Abstract
Ubiquitination regulates a broad array of cellular processes, and defective ubiquitination is implicated in several neurological disorders. Loss of the E3 ubiquitin-protein ligase UBE3A causes Angelman syndrome. Despite its clinical importance, the normal role of UBE3A in neurons is still unclear. As a step toward deciphering its possible functions, we performed high-resolution light and electron microscopic immunocytochemistry. We report a broad distribution of UBE3A in neurons, highlighted by concentrations in axon terminals and euchromatin-rich nuclear domains. Our findings suggest that UBE3A may act locally to regulate individual synapses, while also mediating global, neuron-wide influences through the regulation of gene transcription.
Keywords: Angelman syndrome, E6-AP, ubiquitin-proteasome pathway, axon terminal, mitochondria, euchromatin, heterochromatin, RRID:nif-0000-30467, RRID:AB_10740376
The ubiquitin-proteasome pathway, which provides a mechanism for protein degradation in eukaryotes, is important for a broad array of basic cellular processes. Neurons have long dendrites and axons whose distal regions are remote from the soma, emphasizing the importance of local protein synthesis and degradation in neuronal function. Accordingly, the ubiquitin-proteasome pathways has been shown to be important for appropriate circuit wiring, neuronal morphogenesis, intracellular trafficking, and synaptic plasticity (Hegde, 2004; Acconcia et al., 2009; Cajigas et al., 2010; Schwarz and Patrick, 2012; Hamilton and Zito, 2013; Goo et al., 2015). Conversely, dysregulation of the ubiquitin-proteasome system has been implicated in multiple neurological disorders including Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and autism (Tai and Schuman, 2008; Schwarz and Patrick, 2012).
The covalent attachment of ubiquitin to protein substrates involves sequential reactions catalyzed by a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin-protein ligase (E3). The E3 ligases largely dictate specificity and target protein recognition for the ubiquitin conjugation system; thus the biology of E3 ligases is of broad biological and clinical importance. Based on their distinct domains and mode of action, E3 proteins segregate into three families: RING/RING-like E3s, RING-in-between-RING (RBR) E3s, and Homologous to the E6-AP Carboxyl Terminus (HECT) E3s. The loss of expression or function of UBE3A (also called E6-AP), the founding member of the HECT E3 ligase family, leads to Angelman syndrome (AS), a severe neurological disorder characterized by intellectual disability, absent speech, ataxia, seizures, and hyperactivity. On the other hand, increased UBE3A expression has been associated with autism spectrum disorders (Abrahams and Geschwind, 2008; Glessner et al., 2009). Thus, maintenance of appropriate UBE3A levels is crucial for proper brain development and function.
Deletions or mutations of the maternal UBE3A allele are sufficient to cause AS (Kishino et al., 1997; Matsuura et al., 1997). UBE3A protein is primarily expressed by the maternal allele in mature neurons, due to evolutionarily conserved, tissue-specific mechanisms of imprinting (Rougeulle et al., 1997; Vu and Hoffman, 1997). Mouse models of AS carry maternally-inherited Ube3a deletions, and display many Angelman-like phenotypes, including learning and memory deficits, motor dysfunction, and seizures (Jiang et al., 1998; Miura et al., 2002; van Woerden et al., 2007; Heck et al., 2008; Jiang et al., 2010; Mulherkar and Jana, 2010; Margolis et al., 2015). At the cellular level, AS mice exhibit abnormalities in synaptic transmission and plasticity (Weeber et al., 2003; van Woerden et al., 2007; Yashiro et al., 2009; Greer et al., 2010; Margolis et al., 2010; Wallace et al., 2012), Golgi acidification (Condon et al., 2013), and mitochondrial function (Su et al., 2011; Llewellyn et al., 2015; Santini et al., 2015) . This phenotypic diversity suggests a multiplicity of roles for UBE3A in neural function.
Previous immunohistochemical studies found UBE3A mainly in the nuclei of mature neurons, consistent with a proposed role in the co-regulation of transcription (Nawaz et al., 1999; Bernassola et al., 2008; Pal et al., 2013). However, the majority of putative UBE3A substrates are cytoplasmic proteins, and most cellular phenotypes in AS mice implicate loss of UBE3A function in non-nuclear compartments. This puzzle underscores the need for detailed examination of the subcellular localization of UBE3A, which we achieved in the present study through confocal co-localization analysis and high-resolution pre-embedding immunogold electron microscopy performed in multiple regions of the mouse brain. By highlighting the subcellular compartments in which UBE3A is likely to function, our data provide insight into the diverse functional capacities of this E3 ligase.
MATERIALS AND METHODS
Animals
All procedures related to the care and treatment of animals were in accordance with institutional and NIH guidelines, and all animal use protocols were reviewed and approved by the UNC Institutional Animal Care and Use Committee. Mice carrying the Ube3a knock-out allele were originally generated in the laboratory of A. Beaudet (Jiang et al., 1998) and backcrossed to a congenic C57BL/6J background. AS model mice (Ube3am–/p+), which maternally inherit the Ube3a knock-out allele, were generated by mating wild-type (Ube3am+/p+) male mice to female mice with paternal inheritance of the Ube3a knock-out allele (Ube3am+/p−), which themselves are phenotypically normal (Jiang et al., 1998; Mulherkar and Jana, 2010). To generate Ube3a double knock-out (KO) mice (Ube3am−/p−), female Ube3am+/p− mice were mated to male Ube3am+/p− mice.
Fifteen male P7 and 31 male adult (P60-P100) were used in this study, including 8 P7 and 12 adult WT; 4 P7 and 10 adult Ube3am+/p−, and 3 P7 and 9 adult Ube3am+/p−.
UBE3A antibody
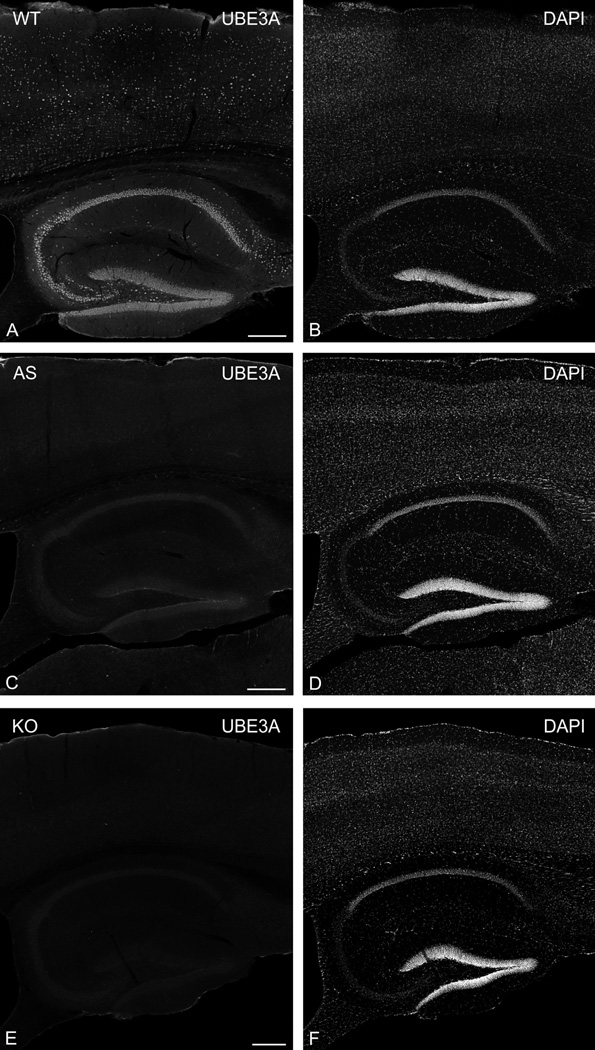
To identify UBE3A we used a mouse monoclonal antibody (3E5, Sigma-Aldrich, Cat# SAB1404508, RRID:AB_10740376) raised against a peptide corresponding to a.a. 315–415 (isoforms 1 and 3) or a.a. 336–436 (isoform 2) of mouse Ube3a. To verify antibody specificity, we performed immunocytochemistry on brain sections from AS model and UBE3A KO adult mice, run with sections from the littermate WT mice (Fig. 1). We observed robust staining in WT mice (Fig. 1A, B), whose overall pattern of immunostaining was consistent with previous observations (Judson et al., 2014). Staining in AS model mice (Figure 1C, D) was greatly reduced, but not completely absent. In contrast, we observed only an extremely weak “background” pattern of UBE3A labeling in KO mice (Fig. 1E, F), which was unrelated to staining patterns observed in WT brain sections. We conclude from these results that the antibody used here reacts specifically with UBE3A protein.
Figure 1.
UBE3A expression in mouse brain from WT (A, B), AS model (C, D), and KO (E, F) mice. Sagittal sections were immunostained for UBE3A (left column, A, C, E) and counterstained with DAPI (right column, B, D, F). UBE3A staining is markedly diminished in the AS mice, and nearly absent in the KO. Scale bars = 250 µm.
Tissue preparation
After inducing deep anesthesia with sodium pentobarbital (80 mg/kg, i.p.), male P7 and adult (P60-P100) mice were intracardially perfused with 50–100 ml of fixative: 4% freshly-depolymerized paraformaldehyde in phosphate buffer (PB, 0.1 M, pH 7.4) for light microscopy (LM), or a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde in PB for electron microscopy (EM). Brains were sectioned at 50 µm and 100 µm on a Vibratome, and collected in cold PB.
Light microscopic immunohistochemistry
Free-floating sections were incubated in 10% normal donkey serum (NDS), and then incubated overnight in a mouse monoclonal anti-UBE3A antibody (UBE3A, 1:10,000, Sigma-Aldrich Cat# SAB1404508, RRID:AB_10740376). Antigenic sites were visualized with donkey IgG conjugated to DyLight 549 (1:200, Jackson ImmunoResearch; West Grove, PA). Some sections were then counterstained with DAPI (1:10,000, Sigma-Aldrich, Cat# D9542) to visualize nuclei, and with NeuroTrace 640–660 (1:1000, Invitrogen, Thermo Fisher Scientific, Rockford, IL; Cat# N21483) to visualize neuronal somata. To verify antibody specificity, we performed immunocytochemistry on brain sections from AS model and Ube3a KO mice, run together with sections from WT mice. Experiments in which the primary antibodies were omitted were performed to control for nonspecific binding of the secondary antibody. Sections were examined with a Zeiss LSM 880 confocal microscope.
Electron microscopy
Floating sections treated with 1% sodium borohydride were blocked with 20% NDS, and incubated in primary antibody (UBE3A, 1:10,000, Sigma-Aldrich, Cat# SAB1404508, RRID:AB_10740376). Sections were then incubated with biotinylated goat-anti mouse IgG (1:2,000, Vector Laboratories, Burlingame, CA; Cat# BA-9200), followed by 1.4 nm gold particles conjugated to streptavidin (1:50; Nanoprobes, Yaphank, NY; Cat# 2016). Sections were washed in 0.05 M sodium acetate (to remove phosphate and chloride ions), followed by silver enhancement with IntenSE-M (Amersham Biosciences, Cat# RPN491, discontinued). Sections were then post-fixed in 1% osmium tetroxide in 0.1M PB for 35–45 min and incubated for 1 hr with 1% uranyl acetate in maleate buffer (0.1M, pH 6.0). After dehydration, sections were infiltrated with a mixture of Epon and Spurr resins (Electron Microscopy Sciences, Hatfield, PA) and flat-mounted between sheets of ACLAR® fluoropolymer (Electron Microscopy Sciences, Hatfield, PA) within glass slides. Seventy-nanometer sections were cut, mounted on 200 mesh copper grids, contrasted with uranyl acetate and Sato’s lead, and examined in a Philips Tecnai electron microscope (Hillsboro, OR) at 80 kV.
Image analysis and preparation of plates
To further test UBE3A antibody specificity for preembedding EM immunohistochemisty, and to evaluate the distribution of labeling in different subcellular compartments, we collected large EM photomontages of neuropil (each ~ 10 × 10 µm, 15 fields per mouse, 3 mice per group) from layer II-III of the primary visual cortex and from CA1 stratum radiatum region of the hippocampus taken from WT, AS model, and KO mice. We also collected EM photomontages of entire neuronal nuclei (15 nuclei per mouse, 3 mice per group) from layer II-III of the primary visual cortex and from CA1 stratum pyramidale region of the hippocampus. We then used ImageJ (Schneider et al., 2012)(ImageJ, RRID:nif-0000–30467) to compute density of gold particles in neuropil and nuclei. Analyses of WT, AS model, and KO mice were carried out in a blinded fashion.
EM photomontages collected from WT mice were further analyzed. Dendritic spines, presynaptic terminals, and dendritic shafts profiles were outlined using ImageJ, and the density of gold particles in each compartment then computed.
To examine the quantitative relationship between staining for UBE3A and DAPI, we performed intensity correlation analysis as described by Li et al. (2004) (Li et al., 2004) using the “Intensity Correlation Analysis” plug-in in ImageJ (see the ImageJ Web site of the Wright Cell Imaging Facility, Toronto Western Research Institute, Ontario, Canada; www.uhnresearch.ca/wcif). For each pixel from the UBE3A and DAPI double-labeled material, DAPI intensity was plotted against the product of (UBE3Apixel value - UBE3Amean) × (DAPIpixel value - DAPImean), with UBE3Apixel value and DAPIpixel value being the current pixel’s intensity, and UBE3Amean and DAPImean being the channel’s mean intensity for the region of interest. Thus, if the values for both channels of a pixel are either above or below the mean, this product will be positive, whereas if one channel is above the mean and the other is below the mean, the product will be negative. Accordingly, the x-axis reflects the covariance of the channels, and the y-axis reflects the intensity distribution of one of the two channels.
We used Corel Draw X7 (Corel, Ontario, Canada) to sharpen images (via unsharp mask), adjust brightness and contrast, and compose final plates. These adjustments were performed uniformly on entire image; they were done exclusively to enhance the presentation quality of figures, without altering the content of the images.
RESULTS
General distribution of UBE3A
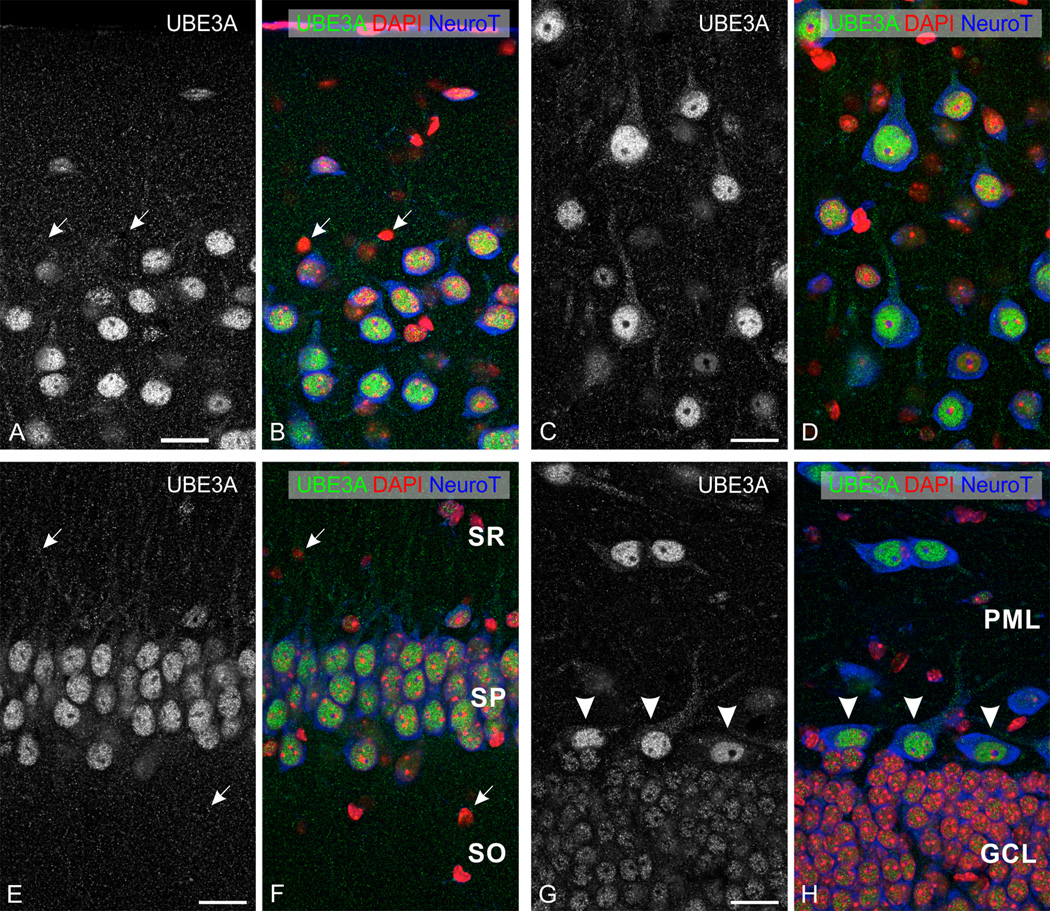
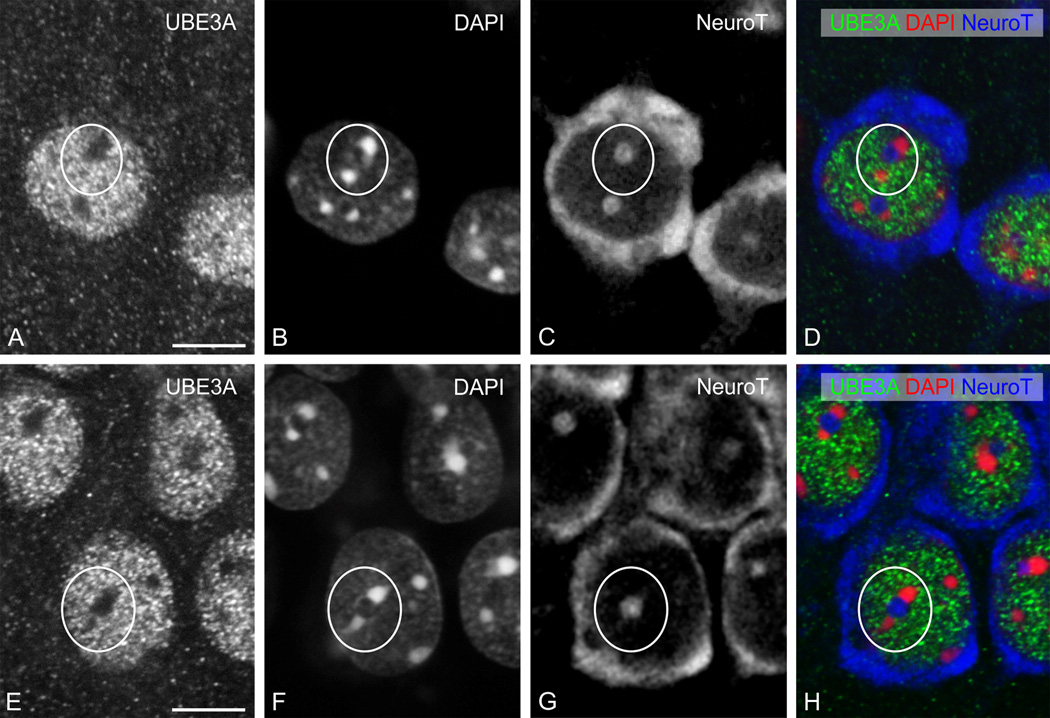
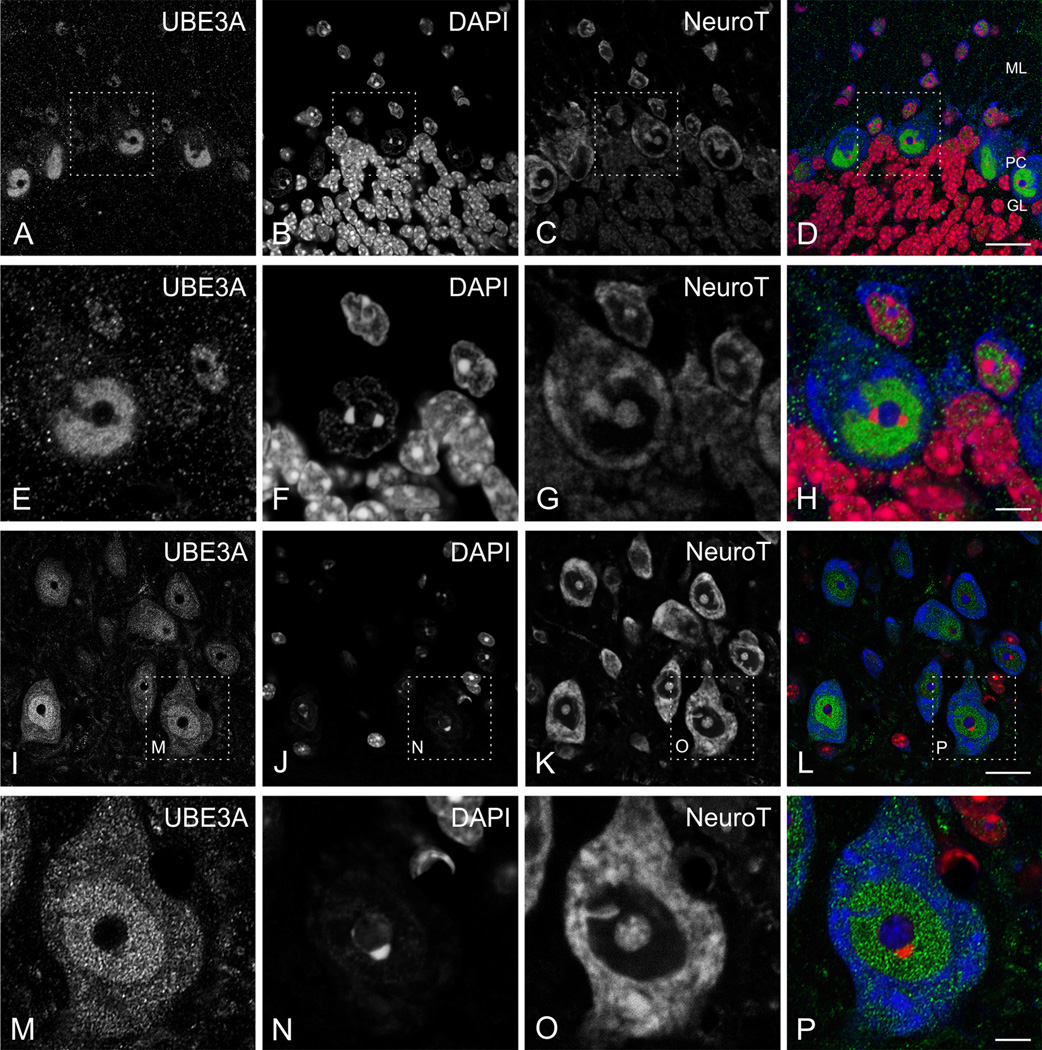
UBE3A was expressed through all layers of the neocortex (Fig. 1A); no obvious differences in the pattern of labeling were seen across neocortical areas. At the subcellular level, UBE3A staining was prominent in nuclei; staining was detectable but weaker in somata and dendrites, and in numerous small puncta throughout the neuropil (Fig. 2 A–D). Counterstaining with NeuroTrace (a fluorescent Nissl stain) and DAPI showed that most neurons were immunopositive, while many non-neuronal cells had little or undetectable UBE3A. Within nuclei, UBE3A staining was typically organized into patches or blobs (Figure 3A). These blobs were interspersed with DAPI “hotspots,” but generally did not overlap with them (Figure 3B). Likewise, nuclear UBE3A staining did not overlap with NeuroTrace patches (Figure 3C), suggesting that UBE3A was excluded from nucleoli.
Figure 2.
Higher magnification view of immunofluorescence staining for UBE3A in neocortex (top row) and hippocampus (bottom row). Sections were counterstained with DAPI (red) to visualize nuclei, and with NeuroTrace 640–660 (blue) to visualize neuronal somata. A, B: cerebral cortex (primary visual cortex, layer I-II); C, D: cerebral cortex (primary visual cortex, layer V); E, F: CA1 hippocampus; and G, H: dentate gyrus. White arrows point to small non-neuronal cells with little to no staining for UBE3A. Arrowheads in G, H point to hilar mossy cells. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum; GCL, granule cell layer; PML, polymorphic cell layer. Scale bars= 20 µm.
Figure 3.
High magnification immunofluorescence, showing UBE3A staining in neocortex (top row) and hippocampus (bottom row), as in Figure 2. UBE3A staining is organized into small puncta that are highly concentrated in neuronal nuclei. White circles: note the relative lack of staining in hotspots of condensed DNA (as defined by DAPI), and in nucleoli (as defined by NeuroTrace). Scale bars = 10 µm.
Expression was stronger in the hippocampus than in neocortex, especially in the pyramidal cell layer of Ammon’s horn and the granule cell layer of the dentate gyrus (Fig. 1A). The overall subcellular distribution resembled that neocortex, with prominent nuclear staining (Fig. 2 E–H, 3 E–H) and weaker staining in the neuropil. The level of nuclear UBE3A in neurons was variable; this was particularly obvious in the dentate gyrus, where the hilar mossy cells were much more strongly stained than the adjacent granule cells (Figure 2 G, H). In contrast, we saw little variation in the level of nuclear UBE3A within a given neuronal type (for example, among pyramidal neurons in CA1).
In the neostriatum, UBE3A was prominently expressed in the gray matter between fascicles of myelinated fibers, which themselves were only very weakly stained (Fig. 4 A–D). At higher magnification, it was clear that UBE3A stained nuclei and small puncta throughout the neuropil (Fig. 4 E–H). UBE3A stained all thalamic regions robustly; staining could be seen in nuclei and neuropil (Fig. 4 I–P). In the cerebellar cortex, UBE3A labeling was strongest in the Purkinje cell layer, where Purkinje cell nuclei were intensely stained (Fig. 5 A–D). In the molecular layer, UBE3A stained scattered nuclei from small neurons, and numerous puncta throughout the neuropil. Staining in the granule cell layer was weak. In contrast to Purkinje cells, the nuclei of granule cells contained very little UBE3A (Figure 5 E–H). UBE3A was detected throughout the brainstem, though staining intensity varied across different regions. Particularly strong staining was found in the reticular formation, where large neurons in the gigantocellular nucleus were intensely stained (Fig. 5 I–P).
Figure 4.
High magnification immunofluorescence, showing UBE3A staining in neostriatum (A–H) and thalamus (I–P). Sections were counterstained with DAPI (red) to visualize nuclei, and with NeuroTrace 640–660 (blue) to visualize neuronal somata. Scale bar = 20 µm in A–D and I–L; 5 µm in E–H and M–P.
Figure 5.
High magnification immunofluorescence, showing UBE3A in cerebellar cortex (A–H) and gigantocellular reticular nucleus (I–P). Sections were counterstained with DAPI (red) to visualize nuclei, and with NeuroTrace 640–660 (blue) to visualize neuronal somata. Scale bar = 20 µm in A–D and I–L; 5 µm in E–H and M–P.
Subcellular distribution of UBE3A
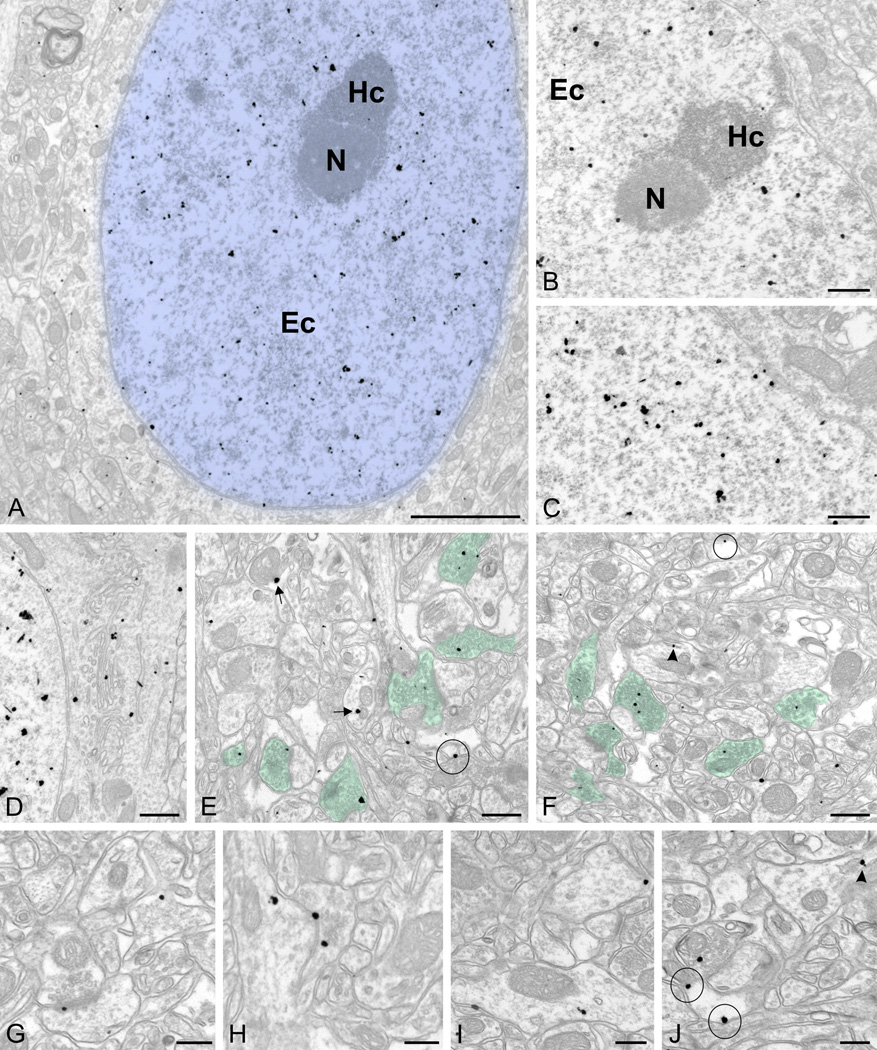
We examined the subcellular organization of UBE3A at higher resolution with electron microscopy, using pre-embedding immunogold labeling followed by silver intensification. We focused our attention on layer II-III of the primary visual cortex and the CA1 region of the hippocampus. Consistent with the LM results, labeling was especially prominent in nuclei (Fig. 6 A–C), but was also present in somata and neuropil (Fig. 6 D–J). To ensure that the labeling faithfully reflected the distribution of antigen rather than nonspecific adsorption (a problem often encountered in nuclei), we quantified labeling in tissue from KO and WT mice. In both neocortex and CA1 hippocampus, the density of gold/silver particles over the nuclei and neuropil was more than 10 times lower in KO than in WT tissue (Table 1). We conclude that the overwhelming majority of the labeling in wild-type mice represented genuine UBE3A protein.
Figure 6.
Pre-embedding immunogold labeling for UBE3A in the adult forebrain.
A, D, E, G, I, J are from cerebral cortex (primary visual cortex, layer I-II). B, C are from CA1 stratum pyramidale region of hippocampus, F and H are from CA1 stratum radiatum region of hippocampus (~40–50 µm from stratum pyramidale).
A–C: UBE3A labeling in nucleus (colorized in blue); labeling is over euchromatin (Ec) domains, but is not associated with heterochromatin (Hc) and nucleoli (N). D: UBE3A labeling associated with endomembranes of the Golgi apparatus. E–F: UBE3A labeling in presynaptic terminals (colorized in green). G: UBE3A labeling close to postsynaptic densities. H: UBE3A labeling in an axodendritic synapse. I: UBE3A labeling in dendrites. J: UBE3A labeling associated with glial membranes. Arrows point to label associated with mitochondria, arrowheads point to label associated with endomembranous structures in dendrites, and circles mark label associated with glial plasma membranes. Scale bar = 2 µm in A; 0.5 µm in B–F; 250 nm in G–I.
Table 1. Relative density of UBE3A staining in AS and KO mice, compared to WT.
The analysis was performed on large EM photomontages of neuropil (each ~ 10 × 10 µm, 15 fields per mouse, 3 mice per group) and on montages of entire neuronal nuclei (15 nuclei per mouse, 3 mice per group) from the CA1 region of the hippocampus and from layer II-III of the primary visual cortex. Data (expressed as percent of label in WT mice) are means ± SEM from three independent experiments.
| Adult | P7 | |||||||
|---|---|---|---|---|---|---|---|---|
| Neocortex | Hippocampus | Neocortex | Hippocampus | |||||
| Nuclei | Neuropil | Nuclei | Neuropil | Nuclei | Neuropil | Nuclei | Neuropil | |
| AS | 8.6% ± 2.3 | 20% ± 3.3 | 2.2% ± 0.8 | 28% ± 2.7 | 9.5% ± 2.3 | 32% ± 1.3 | 5.1% ± 1.6 | 27% ± 0.5 |
| KO | 1.6% ± 0.2 | 8.1% ± 0.1 | 4.1% ± 0.1 | 6.9% ± 0.3 | 4.2% ± 2.1 | 6.5% ± 2.2 | 2.2% ± 1.0 | 6.8% ± 0.4 |
In WT mice, labeling was seen over both glia and neurons. Labeling in glial processes was often associated with the plasma membrane (Figure 6J). Neuronal labeling in dendritic spines and shafts was also frequently associated with the plasma membrane, and with poorly-defined tubulo-vesicular endomembranous structures (Figure 6 G–I). Labeling in neuronal somata was seen decorating the Golgi apparatus (Figure 6D). UBE3A particles were also associated with the outer mitochondrial membrane (Figure 6E). Within the neuropil, the majority of labeling was found in axon terminals from axospinous, axodendritic, and axosomatic synapses. In axon terminals, UBE3A concentrated over the vesicles (colorized in green in Figure 6 E, F).
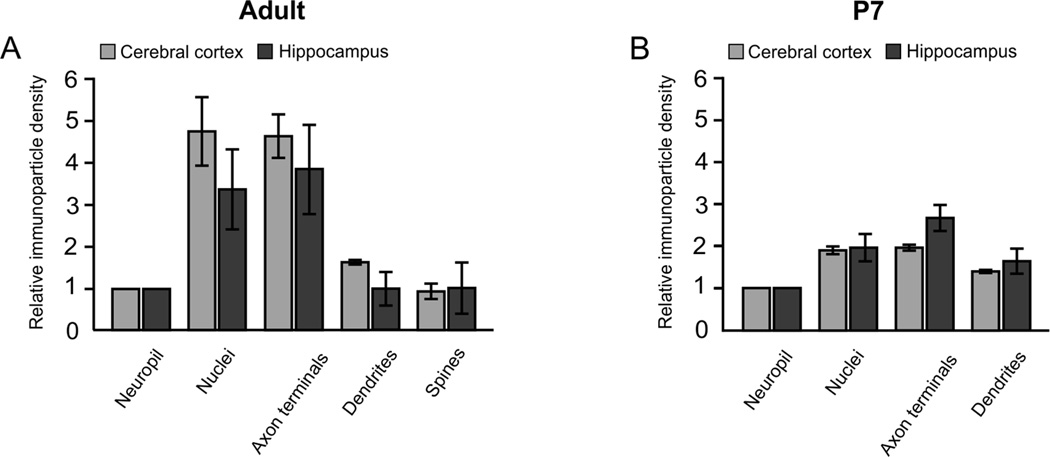
Quantitative analysis confirmed enrichment of UBE3A in both nuclei and axon terminals of the adult brain (Figure 7A). UBE3A concentration in nuclei was on average ~4 times higher than in the general neuropil (4.7 ± 0.8 and 3.3 ± 1.0 in neocortex and CA1 hippocampus, respectively) or dendrites (2.9 ± 0.7 and 3.3 ± 0.4 in neocortex and CA1 hippocampus, respectively), and also on average ~4 times higher in axon terminals than in the general neuropil (4.6 ± 0.5 and 3.8 ± 1.0 in neocortex and CA1 hippocampus, respectively).
Figure 7.
Quantitation of UBE3A immunogold labeling density over different types of profiles in the adult (A) and juvenile (P7, B) mouse. Labeling is generally similar in cerebral cortex (light bars) and hippocampus (dark bars) at both ages. In the adult, labeling is highly concentrated over nuclei and axon terminals; this differentiation is much less pronounced at P7.
The analysis was performed on large EM photomontages of neuropil (each ~ 10 × 10 µm, 15 fields per mice, 3 mice per age group) and entire neuronal nuclei (15 nuclei per mice, 3 mice per age group) from layer II-III of the primary visual cortex and from CA1 region of the hippocampus. Data are means ± SEM from three independent experiments.
We had previously determined by LM analysis that UBE3A expression shifts from the cytoplasm to the nucleus during postnatal development (Judson et al., 2014). To better characterize this phenomenon, we examined the subcellular organization of UBE3A in P7 mice. Quantification of labeling on tissue from KO mice reveals an approximately 10-fold decrease in the density of gold/silver particles over the nuclei and neuropil relative to WT (Table 1), confirming the specificity of labeling. UBE3A was found in the same subcellular compartments in P7 as in adult mice; labeling could be seen in nuclei, somata, dendrites, and axon terminals (Figure 8). Interestingly, we also observed UBE3A gold/silver particles over vesicle clusters that had no obvious relationship to postsynaptic structures; we speculate that these may represent nascent presynaptic sites during early stages of synaptogenesis. UBE3A labeling seemed more uniformly distributed across the different subcellular compartments than in adults. Indeed, quantification in P7 brains, showed that UBE3A did not concentrate nearly as strongly in nuclei and axon terminals; labeling density in nuclei was only 15–25% higher than in dendrites at this age (26.7 ± 0.0 and 16.2 ± 0.04 in neocortex and CA1 hippocampus respectively, Figure 7B). Together, these findings support that the subcellular distribution of UBE3A is relatively uniform in the early postnatal brain, becoming increasingly restricted to specific compartments as development progresses.
Figure 8.
Pre-embedding immunogold labeling for UBE3A in cerebral cortex at P7. A–C: The pattern of nuclear labeling is similar to that seen in the adult (compare with Fig. 4); label avoids heterochromatin patches and the nucleolus. D: General view shows label in nucleus (colorized blue) and proximal dendritic shaft (pink). Some label can be seen in multiple compartments of neuropil, including axon terminals (green). E–F: Label concentrates over developing vesicle-rich axon terminals. G: Label can be seen at the edge of mitochondria. Scale bars = 2 µm in A; 500 nm in B, C; 1 µm in D; 500 nm in E; 250 nm in F; 500 nm in G.
UBE3A staining in AS mouse model
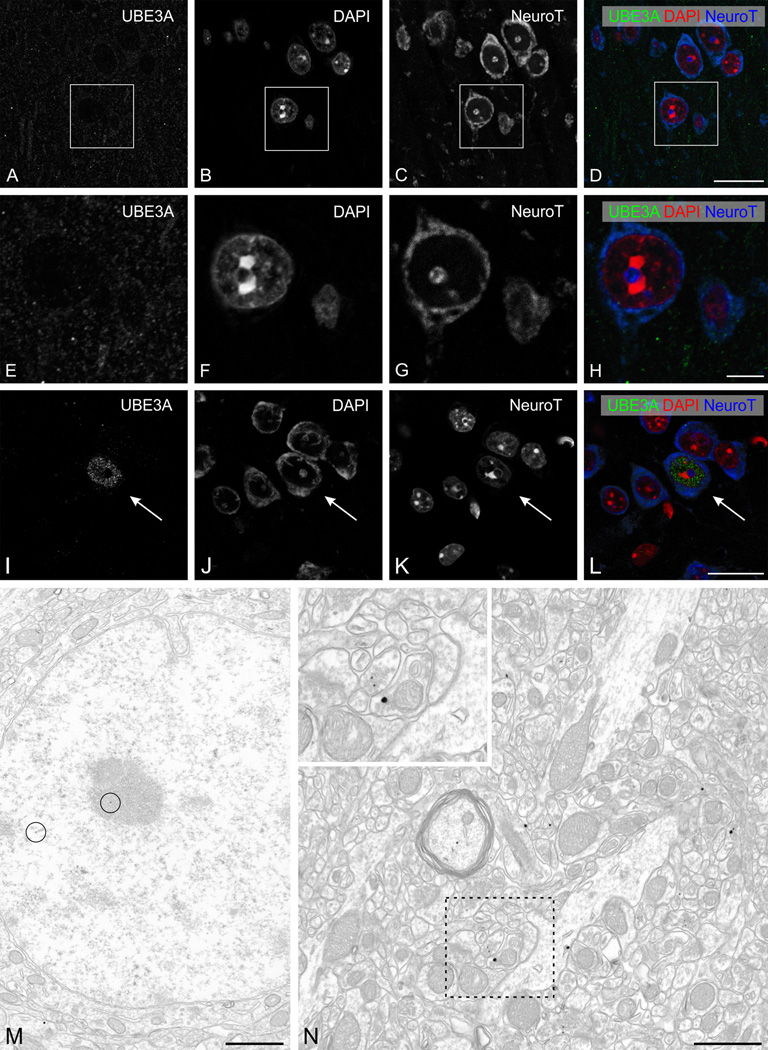
Unlike sections from KO mice, sections from AS model mice were not completely devoid of UBE3A staining (Fig. 1C), leading us to question the total absence of neuronal UBE3A in the AS mouse model. Accordingly, we further investigated UBE3A distribution in AS mice, focusing on the primary visual cortex. High magnification views of UBE3A staining in AS mice showed that neuronal nuclei were devoid of staining, but that small puncta were stained throughout the neuropil (Fig. 9 A–L). The overall intensity of staining was weaker than in the WT, but was still clearly defined. Rare scattered neurons expressed levels of UBE3A staining similar to that of WT animals, in both cytoplasm and nucleus. Pre-embedding immunogold labeling was consistent with the LM results, confirming that staining in nuclei was generally undetectable, but that some staining remained in the neuropil (Fig. 9N). Part of the residual label was associated with small profiles likely to represent glia, which are known to express UBE3A biallelically (Yamasaki et al., 2003; Judson et al., 2014). However, some of the label in AS mice was clearly associated with neuronal elements, especially axon terminals.
Figure 9.
UBE3A staining in the primary visual cortex of adult AS mice model. A–L: High magnification immunofluorescence, showing UBE3A staining in cerebral cortex (primary visual cortex, layer I-II). Sections were counterstained with DAPI (red) to visualize nuclei, and with NeuroTrace 640–660 (blue) to visualize neuronal somata. UBE3A staining is confined to weakly immunoreactive puncta in the neuropil. Rare neurons exhibit nuclear staining similar to that seen in WT mice (arrows, in I–L).
M, N: Pre-embedding immunogold labeling for UBE3A in cerebral cortex (primary visual cortex, layer I–II). Only very rare silver/gold particles are located over neuronal nuclei (circles in M). In the neuropil, labeling can be seen in presynaptic terminals (inset in N). Scale bars = 20 µm in A–D and I–L; 5 µm in E–H; 1 µm in M, N.
Quantification showed that the density of gold/silver particles over nuclei was at least 10-fold lower in AS model mice than in WT mice (Table 1). AS model mice displayed a more modest decrease (reduction of ~70–80%) of labeling for UBE3A in neuropil. The density of labeling over nuclei in P7 AS mice was similarly 10-fold lower than WT, mirroring our observations in adult mice. In contrast, we observed a more modest decrease (65–75%) in neuropil labeling at P7 (Table 1).
The nuclear organization of UBE3A
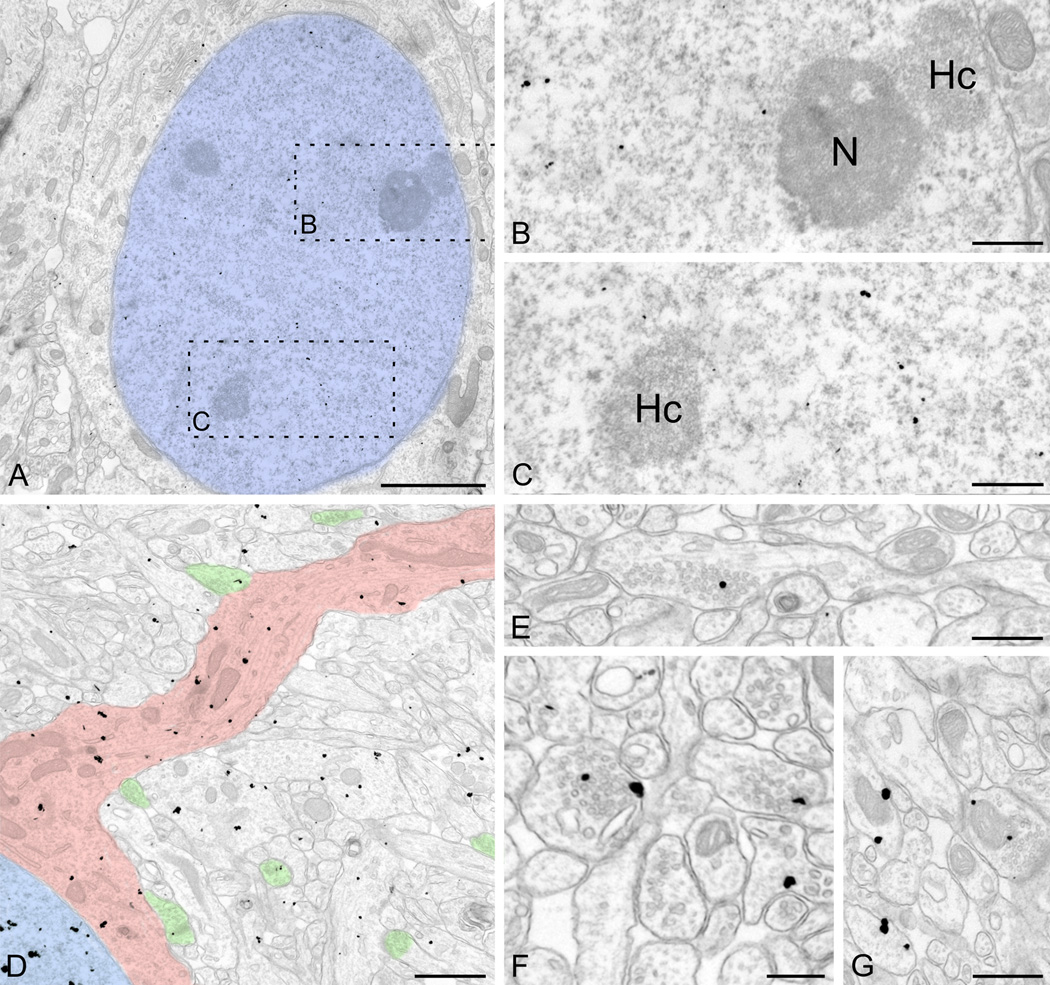
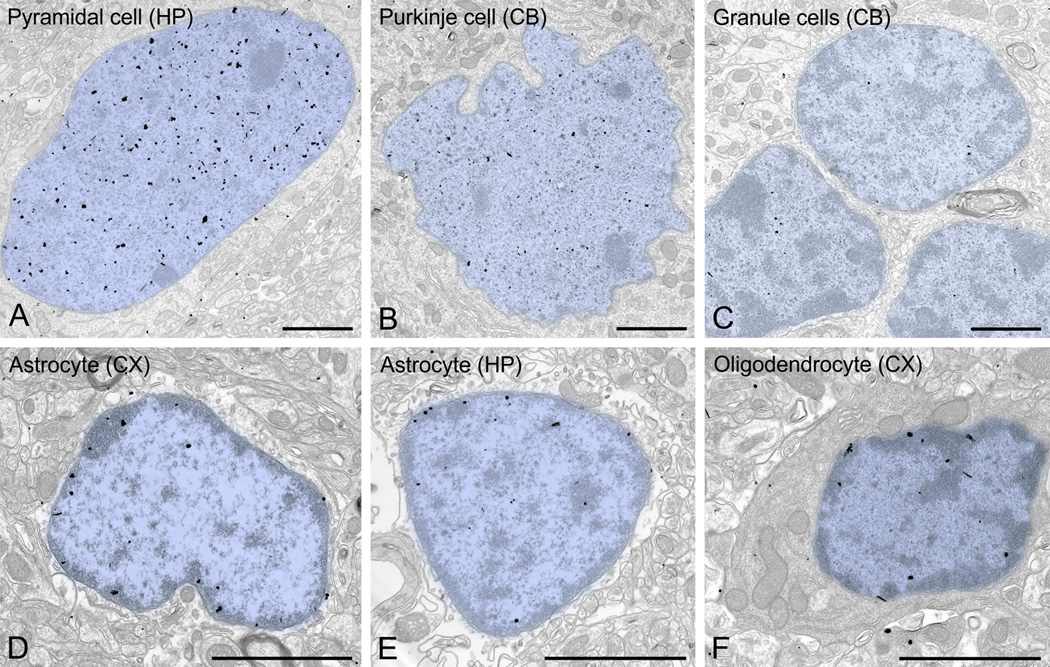
We examined the adult pattern of nuclear labeling in the cerebral cortex and hippocampus in more detail. While occasional gold/silver particles were associated with the nuclear membrane, the vast majority of UBE3A labeling was found over euchromatin domains (Figure 6 A–C). Very little labeling was found over electron-dense zones within the nucleus (interpreted as heterochromatin), or associated with nuclear organelles such as nucleoli. The same general pattern of labeling also applied to other brain regions (Figure 10 A–C). The distribution of UBE3A in glial nuclei differed sharply from that in neuronal nuclei (Figure 10 D–F). In the nuclei of astrocytes and oligodendrocytes, UBE3A hotspots concentrated in and around heterochromatin domains rather than in euchromatin domains, suggesting that nuclear UBE3A plays a different role in glia than in neurons.
Figure 10.
UBE3A in neuronal and glial nuclei. Label in neuronal nuclei (A, CA1 pyramidal neuron; B, Purkinje cell; C, cerebellar granule cells) is denser than in glial nuclei (D, astrocyte from cerebral cortex; E, astrocyte from CA1 hippocampus; F, oligodendrocyte from cerebral cortex), with distinct patterns of distribution. The neuronal label tends to avoid heterochromatin electron-dense zones, whereas label in glia tends to associate with heterochromatin. Scale bars = 2 µm.
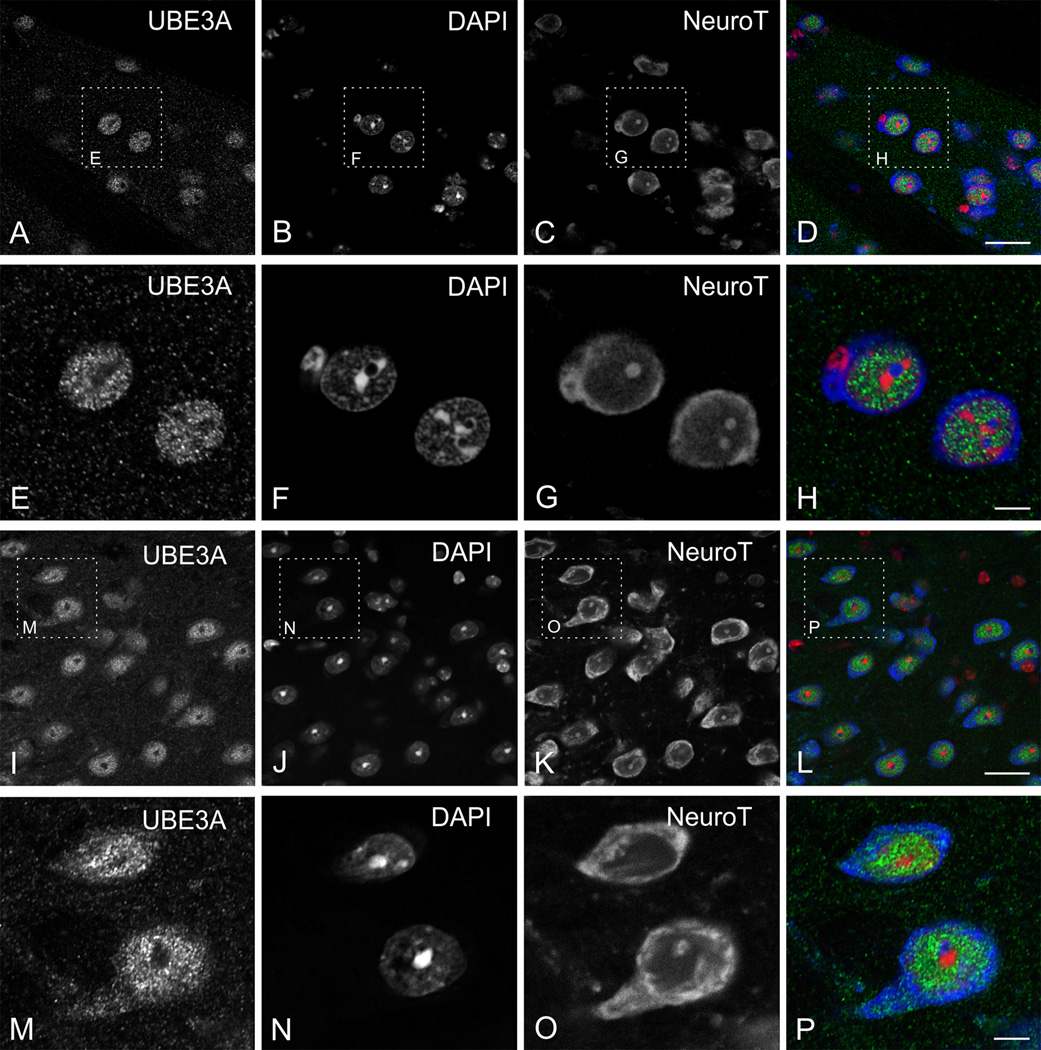
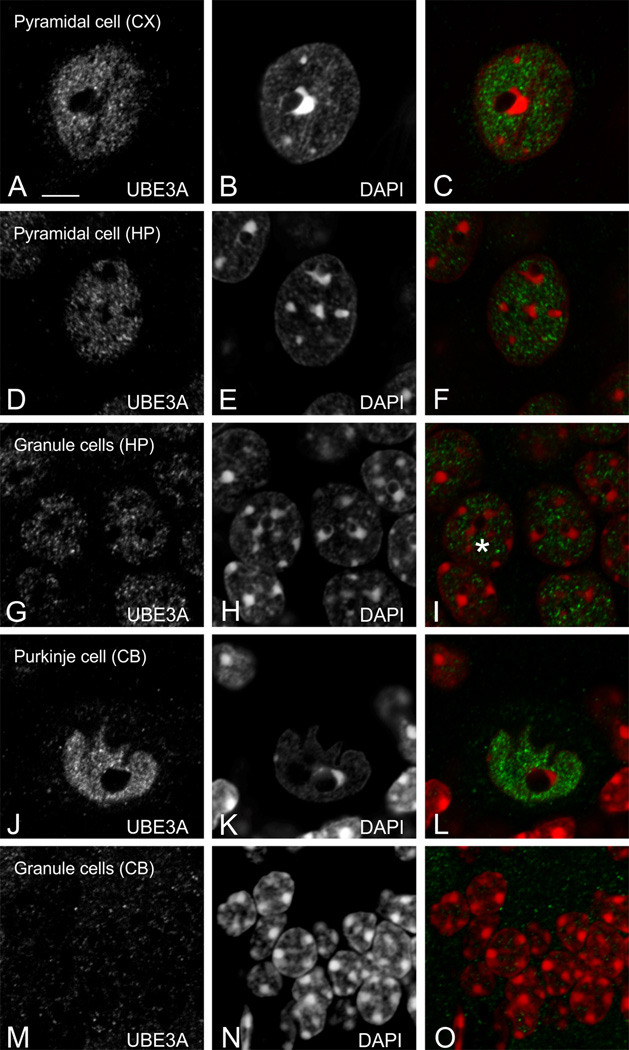
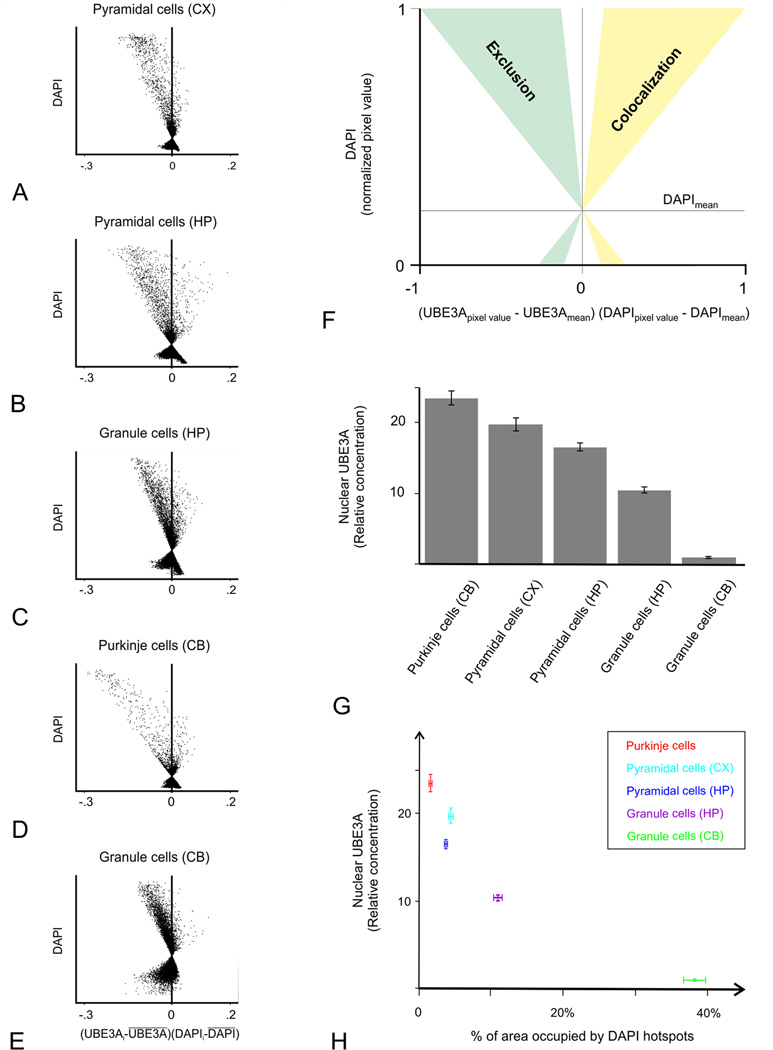
To test the relationship between UBE3A and DNA condensation more directly, we collected high-resolution images of UBE3A and DAPI double labeling (Figure 11), and performed quantitative analysis on these images. Intensity correlation quotients (ICQ, computed such that −0.5 represents perfect negative covariance, and 0.5 represents perfect positive covariance between two antigens) were consistently negative, and intensity correlation plots were systematically skewed toward negative values (Figure 12 A–F). Thus, intensity correlation analysis confirmed that UBE3A and DAPI staining negatively co-vary in multiple brain regions, implying that UBE3A is excluded from regions of condensed DNA. Further analysis showed that UBE3A is more concentrated in neuronal nuclei with less condensed DNA, as indicated by a paucity of DAPI hotspots (Figure 12 G, H). This was especially striking in the cerebellar cortex, where the concentration of UBE3A was more than 20 times higher in the nuclei of Purkinje cells than in the nuclei of cerebellar granule cells (Fig 12G).
Figure 11.
Reciprocal localization of UBE3A and DAPI in neuronal nuclei. UBE3A tends to avoid condensed DNA (defined by DAPI hotspots), as seen in cerebral cortex (A–C), CA1 pyramidal cells (D–F), granule cells of dentate gyrus (G–I), cerebellar Purkinje cells (J–L), and granule cells of the cerebellum (M–O). Scale bars = 5 µm.
Figure 12.
Quantification of the reciprocal localization of UBE3A and DAPI in neuronal nuclei. To analyze the relationship between UBE3A and DAPI in neuronal nuclei, we used the intensity correlation analysis approach of Li et al. (2004). Analyses are presented as intensity correlation plots: Each scatterplot corresponds to the nuclear region of the sections used for illustration in Figure 11. A corresponds to Fig. 11A–C, B to Fig. 11D–F, C to Fig. 11G–I, D to Fig. 11J–L, and E to Fig. 11M–O. (Scatterplots are from raw confocal images, while contrast and brightness were adjusted in the micrographs). The x-value, (UBE3Apixel value - UBE3Amean) × (DAPIpixel value - DAPImean), reflect the covariance of both channels, and the y-value reflects the intensity of DAPI. Pixels with values situated left of the x = 0 line do not colocalize or have inversely-correlated intensities, whereas pixels situated on the right side colocalize (F; for details, see the Materials and Methods). All plots are skewed toward negative values, implying that UBE3A and DAPI intensity covary in opposite directions. Analysis of the nuclear concentration of UBE3A shows an inverse relationship between the extent of DAPI hotspots and UBE3A concentration (G, H).
DISCUSSION
We find that UBE3A in the mouse brain has a broad subcellular distribution, perhaps reflecting diverse roles for this E3 ligase in the regulation of neuronal physiology. The subcellular distribution of UBE3A is not uniform; there are distinct neuronal compartments in which UBE3A preferentially concentrates, including euchromatin-rich domains within the nucleus and presynaptic vesicles within axon terminals. Outside of the nucleus, UBE3A appears to have a special affinity for membranes.
UBE3A appears to occupy the same neuronal compartments during early postnatal development and in adulthood, but relative to its level in the nucleus, UBE3A levels in the neuropil appear to decline with maturation. The factors dictating spatiotemporal patterns of UBE3A localization at the subcellular level remain to be elucidated, though current evidence suggests that amino-terminal sequence variation among UBE3A isoforms helps determine the protein’s subcellular localization (Miao et al., 2013). It is also possible that interacting proteins govern the distribution of UBE3A within cells, and the influence of either factor might change over the course of development.
UBE3A in the nucleus
The ubiquitin-proteasome system promotes protein quality control by degrading misfolded proteins in the nucleus (e.g. (Nielsen et al., 2014)). Nuclear proteasomes have been identified in euchromatin domains and at the periphery of heterochromatin clumps (Rivett et al., 1992). There is also evidence that proteasomes and ubiquitinated proteins accumulate in promyelocytic leukemia nuclear bodies (Fabunmi et al., 2001; Dino Rockel and von Mikecz, 2002). Many of the enzymatic components of the ubiquitin system have nuclear localization signals, and have been identified in the nucleus. Thus, it is not surprising that in addition to its role in degrading misfolded proteins, the ubiquitin-proteasome system has been implicated in regulating chromatin structure and in controlling the location and lifetime of specific transcriptional activators, co-activators, and repressors (Osley et al., 2006; Bader et al., 2007).
Despite this evidence, the function of nuclear UBE3A in neurons is unclear. We found UBE3A in discrete hotspots over euchromatin within neuronal nuclei, well-positioned to regulate the expression of active genes. Like other ubiquitin E3 ligases, UBE3A might modify the turnover of histone modification enzymes (Cao and Yan, 2012). Alternatively, UBE3A might affect the function of specific transcription factors. For example, UBE3A may act by ubiquitinating SOD1 (Superoxide dismutase 1), which can itself act as a nuclear transcription factor (Mishra et al., 2013; Tsang et al., 2014). SOD1 mutations have been linked to autism (Kovac et al., 2014), which in turn has been linked to maternal 15q11–13 copy number variation that may cause UBE3A overexpression, and to modified phosphorylation that can cause UBE3A hyperfunction (Cook et al., 1997; Christian et al., 2008; Glessner et al., 2009; Hogart et al., 2009; Hogart et al., 2010; Iossifov et al., 2014; Yi et al., 2015). UBE3A also exhibits transcriptional effects independent of its role as an E3 ligase (El Hokayem and Nawaz, 2014). For example, UBE3A is actively recruited to the pS2 promoter in response to estrogen (Reid et al., 2003), and serves as a transcriptional coactivator for steroid hormone receptors, irrespective of the functionality of its E3 catalytic domain. In contrast with neuronal UBE3A, we found that glial UBE3A was associated with heterochromatin, suggesting different functional roles for nuclear UBE3A in neurons and glia.
UBE3A in the Golgi apparatus and mitochondria
Loss of Ube3a is linked to alkalinization of the Golgi apparatus, associated cisternal swellings, and deficits in protein sialylation (Condon et al., 2013). Although we did not observe swollen or distended neuronal Golgi within either AS or Ube3a KO mice, we did observe UBE3A in close proximity to Golgi endomembranes, in a position to regulate the turnover of transmembrane proteins that maintain pH in the trans-Golgi network (Condon et al., 2013). The Na+/H+ exchanger isoforms NHE7 and NHE8 preferentially localize to the Golgi apparatus, and are key mediators of ion homeostasis within this endomembrane system. NHE8 overexpression enhances H+ secretion from the Golgi lumen, thereby increasing Golgi luminal pH and phenocopying the effect of Ube3a loss (Nakamura et al., 2005; Condon et al., 2013); NHE8 is thus a plausible candidate ubiquitin substrate of UBE3A, possibly accumulating to pathological levels in AS. Whether UBE3A directly regulates NHE8 or NHE7 is unknown, but supporting a possible link between UBE3A and Na+/H+ exchangers, we note that mutations of the related exchanger NHE6 result in endosomal-lysosomal dysfunction and Christianson syndrome, a disorder phenotypically similar to AS (e.g. (Stromme et al., 2011; Pescosolido et al., 2014).
Several recent studies implicate UBE3A in mitochondrial function. Hippocampal neurons of AS mice reportedly have abnormally small mitochondria with disorganized cristae (Su et al., 2011), though we did not observe such gross structural abnormalities in our study. Loss of Ube3a was also shown to associate with key enzymatic deficits in the mitochondrial respiratory chain (Su et al., 2011; Llewellyn et al., 2015), which may increase the production of reactive oxygen species. Accordingly, mitochondrial superoxide levels appear to be abnormally elevated in the hippocampal neurons of AS mice and may contribute to deficits in synaptic plasticity, learning, and memory (Santini et al., 2015). Our finding that UBE3A localizes to the outer membrane of neuronal mitochondria may provide further insight into the relationship between loss of UBE3A and mitochondrial dysfunction in AS.
UBE3A in synapses
UBE3A deficiency is associated with defects in synaptic plasticity; thus, its synaptic localization is of particular interest. We found UBE3A in the cytoplasm of dendritic spines at levels equivalent to that found in dendritic shafts. Labeling was rarely directly over PSDs, consistent with findings from biochemical fractionation studies (Gustin et al., 2010) and with the negative findings of several proteomic studies (Cheng et al., 2006; Collins et al., 2006; Bayes et al., 2012), and thus is unlikely to reflect an inaccessibility of PSDs to UBE3A antibodies. Despite a relatively low concentration in PSDs, UBE3A may nevertheless participate locally in postsynaptic regulation, perhaps by regulating the turnover of SAP97 and other synaptic proteins within the cytoplasm of dendritic spines (Matsumoto et al., 2006; Li et al., 2014).
We found UBE3A to be enriched in both nascent and mature axon terminals. Loss of UBE3A expression in axon terminals during early stages of postnatal development could potentially lead to wiring defects in AS model mice. Overexpression of the putative UBE3A substrate Ephexin 5 suppresses excitatory synapse formation in developing hippocampal neurons (Margolis et al., 2010), in general agreement with electrophysiological correlates of reduced excitatory synapse number in AS mice (Yashiro et al., 2009; Greer et al., 2010; Wallace et al., 2012). Although Ephexin 5 is expressed in axons and could presumably inhibit excitatory synapse formation via a presynaptic effect, this reduction has been ascribed to postsynaptic functions of Ephexin 5 (Margolis et al., 2010). Within axon terminals, UBE3A was specifically associated with synaptic vesicles. While no integral synaptic vesicle proteins (Takamori et al., 2006) have yet been identified as targets for UBE3A, UBE3A is thought to modulate the degradation of α-synuclein, a protein closely associated with synaptic vesicles (Maroteaux et al., 1988; Jensen et al., 1998; Mulherkar et al., 2009; Ham et al., 2013). The enrichment of UBE3A in axon terminal complements our previous finding that UBE3A deficiency causes a striking accumulation of clathrin-coated vesicles in axon terminals (Wallace et al., 2012) and points to its possible role in synaptic vesicle cycling.
Our results show that some neuronal UBE3A labeling remains in AS mice, perhaps reflecting the unusual imprinting mechanism (Landers et al., 2004; Le Meur et al., 2005). Rather than being uniformly distributed across the different neuronal compartments, UBE3A in AS mice concentrated in neuropil, especially in axon terminals, and was almost entirely absent from nuclei. This phenomenon points to an intriguing differential regulation of UBE3A in axon terminals and nuclei, perhaps related to the properties of different splice variants (Yamamoto et al., 1997).
CONCLUSION
The present evidence demonstrating UBE3A in multiple subcellular compartments offers intriguing clues to its functions. Its association with mitochondria supports previous suggestions that UBE3A helps to regulate oxidative metabolism, and its concentration in axon terminals is likely to be physiologically significant for the function of individual synapses. Conversely, its presence selectively in euchromatin-rich nuclear domains suggests that UBE3A may also mediate global effects on neuronal physiology, by regulating gene transcription. Our results should inform mechanistic interpretations of defects resulting from UBE3A loss or overexpression, and may guide studies of UBE3A–interacting proteins, including candidate substrates.
Acknowledgments
Grant support: This work was supported by grants awarded to B.D.P. from the Simons Foundation (SFARI Award 274426), Angelman Syndrome Foundation, NINDS (R01NS085093), and NIMH (R01MH093372), and to R.J.W. from NINDS (RO1NS039444). M.C.J. was supported by a NARSAD grant from the Brain & Behavior Research Foundation
Other acknowledgments.
Imaging was supported by the Hooker Imaging Core.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Experimental design: AB, MCJ, BDP, RJW. Mouse colony management: MCJ. Immunohistochemistry: AB, SB, KP. Data acquisition and analysis: AB. Drafting of the manuscript: AB. Critical revisions of the manuscript: AB, MCJ, BDP, RJW. Obtained funding: BDP, RJW.
Footnotes
Conflict of interest statement
Authors verify that they have no known or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within 3 years of beginning the submitted work that could inappropriately influence, or be perceived to influence, this work.
LITERATURE CITED
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: the network at work. Experimental cell research. 2009;315(9):1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Bader N, Jung T, Grune T. The proteasome and its role in nuclear protein maintenance. Experimental gerontology. 2007;42(9):864–870. doi: 10.1016/j.exger.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Bayes A, Collins MO, Croning MD, van de Lagemaat LN, Choudhary JS, Grant SG. Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins. PloS one. 2012;7(10):e46683. doi: 10.1371/journal.pone.0046683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer cell. 2008;14(1):10–21. doi: 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Cajigas IJ, Will T, Schuman EM. Protein homeostasis and synaptic plasticity. The EMBO journal. 2010;29(16):2746–2752. doi: 10.1038/emboj.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Yan Q. Histone ubiquitination and deubiquitination in transcription, DNA damage response, and cancer. Frontiers in oncology. 2012;2:26. doi: 10.3389/fonc.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Molecular & cellular proteomics : MCP. 2006;5(6):1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Christian SL, Brune CW, Sudi J, Kumar RA, Liu S, Karamohamed S, Badner JA, Matsui S, Conroy J, McQuaid D, Gergel J, Hatchwell E, Gilliam TC, Gershon ES, Nowak NJ, Dobyns WB, Cook EH., Jr Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biological psychiatry. 2008;63(12):1111–1117. doi: 10.1016/j.biopsych.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. Journal of neurochemistry. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Condon KH, Ho J, Robinson CG, Hanus C, Ehlers MD. The Angelman syndrome protein Ube3a/E6AP is required for Golgi acidification and surface protein sialylation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(9):3799–3814. doi: 10.1523/JNEUROSCI.1930-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, Leventhal BL, Courchesne R, Lincoln A, Shulman C, Lord C, Courchesne E. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. American journal of human genetics. 1997;60(4):928–934. [PMC free article] [PubMed] [Google Scholar]
- Dino Rockel T, von Mikecz A. Proteasome-dependent processing of nuclear proteins is correlated with their subnuclear localization. Journal of structural biology. 2002;140(1–3):189–199. doi: 10.1016/s1047-8477(02)00527-0. [DOI] [PubMed] [Google Scholar]
- El Hokayem J, Nawaz Z. E6AP in the brain: one protein, dual function, multiple diseases. Molecular neurobiology. 2014;49(2):827–839. doi: 10.1007/s12035-013-8563-y. [DOI] [PubMed] [Google Scholar]
- Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN. Interferon gamma regulates accumulation of the proteasome activator PA28 and immunoproteasomes at nuclear PML bodies. Journal of cell science. 2001;114(Pt 1):29–36. doi: 10.1242/jcs.114.1.29. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, Glaberson W, Flory J, Otieno F, Garris M, Soorya L, Klei L, Piven J, Meyer KJ, Anagnostou E, Sakurai T, Game RM, Rudd DS, Zurawiecki D, McDougle CJ, Davis LK, Miller J, Posey DJ, Michaels S, Kolevzon A, Silverman JM, Bernier R, Levy SE, Schultz RT, Dawson G, Owley T, McMahon WM, Wassink TH, Sweeney JA, Nurnberger JI, Coon H, Sutcliffe JS, Minshew NJ, Grant SF, Bucan M, Cook EH, Buxbaum JD, Devlin B, Schellenberg GD, Hakonarson H. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459(7246):569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo MS, Scudder SL, Patrick GN. Ubiquitin-dependent trafficking and turnover of ionotropic glutamate receptors. Frontiers in molecular neuroscience. 2015;8:60. doi: 10.3389/fnmol.2015.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin RM, Bichell TJ, Bubser M, Daily J, Filonova I, Mrelashvili D, Deutch AY, Colbran RJ, Weeber EJ, Haas KF. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiology of disease. 2010;39(3):283–291. doi: 10.1016/j.nbd.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham A, Kim DW, Kim KH, Lee SJ, Oh KB, Shin J, Mar W. Reynosin protects against neuronal toxicity in dopamine-induced SH-SY5Y cells and 6-hydroxydopamine-lesioned rats as models of Parkinson’s disease: Reciprocal up-regulation of E6-AP and down-regulation of alpha-synuclein. Brain research. 2013;1524:54–61. doi: 10.1016/j.brainres.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Hamilton AM, Zito K. Breaking it down: the ubiquitin proteasome system in neuronal morphogenesis. Neural plasticity. 2013;2013:196848. doi: 10.1155/2013/196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Zhao Y, Roy S, LeDoux MS, Reiter LT. Analysis of cerebellar function in Ube3a–deficient mice reveals novel genotype-specific behaviors. Human molecular genetics. 2008;17(14):2181–2189. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Progress in neurobiology. 2004;73(5):311–357. doi: 10.1016/j.pneurobio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hogart A, Leung KN, Wang NJ, Wu DJ, Driscoll J, Vallero RO, Schanen NC, LaSalle JM. Chromosome 15q11–13 duplication syndrome brain reveals epigenetic alterations in gene expression not predicted from copy number. Journal of medical genetics. 2009;46(2):86–93. doi: 10.1136/jmg.2008.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiology of disease. 2010;38(2):181–191. doi: 10.1016/j.nbd.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515(7526):216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. The Journal of biological chemistry. 1998;273(41):26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaud AL, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21(4):799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Pan Y, Zhu L, Landa L, Yoo J, Spencer C, Lorenzo I, Brilliant M, Noebels J, Beaud AL, et al. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PloS one. 2010;5(8):e12278. doi: 10.1371/journal.pone.0012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Sosa-Pagan JO, Del Cid WA, Han JE, Philpot BD. Allelic specificity of Ube3a expression in the mouse brain during postnatal development. The Journal of comparative neurology. 2014;522(8):1874–1896. doi: 10.1002/cne.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nature genetics. 1997;15(1):70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- Kovac J, Macedoni Luksic M, Trebusak Podkrajsek K, Klancar G, Battelino T. Rare single nucleotide polymorphisms in the regulatory regions of the superoxide dismutase genes in autism spectrum disorder. Autism research : official journal of the International Society for Autism Research. 2014;7(1):138–144. doi: 10.1002/aur.1345. [DOI] [PubMed] [Google Scholar]
- Landers M, Bancescu DL, Le Meur E, Rougeulle C, Glatt-Deeley H, Brannan C, Muscatelli F, Lalande M. Regulation of the large (approximately 1000 kb) imprinted murine Ube3a antisense transcript by alternative exons upstream of Snurf/Snrpn. Nucleic acids research. 2004;32(11):3480–3492. doi: 10.1093/nar/gkh670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur E, Watrin F, Landers M, Sturny R, Lalande M, Muscatelli F. Dynamic developmental regulation of the large non-coding RNA associated with the mouse 7C imprinted chromosomal region. Developmental biology. 2005;286(2):587–600. doi: 10.1016/j.ydbio.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Li J, Shi M, Ma Z, Zhao S, Euskirchen G, Ziskin J, Urban A, Hallmayer J, Snyder M. Integrated systems analysis reveals a molecular network underlying autism spectrum disorders. Molecular systems biology. 2014;10:774. doi: 10.15252/msb.20145487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(16):4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn KJ, Nalbandian A, Gomez A, Wei D, Walker N, Kimonis VE. Administration of CoQ10 analogue ameliorates dysfunction of the mitochondrial respiratory chain in a mouse model of Angelman syndrome. Neurobiology of disease. 2015;76:77–86. doi: 10.1016/j.nbd.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, Soskis MJ, Sahin M, Greenberg ME. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143(3):442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Sell GL, Zbinden MA, Bird LM. Angelman Syndrome. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2015;12(3):641–650. doi: 10.1007/s13311-015-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Nakagawa S, Yano T, Takizawa S, Nagasaka K, Nakagawa K, Minaguchi T, Wada O, Ooishi H, Matsumoto K, Yasugi T, Kanda T, Huibregtse JM, Taketani Y. Involvement of a cellular ubiquitin-protein ligase E6AP in the ubiquitin-mediated degradation of extensive substrates of high-risk human papillomavirus E6. Journal of medical virology. 2006;78(4):501–507. doi: 10.1002/jmv.20568. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaud AL, et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nature genetics. 1997;15(1):74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- Miao S, Chen R, Ye J, Tan GH, Li S, Zhang J, Jiang YH, Xiong ZQ. The Angelman syndrome protein Ube3a is required for polarized dendrite morphogenesis in pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(1):327–333. doi: 10.1523/JNEUROSCI.2509-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Maheshwari M, Chhangani D, Fujimori-Tonou N, Endo F, Joshi AP, Jana NR, Yamanaka K. E6-AP association promotes SOD1 aggresomes degradation and suppresses toxicity. Neurobiology of aging. 2013;34(4):1310, e1311–e1323. doi: 10.1016/j.neurobiolaging.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Miura K, Kishino T, Li E, Webber H, Dikkes P, Holmes GL, Wagstaff J. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiology of disease. 2002;9(2):149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- Mulherkar SA, Jana NR. Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiology of disease. 2010;40(3):586–592. doi: 10.1016/j.nbd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Mulherkar SA, Sharma J, Jana NR. The ubiquitin ligase E6-AP promotes degradation of alpha-synuclein. Journal of neurochemistry. 2009;110(6):1955–1964. doi: 10.1111/j.1471-4159.2009.06293.x. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. The Journal of biological chemistry. 2005;280(2):1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O’Malley BW. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Molecular and cellular biology. 1999;19(2):1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SV, Poulsen EG, Rebula CA, Hartmann-Petersen R. Protein quality control in the nucleus. Biomolecules. 2014;4(3):646–661. doi: 10.3390/biom4030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley MA, Fleming AB, Kao CF. Histone ubiquitylation and the regulation of transcription. Results and problems in cell differentiation. 2006;41:47–75. doi: 10.1007/400_006. [DOI] [PubMed] [Google Scholar]
- Pal P, Lochab S, Kanaujiya JK, Kapoor I, Sanyal S, Behre G, Trivedi AK. E3 ubiquitin ligase E6AP negatively regulates adipogenesis by downregulating proadipogenic factor C/EBPalpha. PloS one. 2013;8(6):e65330. doi: 10.1371/journal.pone.0065330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescosolido MF, Stein DM, Schmidt M, El Achkar CM, Sabbagh M, Rogg JM, Tantravahi U, McLean RL, Liu JS, Poduri A, Morrow EM. Genetic and phenotypic diversity of NHE6 mutations in Christianson syndrome. Annals of neurology. 2014;76(4):581–593. doi: 10.1002/ana.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Molecular cell. 2003;11(3):695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Rivett AJ, Palmer A, Knecht E. Electron microscopic localization of the multicatalytic proteinase complex in rat liver and in cultured cells. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1992;40(8):1165–1172. doi: 10.1177/40.8.1619280. [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Glatt H, Lalande M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nature genetics. 1997;17(1):14–15. doi: 10.1038/ng0997-14. [DOI] [PubMed] [Google Scholar]
- Santini E, Turner KL, Ramaraj AB, Murphy MP, Klann E, Kaphzan H. Mitochondrial Superoxide Contributes to Hippocampal Synaptic Dysfunction and Memory Deficits in Angelman Syndrome Model Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(49):16213–16220. doi: 10.1523/JNEUROSCI.2246-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Patrick GN. Ubiquitin-dependent endocytosis, trafficking and turnover of neuronal membrane proteins. Molecular and cellular neurosciences. 2012;49(3):387–393. doi: 10.1016/j.mcn.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme P, Dobrenis K, Sillitoe RV, Gulinello M, Ali NF, Davidson C, Micsenyi MC, Stephney G, Ellevog L, Klungland A, Walkley SU. X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction. Brain : a journal of neurology. 2011;134(Pt 11):3369–3383. doi: 10.1093/brain/awr250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Fan W, Coskun PE, Vesa J, Gold JA, Jiang YH, Potluri P, Procaccio V, Acab A, Weiss JH, Wallace DC, Kimonis VE. Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neuroscience letters. 2011;487(2):129–133. doi: 10.1016/j.neulet.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nature reviews Neuroscience. 2008;9(11):826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nature communications. 2014;5:3446. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nature neuroscience. 2007;10(3):280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Vu TH, Hoffman AR. Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain. Nature genetics. 1997;17(1):12–13. doi: 10.1038/ng0997-12. [DOI] [PubMed] [Google Scholar]
- Wallace ML, Burette AC, Weinberg RJ, Philpot BD. Maternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defects. Neuron. 2012;74(5):793–800. doi: 10.1016/j.neuron.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaud AL, Sweatt JD, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(7):2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Huibregtse JM, Howley PM. The human E6-AP gene (UBE3A) encodes three potential protein isoforms generated by differential splicing. Genomics. 1997;41(2):263–266. doi: 10.1006/geno.1997.4617. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nature neuroscience. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Berrios J, Newbern JM, Snider WD, Philpot BD, Hahn KM, Zylka MJ. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell. 2015;162(4):795–807. doi: 10.1016/j.cell.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]