cancer and mutations in various telomeric proteins are continually being discovered (Table 1). However, the extent to which these aberrations specifically influence metastatic progression remains unclear. To date, many studies that have examined the association between cancer progression and telomere homeostasis have employed descriptive readouts of telomere dynamics, primarily differences in either telomere length or telomerase expression or activity that are then correlated with tumor and/or metastatic progression [64, 66, 135]. In adopting this approach, these studies failed to address two important questions. First, does the identity of the maintenance program (i.e., telomerase versus ALT) influence the natural history of disease progression? Second, at what stage(s) of cancer progression do telomere maintenance mechanisms (TMMs) become activated? We postulate that the selection of TMMs in cancer cells represents a critical determinant of their metastatic capability, such that subsets of TERT-positive cancer cells become more prone to disseminate from primary tumor sites and form overt metastases in distant organs (Fig. 3). Thus, consideration of TMMs may permit more precise diagnostic evaluations of high-risk patients and catalyze the development of novel therapies capable of alleviating these pathways.
Table 1.
Mutations in telomeric proteins are associated with various cancers and cancer predisposition syndromes.
| Protein | Cancer(s) | Reference(s) |
|---|---|---|
| Shelterin | ||
|
| ||
| TRF1/TRF2 | Gastric | [158] |
|
| ||
| POT1 | Leukemia (CLL) | [159] |
| Melanoma | [160] | |
| Glioma | [161] | |
|
| ||
| TPP1 | DC | [162] |
| Melanoma | [163] | |
|
| ||
| TIN2 | DC | [164] |
|
| ||
| RAP1 | Melanoma | [163] |
| Telomere elongation | ||
|
| ||
| TERT | Glioma | [165, 166] |
| Bladder | [165, 167] | |
| Thyroid | [165, 168] | |
| Melanoma | [165, 169] | |
| Breast/Ovarian | [170] | |
|
| ||
| TERC | MDS | [171] |
|
| ||
| MRE11 | Colorectal | [172] |
| Breast | [173] | |
|
| ||
| NBS1 | Prostate | [174] |
| Leukemia (ALL) | [175] | |
|
| ||
| RAD50 | Breast | [176] |
Abbreviations: ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; DC, dyskeratosis congenita; MDS, myelodysplastic syndrome.
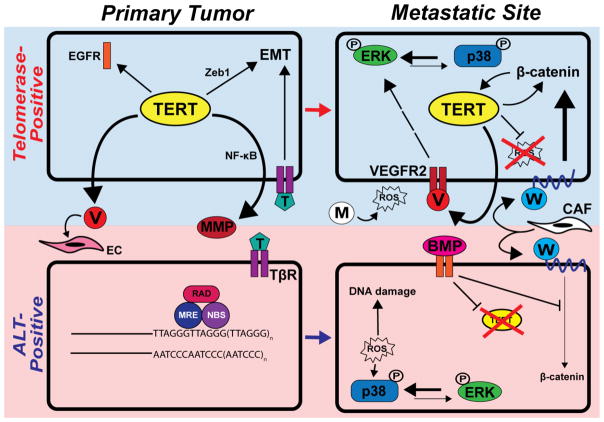
Fig. 3.
Hypothetical model of TMM selection and therapeutic targeting during metastasis. The top row (blue box) depicts the dissemination of a telomerase-positive cancer cell from the primary tumor site to a distant organ, while the bottom row (pink box) depicts these same events in an ALT-positive cell. See Section 5. “Telomere homeostasis: Determinant or consequence of metastatic progression?” for details.
It should be noted that while TERT- and ALT-positive cancer cells both readily respond to metastasis-promoting signals (e.g., TGF-β, Wnt, etc.), TERT uniquely functions in binding ZEB1 and amplifying EMT programs stimulated by TGF-β [121, 122]. TERT also interacts with NF-κB to enhance the production of MMPs, thereby promoting the liberation of carcinoma cells from the primary tumor en route to the systemic circulation [115, 116]. These events are complemented by the formation of nascent blood vessels in response to TERT-driven VEGF production and secretion within the TME [106, 108, 109] (Fig. 3). Interestingly, although ALT-positive cells are incapable of activating the aforementioned ancillary pathways, they nevertheless remain competent in responding to TERT-independent microenvironmental cues that support carcinoma dissemination. Within the metastatic niche, TERT-positive DTCs to undergo self-renewal via the continued secretion of VEGF and resultant activation of a VEGFR2→ERK1/2 signaling axis. A potential consequence of these events manifests in the reactivation of proliferative programs in previously dormant micrometastases, as reflected by high ERK1/2:p38 MAPK activation ratios [41]. Conversely, microenvironmental stressors, such as the production of ROS by resident macrophages, can inversely skew the activation ratio between ERK1/2:p38 MAPK by stimulating p38 MAPK activity, thereby enhancing metastatic latency [40, 45–48]. Protection from oxidative damage is imparted by mitochondrial translocation of TERT [126], and by activation of the Wnt/β-catenin driven survival programs. We posit that in the absence of competing signals that arise from TERT that ALT-positive DTCs may possess elevated susceptibility to cytostatic signaling, particularly those stimulated by BMPs that (i) antagonize Wnt/β-catenin signaling, (ii) repress TERT expression, and (iii) promote DTC dormancy [49, 50, 124, 125]. Moreover, diminished TERT function may render ALT-positive DTCs more susceptible to DNA damage induced by oxidative stress, and to the acquisition of dormant phenotypes via ROS-mediated p38 MAPK activation (Fig. 3). Importantly, our model of telomere homeostasis during metastatic progression does not limit individual cancers, or even single cancer cells, to a singular mode of telomere maintenance. Indeed, we envision a scenario wherein DTCs experience a dynamic fluctuation between TERT- and ALT-based signaling, particularly in response to various selective pressures encountered during dissemination, and potentially in response to various therapeutic regimens. Likewise, it remains plausible that ALT-positive cells may express TERT in the absence of TR, thus devoting TERT entirely to the performance of extratelomeric functions (Fig. 3).
There is a dearth of mechanistic data available regarding the link between ALT activation and cancer progression beyond the notion that TMMs drive cellular immortalization. Specifically, the factors (both cell-intrinsic and microenvironmental) that preferentially activate ALT versus telomerase remain largely uncharacterized. Clinical observations have revealed that ALT-driven primary tumors tend to be of mesenchymal origin, although carcinomas of the lung, liver, kidney, esophagus, breast, and brain are also observed [99, 136, 137]. Importantly, evidence of ALT is present in only a subset of these cancers, and the presence of ALT carries prognostic value, specifically with respect to disease progression and patient survival [99, 138–140]. In particular, ALT generally predicts for a more indolent clinical course [138, 139]; however, ALT coupled with lost expression of either Daxx (death domain-associated protein) or ATRX (alpha thalassemia/mental retardation X-linked) is strongly associated with shorter disease-free and disease-specific survival, thereby implicating ALT in mediating metastatic progression [140]. Taken together, these findings clearly implicate ALT in mediating metastatic progression. Likewise, tumors that exhibit telomerase activity have been associated with both reduced survival and increased lymph node metastasis [141, 142]. While the mechanisms that underlie these observations remain to be elucidated, there is mounting evidence to suggest that TMM selection substantially influences the rate and severity of cancer progression.
TMMs are classically considered to be static properties of specific cancers, such that tumors typically adopt a single TMM during malignant transformation and maintain this program indefinitely. However, recent studies offer two alternatives to this model. First, ALT may serve as an adaptive mechanism of chemoresistance in some cancers treated with anti-telomerase agents [143]. Second, matched primary tumors and metastases may rely on different TMMs [144]. Collectively, these findings suggest that telomere elongation may not simply be a necessary condition for tumorigenesis, but instead may be a spatiotemporally regulated balance between telomerase and ALT that gives rise to genetically distinct cancer cells that possess differential metastatic capabilities [145, 146]. Further studies are needed to determine whether telomerase and ALT activation may occur throughout the metastatic cascade, or whether cells utilizing either telomere maintenance program defines distinct populations within primary tumors that become enriched or depleted in metastatic lesions through clonal selection.
6. Future directions: Telomere-directed therapy for metastasis
The deadly nature of metastatic disease necessitates the development of therapies that specifically target essential pathways operant during dissemination. Current efforts aimed at anti-telomerase therapy have adopted several approaches, including direct enzyme inhibition, telomere destabilization, anti-telomerase immunotherapy, and telomerase-driven suicide gene therapy [147]. Enzyme inhibitors include both small molecules and RNA template antagonists, both of which have demonstrated robust antitumor activity (i.e., against primary and metastatic tumors) in preclinical models of breast and lung cancer [148, 149]. Clinical studies indicate potential telomere length-dependent therapeutic benefit with these agents, although results to date have been inconsistent [150]. Telomere destabilization can be accomplished by incorporating mutant-template TR into catalytically active TERT, resulting in nucleotide misincorporation into telomeric repeat sequences. The effects of telomere destabilization on cancer cells have only begun to be investigated in preclinical settings [151], and methods for clinically implementing such therapies may be difficult to devise. Immunotherapy consists of injection of either a recombinant TERT peptide (i.e., vaccination) or dendritic cells exhibiting constitutive TERT expression. Each of these approaches have been assessed in clinical trials, and while a immunologic response may be achieved [152, 153], this response does not appear to confer a survival advantage [154]. Lastly, gene therapy seeks to parlay telomerase overexpression in cancer cells in order to drive expression of a cytotoxic or oncolytic gene product under the control of the TERT promoter [155]. Unfortunately, gene therapy as a means to target cells with aberrant telomerase activity remains in its infancy and has not been rigorously validated in either preclinical investigations or clinical trials.
ALT has recently emerged as a novel therapeutic target in cancer, as some malignancies rely on ALT as their primary TMM, while others rely upon telomerase or ALT in a context-dependent manner [136, 144]. Moreover, pathways that are preferentially active during ALT may serve as effective drug targets in these cancers [156, 157]. As the relative contributions of each TMM to metastatic progression become clearer, anti-telomerase and anti-ALT therapies may emerge as viable strategies to target metastatic disease (Fig. 3). Theoretically, administering TERT inhibitors should provide clinical benefit to patients whose metastatic tumors are TERT-positive, particularly since progression through the metastatic cascade is reliant upon pathways and effectors regulated by TERT (Fig. 2). Unfortunately, existing TERT inhibitors only target the enzymatic activity of this polymerase, resulting in modest clinical efficacy due in part to the (i) extratelomeric functions of TERT, and (ii) potential compensatory activation of ALT machinery in TERT-inhibited cells. Moreover, we submit that future ALT-directed therapies should aim to maintain DTCs in a perpetual quiescent/senescent state, doing so either by delivering “ALT activators,” or by providing agents capable of shifting telomere regulatory networks towards ALT (e.g., BMP agonists). Along these lines, combining anti-TERT and anti-ALT agents holds tremendous promise to eradicate metastatic disease, thereby providing a two-pronged approach to simultaneously target quiescent (ALT-positive) and proliferating (TERT-positive) DTCs, and to prevent chemotherapy-induced compensatory transitions between these mechanisms. Moving forward, significant attention should be devoted to (i) exploring improved therapies that target telomerase and ALT, including specific modulation of telomerase extratelomeric functions; (ii) propelling these drugs into clinical trials; and (iii) developing methods for longitudinal assessment of patient TMM status in both primary tumors and metastases. These advances will shed light on the dynamic interplay between telomerase and ALT in cancer progression and, potentially, improve the survival of patients afflicted with one of the world’s most lethal diseases.
Acknowledgments
Research support was provided in part by the National Institutes of Health to NJR (T32GM007250 and TL1TR000441) and WPS (CA129359, CA177069, and CA194518), and by METAvivor to WPS.
Footnotes
Financial disclosures/conflicts of interest
The authors declare that they have no competing interests, nor conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 4.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 7.Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mina LA, Sledge GW., Jr Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol. 2011;8:325–332. doi: 10.1038/nrclinonc.2011.59. [DOI] [PubMed] [Google Scholar]
- 9.Massague J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 11.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 14.Talmadge JE. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 2007;67:11471–11475. doi: 10.1158/0008-5472.CAN-07-2496. [DOI] [PubMed] [Google Scholar]
- 15.Torres L, Ribeiro FR, Pandis N, Andersen JA, Heim S, Teixeira MR. Intratumor genomic heterogeneity in breast cancer with clonal divergence between primary carcinomas and lymph node metastases. Breast Cancer Res Treat. 2007;102:143–155. doi: 10.1007/s10549-006-9317-6. [DOI] [PubMed] [Google Scholar]
- 16.Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel WT, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell. 2008;13:441–453. doi: 10.1016/j.ccr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Klein CA. Selection and adaptation during metastatic cancer progression. Nature. 2013;501:365–372. doi: 10.1038/nature12628. [DOI] [PubMed] [Google Scholar]
- 18.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15:473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 19.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 21.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 22.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, Stack MS, Hudson LG. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent E-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68:4606–4613. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 27.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res. 2012;347:85–101. doi: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Downregulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22:2423–2435. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-β-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485–6498. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- 34.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 37.Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008;10:R80. doi: 10.1186/bcr2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibue T, Weinberg RA. Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim D, Rath O, Kolch W, Cho KH. A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways. Oncogene. 2007;26:4571–4579. doi: 10.1038/sj.onc.1210230. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Pathak PS, Fukumura D. Hypoxia-induced activation of p38 mitogen-activated protein kinase and phosphatidylinositol 3'-kinase signaling pathways contributes to expression of interleukin 8 in human ovarian carcinoma cells. Clin Cancer Res. 2004;10:701–707. doi: 10.1158/1078-0432.ccr-0953-03. [DOI] [PubMed] [Google Scholar]
- 46.Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 47.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5:729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiacchiera F, Grossi V, Cappellari M, Peserico A, Simonatto M, Germani A, Russo S, Moyer MP, Resta N, Murzilli S, et al. Blocking p38/ERK crosstalk affects colorectal cancer growth by inducing apoptosis in vitro and in preclinical mouse models. Cancer Lett. 2012;324:98–108. doi: 10.1016/j.canlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voorneveld PW, Kodach LL, Jacobs RJ, van Noesel CJ, Peppelenbosch MP, Korkmaz KS, Molendijk I, Dekker E, Morreau H, van Pelt GW, et al. The BMP pathway either enhances or inhibits the Wnt pathway depending on the SMAD4 and p53 status in CRC. Br J Cancer. 2015;112:122–130. doi: 10.1038/bjc.2014.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 52.Bienz M. β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 53.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Vlad-Fiegen A, Langerak A, Eberth S, Muller O. The Wnt pathway destabilizes adherens junctions and promotes cell migration via β-catenin and its target gene cyclin D1. FEBS Open Bio. 2012;2:26–31. doi: 10.1016/j.fob.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon M, Lee SJ, Wang Y, Rybak Y, Luna A, Reddy S, Adem A, Beaty BT, Condeelis JS, Libutti SK. Filamin A interacting protein 1-like inhibits WNT signaling and MMP expression to suppress cancer cell invasion and metastasis. Int J Cancer. 2014;135:48–60. doi: 10.1002/ijc.28662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–712. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS genetics. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diala I, Wagner N, Magdinier F, Shkreli M, Sirakov M, Bauwens S, Schluth-Bolard C, Simonet T, Renault VM, Ye J, et al. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep. 2013;14:356–363. doi: 10.1038/embor.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelland L. Targeting the limitless replicative potential of cancer: the telomerase/telomere pathway. Clin Cancer Res. 2007;13:4960–4963. doi: 10.1158/1078-0432.CCR-07-0422. [DOI] [PubMed] [Google Scholar]
- 63.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 65.Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell. 2003;113:881–889. doi: 10.1016/s0092-8674(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 66.Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbert B, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L, DePinho RA. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;2:149–155. doi: 10.1016/s1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 71.Murnane JP. Telomere dysfunction and chromosome instability. Mutat Res. 2012;730:28–36. doi: 10.1016/j.mrfmmm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 74.Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97:419–422. doi: 10.1016/s0092-8674(00)80750-3. [DOI] [PubMed] [Google Scholar]
- 75.Yang Q, Zheng YL, Harris CC. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol. 2005;25:1070–1080. doi: 10.1128/MCB.25.3.1070-1080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palm W, de Lange T. How shelterin protects mammalian telomeres. Ann Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 77.Bianchi A, Stansel RM, Fairall L, Griffith JD, Rhodes D, de Lange T. TRF1 binds a bipartite telomeric site with extreme spatial flexibility. EMBO J. 1999;18:5735–5744. doi: 10.1093/emboj/18.20.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, de Lange T. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem. 2004;279:47264–47271. doi: 10.1074/jbc.M409047200. [DOI] [PubMed] [Google Scholar]
- 80.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 82.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 83.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 84.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 85.Cosme-Blanco W, Shen MF, Lazar AJ, Pathak S, Lozano G, Multani AS, Chang S. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 2007;8:497–503. doi: 10.1038/sj.embor.7400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davoli T, de Lange T. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell. 2012;21:765–776. doi: 10.1016/j.ccr.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Londono-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64:2324–2327. doi: 10.1158/0008-5472.can-03-4035. [DOI] [PubMed] [Google Scholar]
- 88.Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, De S, Petrini JH, Sung PA, Jasin M, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8:e1002772. doi: 10.1371/journal.pgen.1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bailey SM, Brenneman MA, Goodwin EH. Frequent recombination in telomeric DNA may extend the proliferative life of telomerase-negative cells. Nucleic Acids Res. 2004;32:3743–3751. doi: 10.1093/nar/gkh691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 91.Henson JD, Cao Y, Huschtscha LI, Chang AC, Au AY, Pickett HA, Reddel RR. DNA C-circles are specific and quantifiable markers of alternative-lengthening-of-telomeres activity. Nat Biotechnol. 2009;27:1181–1185. doi: 10.1038/nbt.1587. [DOI] [PubMed] [Google Scholar]
- 92.Lamarche BJ, Orazio NI, Weitzman MD. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010;584:3682–3695. doi: 10.1016/j.febslet.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Draskovic I, Arnoult N, Steiner V, Bacchetti S, Lomonte P, Londono-Vallejo A. Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci USA. 2009;106:15726–15731. doi: 10.1073/pnas.0907689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 95.Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 96.Stagno D'Alcontres M, Mendez-Bermudez A, Foxon JL, Royle NJ, Salomoni P. Lack of TRF2 in ALT cells causes PML-dependent p53 activation and loss of telomeric DNA. J Cell Biol. 2007;179:855–867. doi: 10.1083/jcb.200703020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu J, Lan J, Wang C, Wu Q, Zhu Y, Lai X, Sun J, Jin C, Huang H. PML3 interacts with TRF1 and is essential for ALT-associated PML bodies assembly in U2OS cells. Cancer Lett. 2010;291:177–186. doi: 10.1016/j.canlet.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Potts PR, Yu H. The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol. 2007;14:581–590. doi: 10.1038/nsmb1259. [DOI] [PubMed] [Google Scholar]
- 99.Chen YJ, Hakin-Smith V, Teo M, Xinarianos GE, Jellinek DA, Carroll T, McDowell D, MacFarlane MR, Boet R, Baguley BC, et al. Association of mutant TP53 with alternative lengthening of telomeres and favorable prognosis in glioma. Cancer Res. 2006;66:6473–6476. doi: 10.1158/0008-5472.CAN-06-0910. [DOI] [PubMed] [Google Scholar]
- 100.Razak ZR, Varkonyi RJ, Kulp-McEliece M, Caslini C, Testa JR, Murphy ME, Broccoli D. p53 differentially inhibits cell growth depending on the mechanism of telomere maintenance. Mol Cell Biol. 2004;24:5967–5977. doi: 10.1128/MCB.24.13.5967-5977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmidt JC, Dalby AB, Cech TR. Identification of human TERT elements necessary for telomerase recruitment to telomeres. Elife. 2014;3:e03563. doi: 10.7554/eLife.03563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hrdlickova R, Nehyba J, Bose HR., Jr Alternatively spliced telomerase reverse transcriptase variants lacking telomerase activity stimulate cell proliferation. Mol Cell Biol. 2012;32:4283–4296. doi: 10.1128/MCB.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khattar E, Kumar P, Liu CY, Akincilar SC, Raju A, Lakshmanan M, Maury JJ, Qiang Y, Li S, Tan EY, et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J Clin Invest. 2016 doi: 10.1172/JCI86042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer. 2011;11:161–176. doi: 10.1038/nrc3025. [DOI] [PubMed] [Google Scholar]
- 105.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 106.Zhou L, Zheng D, Wang M, Cong YS. Telomerase reverse transcriptase activates the expression of vascular endothelial growth factor independent of telomerase activity. Biochem Biophys Res Commun. 2009;386:739–743. doi: 10.1016/j.bbrc.2009.06.116. [DOI] [PubMed] [Google Scholar]
- 107.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–919. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 108.Coleman C, Levine D, Kishore R, Qin G, Thorne T, Lambers E, Sasi SP, Yaar M, Gilchrest BA, Goukassian DA. Inhibition of melanoma angiogenesis by telomere homolog oligonucleotides. J Oncol. 2010;2010:928628. doi: 10.1155/2010/928628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pallini R, Sorrentino A, Pierconti F, Maggiano N, Faggi R, Montano N, Maira G, Larocca LM, Levi A, Falchetti ML. Telomerase inhibition by stable RNA interference impairs tumor growth and angiogenesis in glioblastoma xenografts. Int J Cancer. 2006;118:2158–2167. doi: 10.1002/ijc.21613. [DOI] [PubMed] [Google Scholar]
- 110.Zaccagnini G, Gaetano C, Della Pietra L, Nanni S, Grasselli A, Mangoni A, Benvenuto R, Fabrizi M, Truffa S, Germani A, et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790–14798. doi: 10.1074/jbc.M414644200. [DOI] [PubMed] [Google Scholar]
- 111.Stower H. Telomeres: stem cells cancer and telomerase linked by WNT. Nat Rev Genet. 2012;13:521. doi: 10.1038/nrg3286. [DOI] [PubMed] [Google Scholar]
- 112.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, Kemler R. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 113.Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 114.Chung J, Khadka P, Chung IK. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci. 2012;125:2684–2697. doi: 10.1242/jcs.099267. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, et al. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270–1281. doi: 10.1038/ncb2621. [DOI] [PubMed] [Google Scholar]
- 116.Ding D, Xi P, Zhou J, Wang M, Cong YS. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-κB-dependent transcription. FASEB J. 2013;27:4375–4383. doi: 10.1096/fj.13-230904. [DOI] [PubMed] [Google Scholar]
- 117.Goldblatt EM, Gentry ER, Fox MJ, Gryaznov SM, Shen C, Herbert BS. The telomerase template antagonist GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits growth, and augments the effects of paclitaxel. Mol Cancer Ther. 2009;8:2027–2035. doi: 10.1158/1535-7163.MCT-08-1188. [DOI] [PubMed] [Google Scholar]
- 118.Jackson SR, Zhu CH, Paulson V, Watkins L, Dikmen ZG, Gryaznov SM, Wright WE, Shay JW. Antiadhesive effects of GRN163L--an oligonucleotide N3'->P5' thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67:1121–1129. doi: 10.1158/0008-5472.CAN-06-2306. [DOI] [PubMed] [Google Scholar]
- 119.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neil JR, Schiemann WP. Altered TAB1:IκB kinase interaction promotes transforming growth factor β-mediated nuclear factor-κB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qin Y, Tang B, Hu CJ, Xiao YF, Xie R, Yong X, Wu YY, Dong H, Yang SM. An hTERT/ZEB1 complex directly regulates E-cadherin to promote epithelial-to-mesenchymal transition (EMT) in colorectal cancer. Oncotarget. 2016;7:351–361. doi: 10.18632/oncotarget.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Z, Li Q, Li K, Chen L, Li W, Hou M, Liu T, Yang J, Lindvall C, Bjorkholm M, et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene. 2013;32:4203–4213. doi: 10.1038/onc.2012.441. [DOI] [PubMed] [Google Scholar]
- 123.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res. 2002;62:3876–3882. [PubMed] [Google Scholar]
- 124.Cassar L, Li H, Pinto AR, Nicholls C, Bayne S, Liu JP. Bone morphogenetic protein-7 inhibits telomerase activity, telomere maintenance, and cervical tumor growth. Cancer Res. 2008;68:9157–9166. doi: 10.1158/0008-5472.CAN-08-1323. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121:1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 127.Lamb R, Ozsvari B, Bonuccelli G, Smith DL, Pestell RG, Martinez-Outschoorn UE, Clarke RB, Sotgia F, Lisanti MP. Dissecting tumor metabolic heterogeneity: Telomerase and large cell size metabolically define a sub-population of stem-like, mitochondrial-rich, cancer cells. Oncotarget. 2015;6:21892–21905. doi: 10.18632/oncotarget.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaheen F, Grammatopoulos DK, Muller J, Zammit VA, Lehnert H. Extra-nuclear telomerase reverse transcriptase (TERT) regulates glucose transport in skeletal muscle cells. Biochim Biophys Acta. 2014;1842:1762–1769. doi: 10.1016/j.bbadis.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 129.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 130.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei M, Zaug AJ, Podell ER, Cech TR. Switching human telomerase on and off with hPOT1 protein in vitro. J Biol Chem. 2005;280:20449–20456. doi: 10.1074/jbc.M502212200. [DOI] [PubMed] [Google Scholar]
- 132.Sexton AN, Regalado SG, Lai CS, Cost GJ, O'Neil CM, Urnov FD, Gregory PD, Jaenisch R, Collins K, Hockemeyer D. Genetic and molecular identification of three human TPP1 functions in telomerase action: recruitment, activation, and homeostasis set point regulation. Genes Dev. 2014;28:1885–1899. doi: 10.1101/gad.246819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El Mai M, Wagner KD, Michiels JF, Ambrosetti D, Borderie A, Destree S, Renault V, Djerbi N, Giraud-Panis MJ, Gilson E, et al. The telomeric protein TRF2 regulates angiogenesis by binding and activating the PDGFRβ promoter. Cell Rep. 2014;9:1047–1060. doi: 10.1016/j.celrep.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 134.Chen LY, Zhang Y, Zhang Q, Li H, Luo Z, Fang H, Kim SH, Qin L, Yotnda P, Xu J, et al. Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol Cell. 2012;47:839–850. doi: 10.1016/j.molcel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kammori M, Sugishita Y, Okamoto T, Kobayashi M, Yamazaki K, Yamada E, Yamada T. Telomere shortening in breast cancer correlates with the pathological features of tumor progression. Oncol Rep. 2015;34:627–632. doi: 10.3892/or.2015.4063. [DOI] [PubMed] [Google Scholar]
- 136.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL, Westra WH, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Subhawong AP, Heaphy CM, Argani P, Konishi Y, Kouprina N, Nassar H, Vang R, Meeker AK. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with HER-2 overexpression. Mod Pathol. 2009;22:1423–1431. doi: 10.1038/modpathol.2009.125. [DOI] [PubMed] [Google Scholar]
- 138.McDonald KL, McDonnell J, Muntoni A, Henson JD, Hegi ME, von Deimling A, Wheeler HR, Cook RJ, Biggs MT, Little NS, et al. Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol. 2010;69:729–736. doi: 10.1097/NEN.0b013e3181e576cf. [DOI] [PubMed] [Google Scholar]
- 139.Villa R, Daidone MG, Motta R, Venturini L, De Marco C, Vannelli A, Kusamura S, Baratti D, Deraco M, Costa A, et al. Multiple mechanisms of telomere maintenance exist and differentially affect clinical outcome in diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2008;14:4134–4140. doi: 10.1158/1078-0432.CCR-08-0099. [DOI] [PubMed] [Google Scholar]
- 140.Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, Tsung A, Marsh JW, Lee KK, Hogg ME, et al. Alterative lengthening of telomeres and loss of DAXX/ATRX expression predicts metastatic disease and poor survival in patients with pancreatic neuroendocrine tumors. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1113. pii: clincanres.1113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sanders RP, Drissi R, Billups CA, Daw NC, Valentine MB, Dome JS. Telomerase expression predicts unfavorable outcome in osteosarcoma. J Clin Oncol. 2004;22:3790–3797. doi: 10.1200/JCO.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 142.Mokbel K, Ghilchik M, Williams G, Akbar N, Parris C, Newbold R. The association between telomerase activity and hormone receptor status and p53 expression in breast cancer. International journal of surgical investigation. In J Surg Investig. 2000;1:509–516. [PubMed] [Google Scholar]
- 143.Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sotillo-Pineiro E, Sierrasesumaga L, Patinno-Garcia A. Telomerase activity and telomere length in primary and metastatic tumors from pediatric bone cancer patients. Pediatr Res. 2004;55:231–235. doi: 10.1203/01.PDR.0000102455.36737.3C. [DOI] [PubMed] [Google Scholar]
- 145.Chang S, Khoo CM, Naylor ML, Maser RS, DePinho RA. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 2003;17:88–100. doi: 10.1101/gad.1029903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 148.Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, Sledge GW, Herbert BS. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184–3192. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 149.Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, Wright WE, Shay JW. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 150.Chiappori AA, Kolevska T, Spigel DR, Hager S, Rarick M, Gadgeel S, Blais N, Von Pawel J, Hart L, Reck M, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol. 2015;26:354–362. doi: 10.1093/annonc/mdu550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Goldkorn A, Blackburn EH. Assembly of mutant-template telomerase RNA into catalytically active telomerase ribonucleoprotein that can act on telomeres is required for apoptosis and cell cycle arrest in human cancer cells. Cancer Res. 2006;66:5763–5771. doi: 10.1158/0008-5472.CAN-05-3782. [DOI] [PubMed] [Google Scholar]
- 152.Domchek SM, Recio A, Mick R, Clark CE, Carpenter EL, Fox KR, DeMichele A, Schuchter LM, Leibowitz MS, Wexler MH, et al. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- 153.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 154.Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829–840. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 155.Shay JW, Keith WN. Targeting telomerase for cancer therapeutics. Br J Cancer. 2008;98:677–683. doi: 10.1038/sj.bjc.6604209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Flynn RL, Cox KE, Jeitany M, Wakimoto H, Bryll AR, Ganem NJ, Bersani F, Pineda JR, Suva ML, Benes CH, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347:273–277. doi: 10.1126/science.1257216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsai HJ, Huang WH, Li TK, Tsai YL, Wu KJ, Tseng SF, Teng SC. Involvement of topoisomerase III in telomere-telomere recombination. J Biol Chem. 2006;281:13717–13723. doi: 10.1074/jbc.M600649200. [DOI] [PubMed] [Google Scholar]
- 158.Hu H, Zhang Y, Zou M, Yang S, Liang XQ. Expression of TRF1, TRF2, TIN2, TERT, KU70, and BRCA1 proteins is associated with telomere shortening and may contribute to multistage carcinogenesis of gastric cancer. J Cancer Res Clin Oncol. 2010;136:1407–1414. doi: 10.1007/s00432-010-0795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ramsay AJ, Quesada V, Foronda M, Conde L, Martinez-Trillos A, Villamor N, Rodriguez D, Kwarciak A, Garabaya C, Gallardo M, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet. 2013;45:526–530. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- 160.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, Pooley KA, Pritchard AL, Tiffen JC, Petljak M, et al. POT1 loss-of-function variants predispose to familial melanoma. Nat Genet. 2014;46:478–481. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bainbridge MN, Armstrong GN, Gramatges MM, Bertuch AA, Jhangiani SN, Doddapaneni H, Lewis L, Tombrello J, Tsavachidis S, Liu Y, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2015;107:384. doi: 10.1093/jnci/dju384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kocak H, Ballew BJ, Bisht K, Eggebeen R, Hicks BD, Suman S, O'Neil A, Giri N, Maillard I, Alter BP, et al. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes Dev. 2014;28:2090–2102. doi: 10.1101/gad.248567.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aoude LG, Pritchard AL, Robles-Espinoza CD, Wadt K, Harland M, Choi J, Gartside M, Quesada V, Johansson P, Palmer JM, et al. Nonsense mutations in the shelterin complex genes ACD and TERF2IP in familial melanoma. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 166.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rachakonda PS, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, Wiklund NP, Steineck G, Schadendorf D, Hemminki K, et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc Natl Acad Sci USA. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, Ghossein RA, Fagin JA. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–1566. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen HC, Smart CE, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. 384e371–372. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 172.Wang Z, Cummins JM, Shen D, Cahill DP, Jallepalli PV, Wang TL, Parsons DW, Traverso G, Awad M, Silliman N, et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 2004;64:2998–3001. doi: 10.1158/0008-5472.can-04-0587. [DOI] [PubMed] [Google Scholar]
- 173.Bartkova J, Tommiska J, Oplustilova L, Aaltonen K, Tamminen A, Heikkinen T, Mistrik M, Aittomaki K, Blomqvist C, Heikkila P, et al. Aberrations of the MRE11-RAD50-NBS1 DNA damage sensor complex in human breast cancer: MRE11 as a candidate familial cancer-predisposing gene. Mol Oncol. 2008;2:296–316. doi: 10.1016/j.molonc.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cybulski C, Gorski B, Debniak T, Gliniewicz B, Mierzejewski M, Masojc B, Jakubowska A, Matyjasik J, Zlowocka E, Sikorski A, et al. NBS1 is a prostate cancer susceptibility gene. Cancer Res. 2004;64:1215–1219. doi: 10.1158/0008-5472.can-03-2502. [DOI] [PubMed] [Google Scholar]
- 175.Varon R, Reis A, Henze G, von Einsiedel HG, Sperling K, Seeger K. Mutations in the Nijmegen Breakage Syndrome gene (NBS1) in childhood acute lymphoblastic leukemia (ALL) Cancer Res. 2001;61:3570–3572. [PubMed] [Google Scholar]
- 176.Heikkinen K, Rapakko K, Karppinen SM, Erkko H, Knuutila S, Lundan T, Mannermaa A, Borresen-Dale AL, Borg A, Barkardottir RB, et al. RAD50 and NBS1 are breast cancer susceptibility genes associated with genomic instability. Carcinogenesis. 2006;27:1593–1599. doi: 10.1093/carcin/bgi360. [DOI] [PMC free article] [PubMed] [Google Scholar]