Abstract
Both lipopolysaccharide (LPS) and interleukin (IL)-1β activate the MyD88-dependent signaling pathways to stimulate proinflammatory cytokine expression. However, it remains unknown how LPS and IL-1β interact with each other to coordinate the stimulation. In this study, we sought to investigate the interaction between LPS and IL-1β on MyD88-dependent signaling pathways in human gingival fibroblasts (HGFs). Results showed that LPS derived from Porphyromonas gingivalis (Pg LPS) and IL-1β cooperatively stimulated mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) signaling pathways, and subsequent expression of proinflammatory cytokine expression. Furthermore, our results showed that Pg LPS and IL-1β exerted a synergy on MyD88 expression and knockdown of MyD88 expression by small interfering RNA diminished the synergistic effect of Pg LPS and IL-1β on IL-6 expression, suggesting that upregulation of MyD88 is involved in the coordinated stimulation by Pg LPS and IL-1β of proinflammatory cytokine expression. Finally, our results showed that pharmacological inhibitors for MAPK and NFκB significantly reduced IL-6 secretion stimulated by Pg LPS and IL-1β, indicating that the MyD88-dependent MAPK and NFκB signaling pathways are essential for the upregulation of proinflammatory cytokine expression by Pg LPS and IL-1β. Taken together, this study showed that LPS and IL-1β coordinate a synergy on cytokine production by upregulating MyD88 expression in HGFs.
Keywords: Lipopolysaccharide, Interleukin-1 beta, Inflammation, MyD88
1. Introduction
Toll-like receptors (TLRs) and interleukin-1 receptor (IL-1R) share a conserved cytoplasmic domain of approximately 200 amino acids, known as the Toll/IL-1R (TIR) homology region that recruits cytoplasmic adaptor protein MyD88 [1]. The major function of MyD88 is to link TLRs or IL-1R to downstream IL-1R-associated kinases (IRAKs)-dependent activation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) pathways, and subsequent upregulation of proinflammatory cytokines [2, 3]. Lipopolysaccharide (LPS) and IL-1β activate MyD88-mediated signaling pathways via TLR4 and IL-1R, respectively, and play a key role in host innate immune response [3].
The phenomenon that exogenous bacteria-derived LPS and endogenous host cell-secreted IL-1β activate the same TIR/MyD88-mediated signaling is intriguing. It is conceivable that when infections with Gram-negative bacteria occur, bacteria-derived LPS initiates the activation of MyD88-dependent signaling via TLR4 to induce expression and secretion of proinflammatory cytokines including IL-1β. The secreted IL-1β in turn stimulates MyD88-dependent signaling again via IL-1R, leading to an increased and persistent release of proinflammatory cytokines. Since it has been reported that inflammatory cytokines such as IL-1β are much more potent than LPS in stimulating cytokine production [4], it is likely that IL-1β release in response to the initial LPS stimulation would amplify LPS-triggered MyD88-dependent signaling and subsequent proinflammatory cytokine production. Moreover, if both LPS and IL-1β are present simultaneously in inflamed tissue, they are likely to act in concert via TLR4 and IL-1R, respectively, on MyD88-dependent signaling, resulting in a robust secretion of proinflammatory cytokines.
Although it is believed that the coordination by LPS and IL-1β on the stimulation of MyD88-dependent signaling pathways leads to a powerful upregulation of host innate immune response, the mechanisms underlying the coordination are not well understood. In the current study, we sought to determine how LPS and IL-1β interact with each other to stimulate MyD88-dependent signaling pathways and subsequent expression of proinflammatory cytokines in human gingival fibroblasts.
2. Materials and Methods
2.1. Cell culture
Human gingival fibroblasts were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing 10% fetal calf serum, 1% MEM non-essential amino acid solution, and 0.6 g/100 ml of HEPES. The medium was changed every 2–3 days. Fibroblasts were grown to 80% confluence before treatments with IL-1β (Sigma-Aldrich Corp., St. Louis, MO) or/and LPS from Porphyromonas gingivalis (InvivoGen, San Diego, CA) (Pg).
2.2. Enzyme-linked immunosorbent assay (ELISA)
IL-6, IL-1β and GM-CSF in conditioned medium was quantified using sandwich ELISA kits according to the protocol provided by the manufacturer (R&D System, Minneapolis, MN).
2.3. Real-time polymerase chain reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen, Santa Clarita, CA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA) using 20 µl of reaction mixture containing 1 µg of total RNA, 4 µl of 5× iScript reaction mixture, and 1 µl of iScript reverse transcriptase. The complete reaction was cycled for 5 minutes at 25 °C, 30 minutes at 42 °C and 5 minutes at 85°C using a PTC-200 DNA Engine (MJ Research, Waltham, MA). The reverse transcription (RT) reaction mixture was then diluted 1:10 with nuclease-free water and used for PCR amplification of cDNA in the presence of the primers. The Beacon designer software (PREMIER Biosoft International, Palo Alto, CA) was used for primer designing (human MyD88: 5’ primer sequence, CGGATGGTGGTG GTTGTCTC; 3’ primer sequence, CGCTTCTGATGGGCACCT). Primers were synthesized (Integrated DNA Technologies, Inc., Coralville, IA) and real-time PCR was performed in duplicate using 25 µl of reaction mixture containing 10 µl of RT mixture, 0.2 µM of both primers, and 12.5 µl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad) with a twostep method. The hot-start enzyme was activated (95°C for 2 min) and cDNA was then amplified for 40 cycles consisting of denaturation at 95°C for 10 sec and annealing/extension at 52.5°C for 45 sec. A melt-curve assay was then performed (55°C for 1 min and then temperature was increased by 0.5°C every 10 sec) to detect the formation of primer-derived trimers and dimers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a control (5’ primer sequence, CTGAGTACGTCG TGGAGTC; 3’ primer sequence, AAATGAGCCCCAGCCTTC). Data were analyzed with the iCycler iQ™ software. The average starting quantity (SQ) of fluorescence units was used for analysis. Quantification was calculated using the SQ of targeted cDNA relative to that of GAPDH cDNA in the same sample.
2.4. PCR arrays
First-strand cDNA was synthesized from RNA using RTÇ First Strand Kit (SuperArray Bioscience Corp., Frederick, MD). Human TLR pathway-focused PCR arrays (SuperArray Bioscience Corp.) were performed using 2× SuperArray RTÇ qPCR master mix and the first strand cDNA by following the instruction from the manufacturer. Data analysis is based on the delta-delta threshold cycle (ΔΔCt) method as described in the instruction. Briefly, the ΔCt was calculated by subtracting Ct for GAPDH from Ct for genes of interest and the ΔΔCt was calculated by subtracting the ΔCt for control cells from ΔCt for treated cells. The fold change was calculated as 2−ΔΔCt.
2.5. Transfection and luciferase activity assay
Human gingival fibroblasts were transfected with 1 µg of NFκB Cignal Luciferase Reporter (Qiagen Inc., Valencia, CA) using FuGENE® HD as transfection reagent for 18 h. The renilla luciferase constructs were used as control. The cells were then treated with fresh medium containing Pg LPS, IL-1β or both for 24 h. After the treatment, the cells were rinsed with cold PBS and lysed with the buffer from Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Both firefly and renilla luciferase levels were measured in a luminometer according to the instruction from the manufacturer. The firefly luciferase levels were normalized to the renilla luciferase levels.
2.6. Immunoblotting of MyD88 and MAPK
For immunoblotting of MyD88, cell lysate containing 25–50 µg protein was electrophoresed in a 10% polyacrylamide gel. After transferring proteins to a PVDF membrane, MyD88 was immunoblotted with goat anti-human MyD88 primary antibody (R&D Systems) and horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA). MyD88 were visualized by incubating the membrane with chemiluminescence reagent (NEN Life Science Products) for 1 min and exposing it to x-ray film for 1–10 min. The X-ray films were scanned using an Epson scanner (Perfection 1200U). For mitogen-activaetd protein kinase (MAPK) immunoblotting, anti-total or anti-phosphorylated extracellular regulated kinase (ERK), c-Jun N-terminal kinases (JNK) or p38 MAPK primary antibodies and HRPconjugated secondary antibody were used. The density of bands on the images was quantified using Adobe Photoshop version 10.0.1. The results were presented as the ratios of phosphorylated ERK, JNK or p38 vs. total ERK, JNK or p38.
2.7. Treatment of cells with the inhibitors of signaling pathways
Human gingival fibroblasts were treated with Pg LPS, IL-1β or the combination of Pg LPS and IL-1β in the absence or presence of 5 or 10 µM of ERK pathway inhibitor PD98059, JNK pathway inhibitor SP600125, or p38 MAPK pathway inhibitor SB203580, or 2.5 or 5 µM of NFκB pathway inhibitor Bay117085 (Calbiochem/EMD Biosciences, Inc., San Diego, CA) for 24 h. After the treatment, IL-6 in medium was quantified using ELISA.
2.8. RNA interference
Human gingival fibroblasts were transiently transfected with 200 nM of MyD88 siRNA (Thermo-Fisher Scientific, Waltham, MA) or the scrambled control siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX) using Lipofectamine RNAi MAX reagent (Thermo-Fisher Scientific) for 24 h by following the manufacturer's instructions. After the transfection, fibroblasts were treated with Pg LPS, IL-1β or both Pg LPS and IL-1β for 24 h.
2.9. Statistic analysis
Data were presented as mean ± SD. Student t tests were performed to determine the statistical significance of gene expression or luciferase activity among different experimental groups. A value of P< 0.05 was considered significant.
3. Results
3.1. LPS and IL-1β synergistically stimulate expression of IL-1β, IL-6 and other inflammatory cytokines
In this study, we first employed a TLR pathway-focused PCR array to assess the effect of LPS, IL-1β or the combination of LPS and IL-1β on proinflammatory gene expression in human gingival fibroblasts. As shown in Table 1, Pg LPS alone stimulated expression of several cytokines and chemokines such as IL-6, chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte-chemotactic protein (MCP)-1, and IL-8, but had no significant effect on molecules involved in the signaling transduction and transcription factors. In contrast, IL-1β is a stronger stimulator as it stimulated not only cytokines and chemokines, but also molecules involved in the signal transduction and transcription such as interferon regulatory transcription factor (IRF)1, NFKB1, NFKB1A and prostaglandin-endoperoxide synthase (PTGS) 2, also known as cyclooxygenase-2 (COX-2). Strikingly, the combination of Pg LPS and IL-1β is much more potent than Pg LPS or IL-1β alone in the stimulation of the proinflammatory molecules. For example, while Pg LPS and IL-1β stimulated IL-6 expression by 2- and 74-fold, respectively, the combination of Pg LPS and IL-1β led to a 201-fold increase. These data revealed a strongly synergy between Pg LPS and IL-1β in the upregulation of proinflammatory gene expression.
Table 1.
Synergistic effect of Pg LPS and IL-1β on expression of gene expression
| Genes | Ct | ΔCt | Fold of control in cells treated with: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (Ctl) |
Pg LPS | IL-1β |
Pg LPS + IL-1β |
Pg LPS vs Ctl |
IL-1β vs Ctl |
(Pg LPS +IL-1) vs Ctl |
Pg LPS | IL-1β |
Pg LPS +IL-1β |
|
| I. Genes for cytokines and chemokines | ||||||||||
| IL6 | 30.76 | 29.71 | 24.55 | 23.10 | 1.04 | 6.21 | 7.65 | 2.1 | 74.0 | 201.4 |
| IL1B | 33.66 | 32.99 | 28.40 | 27.66 | 0.67 | 5.26 | 6.01 | 1.6 | 38.4 | 64.2 |
| CCL2 | 33.16 | 30.81 | 26.94 | 25.92 | 2.35 | 6.22 | 7.24 | 5.1 | 74.7 | 151.3 |
| CSF2 | 33.35 | 32.92 | 26.94 | 26.56 | 0.42 | 6.41 | 6.79 | 1.3 | 84.9 | 110.5 |
| CSF3 | 31.84 | 32.57 | 27.44 | 27.02 | -0.73 | 4.40 | 4.82 | 0.6 | 21.2 | 28.3 |
| IL8 | 32.45 | 28.78 | 22.03 | 21.46 | 3.67 | 10.42 | 10.99 | 12.7 | 1366.6 | 2032.3 |
| II. Genes for signaling molecules, transcription factors and receptor | ||||||||||
| MYD88 | 28.73 | 28.34 | 28.65 | 27.12 | 0.39 | 0.07 | 1.61 | 1.3 | 1.1 | 3.1 |
| IRF1 | 31.16 | 31.02 | 29.14 | 28.66 | 0.14 | 2.02 | 2.51 | 1.1 | 4.1 | 5.7 |
| NFKB1 | 31.05 | 30.70 | 28.41 | 27.75 | 0.36 | 2.65 | 3.30 | 1.3 | 6.3 | 9.8 |
| NFKBIA | 28.71 | 28.18 | 25.46 | 25.19 | 0.54 | 3.26 | 3.52 | 1.5 | 9.6 | 11.5 |
| PTGS2 | 31.13 | 31.57 | 28.39 | 28.05 | −0.44 | 2.74 | 3.08 | 0.7 | 6.7 | 8.5 |
| REL | 33.27 | 32.91 | 31.64 | 30.89 | 0.36 | 1.63 | 2.38 | 1.3 | 3.1 | 5.2 |
| RIPK2 | 29.71 | 29.50 | 28.21 | 27.23 | 0.21 | 1.50 | 2.48 | 1.2 | 2.8 | 5.6 |
| TBK1 | 29.53 | 29.48 | 29.31 | 27.85 | 0.05 | 0.22 | 1.68 | 1.0 | 1.2 | 3.2 |
| TLR1 | 36.92 | 36.34 | 34.51 | 33.51 | 0.58 | 2.41 | 3.41 | 1.5 | 5.3 | 10.6 |
| III. Housekeeping genes (control) | ||||||||||
| ACTB | 20.47 | 20.53 | 20.58 | 20.34 | 0.06 | 0.11 | −0.12 | 0.9 | 1.0 | 1.1 |
| GAPDH | 21.71 | 21.71 | 21.71 | 21.71 | 0 | 0 | 0 | 1.0 | 1.0 | 1.0 |
Human gingival fibroblasts were treated with or without 100 pg/ml of IL-1β, µg/ml of Pg LPS or both for 24 h. RNA was isolated from duplicate samples, combined and subjected to PCR array. Human toll-like receptor (TLR) signal pathway PCR array (Qiagen, Santa Clarita, CA) was used to profile gene expression according to the instructions from the manufacturer. RNA isolated from duplicate wells was combined and converted to cDNA and then amplified by PCR. Data analysis is based on the delta-delta threshold cycle (ΔΔCt) method: The ΔCt was calculated by subtracting Ct for GAPDH from Ct for genes of interest and the ΔΔCt was calculated by subtracting the ΔCt for control cells from ΔCt for treated cells. The fold change was calculated as 2−ΔΔCt.
Full names for the abbreviations: CCL2, chemokine (C-C motif) ligand 2; CSF2, colony stimulating factor 2; CSF3, colony stimulating factor 3; MyD88, Myeloid differentiation primary response 88; IRF1, interferon regulatory transcription factor; NFKB1, nuclear factor kappa B1; NFKB1A, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; PTGS2, prostaglandin-endoperoxide synthase 2; BEL, V-rel reticuloendotheliosis viral oncogene homolog (avian); RIPK2, receptor-interacting serine-threonine kinase 2; TBK1, TANK-Binding Kinase 1; TLR1, Toll-like receptor 1; ACTB, beta actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
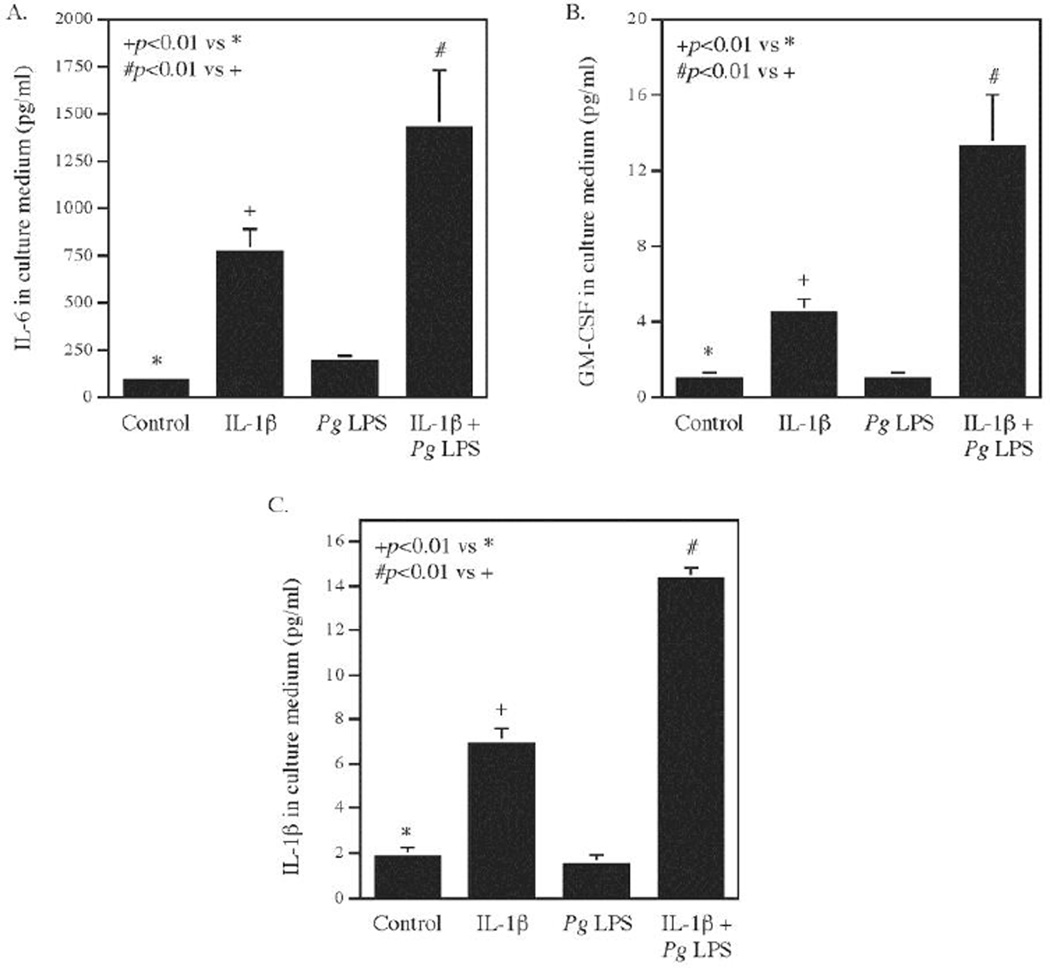
To confirm the findings from the PCR array shown in Table 1, we assessed the effect of the combination of Pg LPS and IL-1β on inflammatory cytokine secretion from gingival fibroblasts. Results showed that Pg LPS and IL-1β synergistically increased secretion of IL-6 secretion (Fig. 1A), GM-CSF, also known as CSF2 (Fig. 1B), and IL-1β (Fig. 1C). All these results are consistent with the findings from the PCR array as shown in Table 1. Since the expression of 84 genes was amplified at the same time and under the same temperature by the PCR array, the fold changes of gene expression may not be same as those by quantitative realtime PCR. However, both the data from the PCR array and real-time PCR showed a synergistic effect of LPS and IL-1β on the expression of proinflammatory cytokines, chemokines, signaling molecules and transcription factors.
Figure 1.
The synergistic effect of IL-1β and Pg LPS on proinflammatory cytokine expression. Human gingival fibroblasts were treated with 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 24 h and the secreted IL-6 (A), granulocyte-macrophage colony-stimulating factor (GM-CSF) (B) and IL-1β (C) in culture medium were quantified using ELISA. The data (mean ± SD) presented are from one of three independent experiments with similar results.
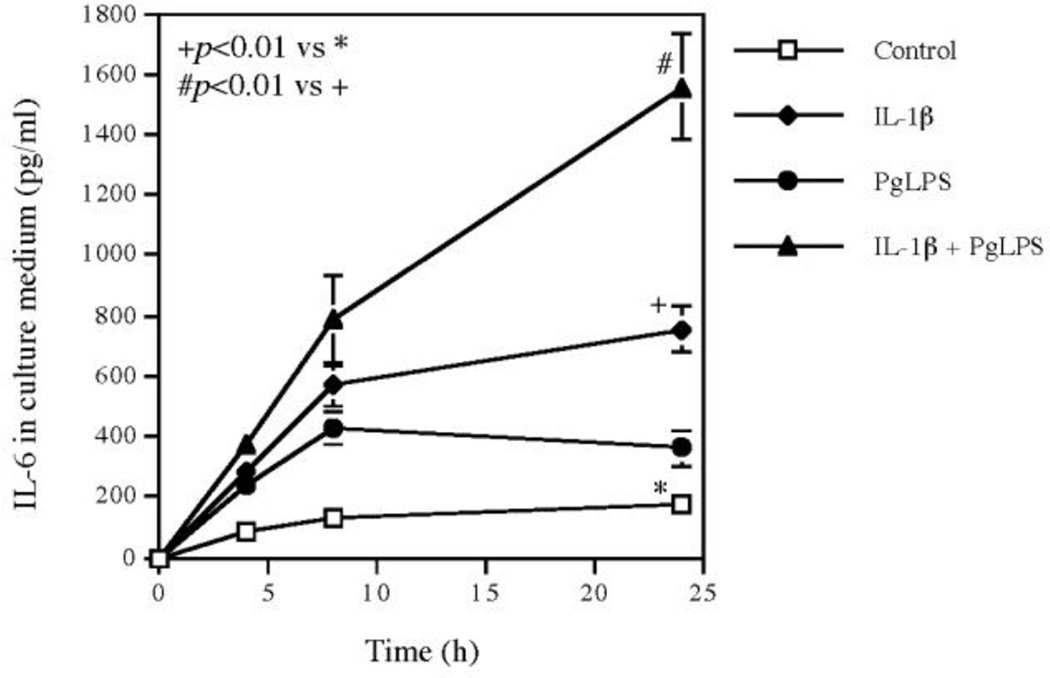
3.2. The kinetics of IL-6 secretion in response to Pg LPS, IL-1β or both Pg LPS and IL-1β
To further characterize the upregulation of cytokines by the combination of Pg LPS and IL-1β, we performed time course study on IL-6 secretion since IL-6 is known to be a key player in inflammation [5, 6]. Results showed that while Pg LPS and IL-1β induced IL-6 secretion by 2- and 6-fold, respectively, at 24 h, the combination of Pg LPS and IL-1β increased IL-6 secretion by 16-fold (Fig. 2). Interestingly, the kinetics of IL-6 secretion showed that the IL-6 secretion stimulated by Pg LPS or IL-1β was increased before 8 h and became either plateaued or decreased after 8 h. In contrast, the IL-6 secretion stimulated by the combination of Pg LPS and IL-1β continued to increase during 8–24 h and reached to a high level at 24 h (Fig. 2). The kinetics of IL-6 release clearly revealed the synergistic effect of Pg LPS and IL-1β on IL-6 secretion.
Figure 2.
Time course of IL-6 secretion from human gingival fibroblasts stimulated with IL-1β, Pg LPS or both. Human gingival fibroblasts were treated with 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 24 h and the secreted IL-6 in culture medium was quantified using ELISA at 4, 8, 24 h during the treatment. The data (mean ± SD) presented are from one of three independent experiments with similar results.
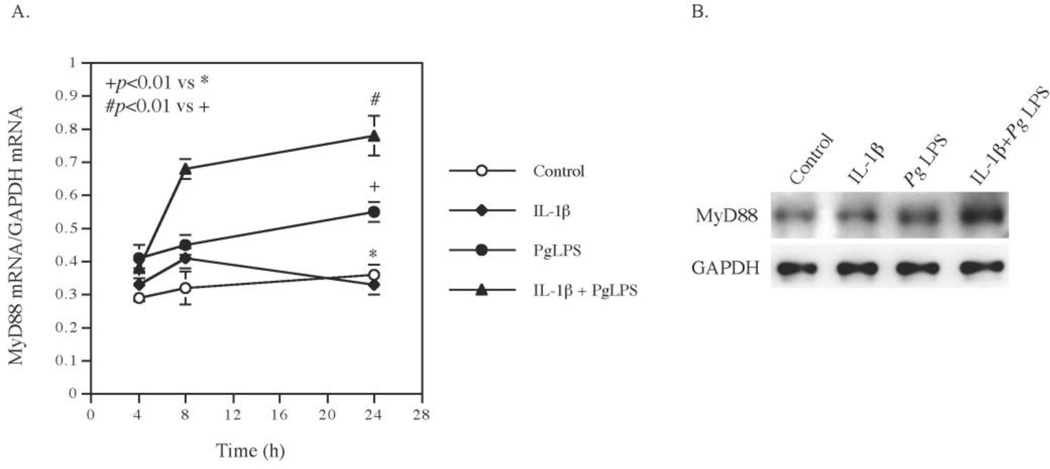
3.3. Pg LPS and IL-1β exert a synergy on MyD88 expression
The results from the PCR array study in Table 1 showed that while Pg LPS or IL-1β alone had no significant stimulation on MyD88 expression, the combination of Pg LPS and IL-1β increased MyD88 expression by 3-fold. To confirm the above results from the PCR array, we performed a time course study and quantified MyD88 mRNA using real-time PCR. Result showed that Pg LPS increased MyD88 mRNA expression by 50% and IL-1β had no effect at 24 h, but the combination of Pg LPS and IL-1β significantly increased MyD88 expression by 2.7-fold at 24 h (Fig. 3A), which is consistent with the results from the PCR array (Table 1). Interestingly, the time course study also showed that the combination of Pg LPS and IL-1β increased MyD88 expression as early as 8 h, which was before the persistent increase in IL-6 secretion during 8–24 h, suggesting the involvement of the MyD88 upregulation in the synergistic stimulation of IL-6 expression by Pg LPS and IL-1β. To further confirm the effect of Pg LPS and IL-1β expression, we performed Western blot to detect MyD88 protein level. Results showed that the combination of Pg LPS and IL-1β much potent than Pg LPS or IL-1β alone in stimulating MyD88 protein expression (Fig. 3B), which is in agreement with the upregulation of MyD88 mRNA expression (Fig. 3A).
Figure 3.
The synergistic effect of IL-1β and Pg LPS on MyD88 mRNA and protein expression. A. Human gingival fibroblasts were treated with 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 24 h and MyD88 mRNA was quantified at 4, 8, 24 h using real-time PCR during the treatment. The data (mean ± SD) presented are from one of three independent experiments with similar results. B. Human gingival fibroblasts were treated with 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 12 h and MyD88 was detected using immunoblotting.
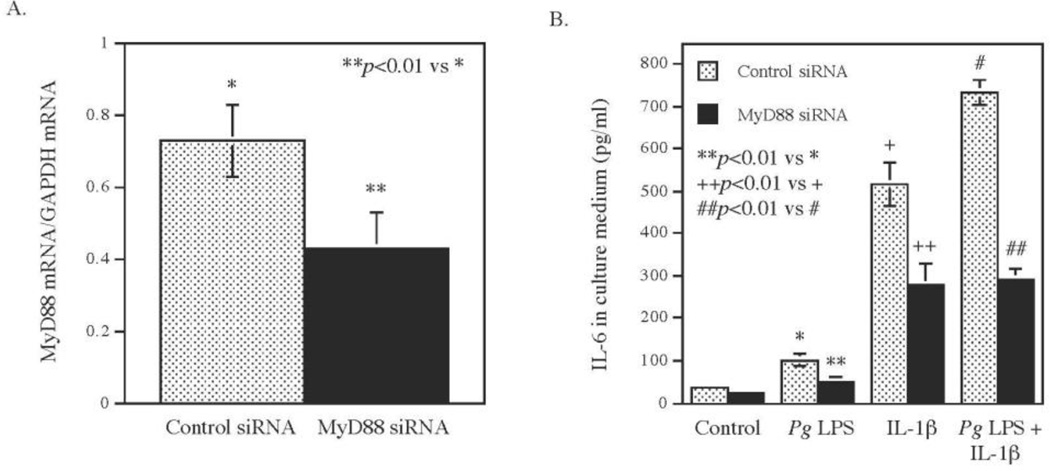
3.4. MyD88 upregulation is involved in the synergy of Pg LPS and IL-1β IL-6 expression
To further define the role of the MyD88 upregulation in the stimulation of IL-6 by Pg LPS and IL-1β, we employed small interfering RNA (siRNA) technique to knock down MyD88 mRNA expression in fibroblasts before treating cells with Pg LPS or/and IL-1β. Results showed that the transfection of fibroblast with MyD88 siRNA led to a 40% reduction of MyD88 mRNA (Fig. 4A). Quantification of IL-6 secreted into medium after treating cells with Pg LPS or/and IL-1β for 24 h showed that knockdown MyD88 reduced the effect of Pg LPS, IL-1β and the combination of IL-1β and Pg LPS on IL-6 secretion by 50%, 44% and 60%, respectively (Fig. 4B). Furthermore, results showed that in cells transfected with MyD88 siRNA, the amount of IL-6 secreted from cells stimulated with the combination of Pg LPS and IL-1β was same as that from cells stimulated with IL-1β alone (Fig. 4B), indicating that MyD88 mRNA knockdown abolished the synergistic effect of Pg LPS and IL-1β on IL-6 secretion. These findings support the role of MyD88 upregulation in the synergy of Pg LPS and IL-1β on IL-6 expression.
Figure 4.
The effect of MyD88 knockdown on IL-6 secretion induced by Pg LPS, IL-1β or both. Human gingival fibroblasts were transfected with 200 nM of MyD88 siRNA or scrambled siRNA (control siRNA) for 24 h. After the transfection, MyD88 knockdown by siRNA was confirmed using real-time PCR (A). The transfected cells were then treated with or without 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 24 h and IL-6 in culture medium was quantified using ELISA (B). The data (mean ± SD) presented are from one of three independent experiments with similar results.
3.5. Pg LPS and IL-1β cooperatively stimulate MAPK and NFκB signaling
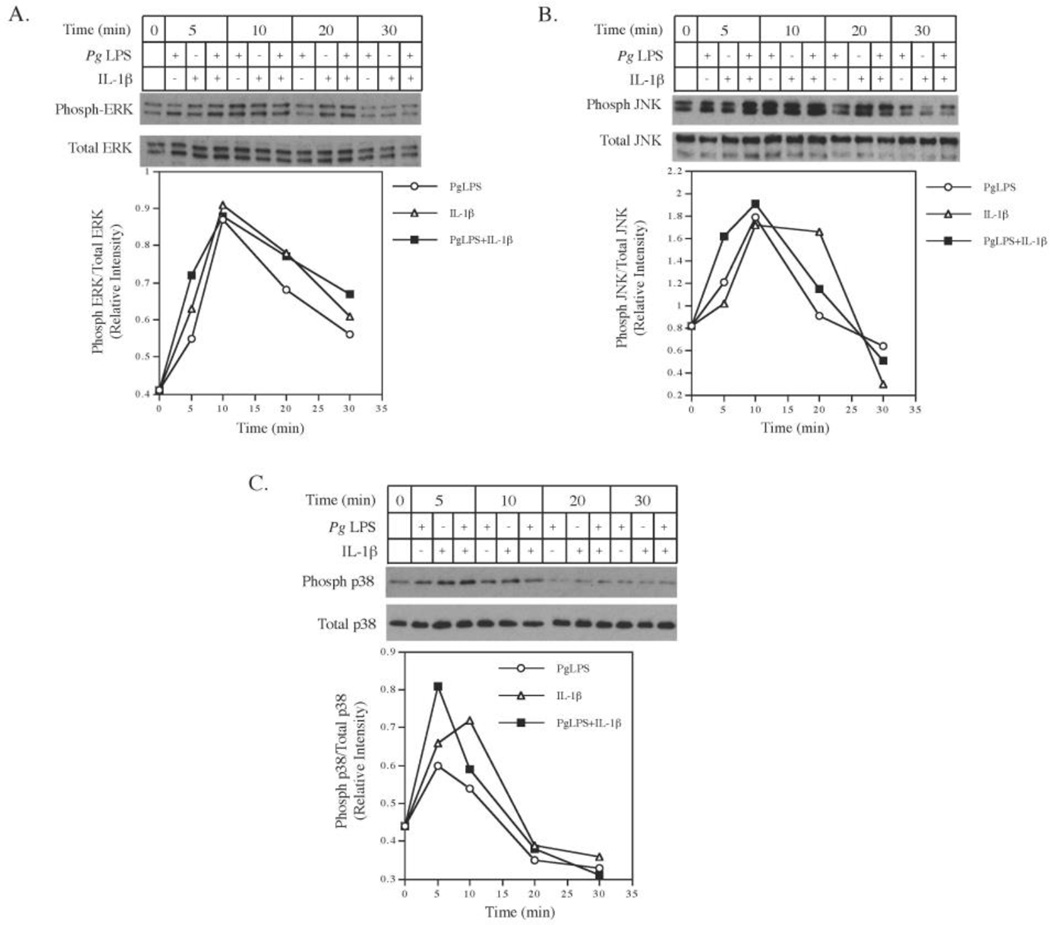
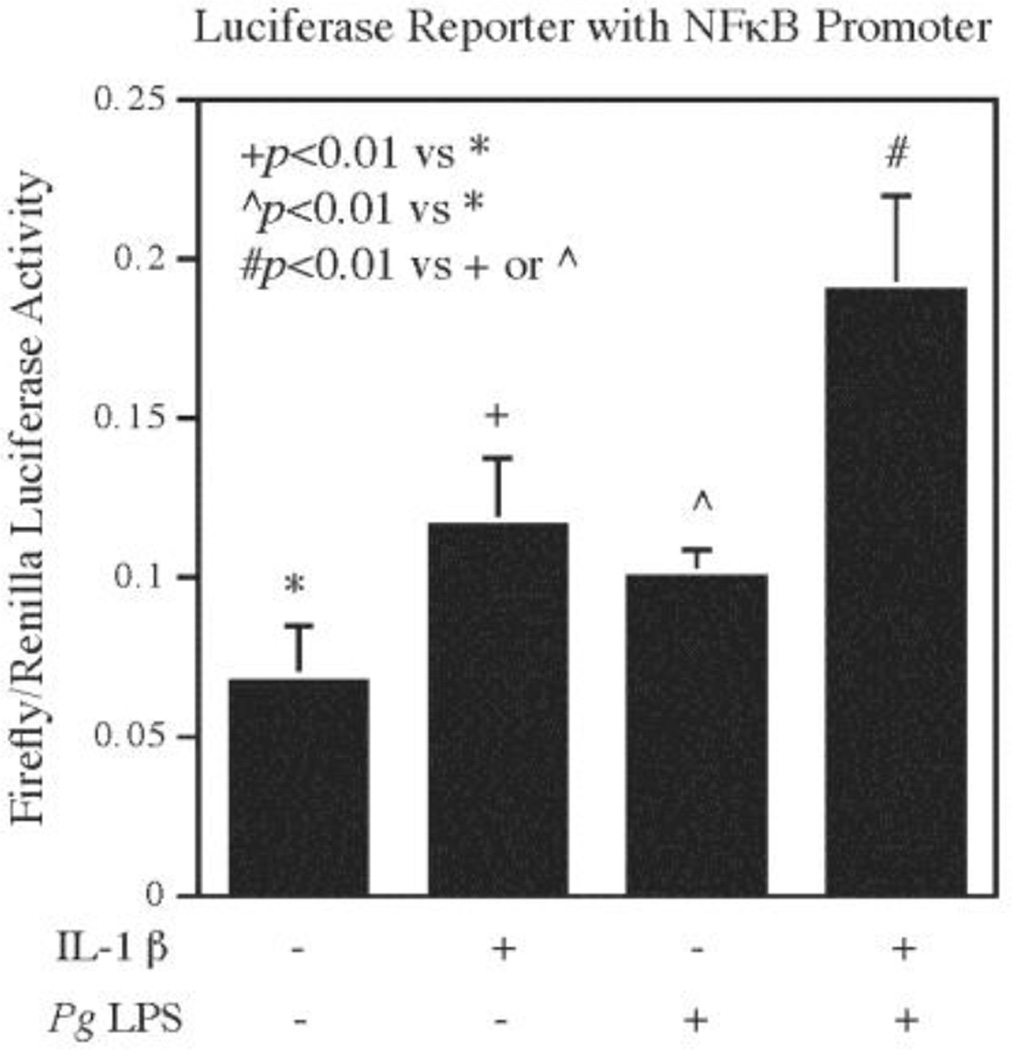
Since it is known that the MyD88 mediates the activation of MAPK and NFκB signaling [7, 8], we tested our hypothesis that Pg LPS and IL-1β cooperatively stimulate MAPK and NFκB activation. Results from our time course study showed that while Pg LPS or IL-1β alone stimulated phosphorylation of ERK (Fig. 5A), JNK (Fig. 5B), and p38 (Fig. 5C), the combination of Pg LPS and IL-1β had a cooperative effect on ERK (Fig. 5A), JNK (Fig. 5B), and p38 phosphorylation (Fig. 5C) at 5 min. In addition to the MAPK pathways, results from our luciferase activity assay showed that while Pg LPS or IL-1β alone stimulated NFκB activity, the combination of Pg LPS and IL-1β stimulated NFκB activity to a higher level (Fig. 6), indicating that Pg LPS and IL-1β also coordinate a stimulation of NFκB pathway.
Figure 5.
The cooperative effect of Pg LPS and IL-1β on phosphorylation of ERK, JNK and p38 MAPK. Human gingival fibroblasts were treated with or without 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 3, 10, 20, and 30 min. At each time point, cells were harvested for immunoblotting of phosphorylated and total ERK (A), JNK (B) and p38 MAPK (C). The intensity of phosphorylated ERK, JNK and p38 MAPK was quantified and normalized to that of total ERK, JNK and p38 MAPK, respectively. The data (mean ± SD) presented are from one of three independent experiments with similar results.
Figure 6.
The cooperative effect of Pg LPS and IL-1β on NFκB transcriptional activity. Human gingival fibroblasts were transfected with NFκB promoter-containing luciferase reporter vectors for 24 h and then treated with or without 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both for 24 h. After the treatment, cells were harvested for luciferase activity assays. The data are mean ± SD of three independent experiments.
3.6. NFκB and MAPK signaling pathways are involved in the synergy between Pg LPS and IL-1β on IL-6 expression
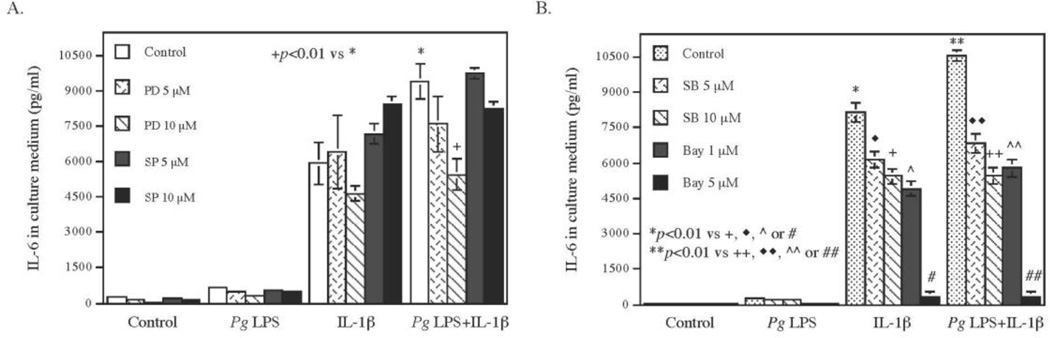
To determine if NFκB and MAPK pathways are involved in the synergy between Pg LPS and IL-1β on IL-6 expression, we employed pharmacological and specific inhibitors of the pathways. Results (Fig. 7) showed that the ERK pathway inhibitor PD98059, the p38 MAPK pathway inhibitor SB203580, and the NFκB pathway inhibitor Bay 11-7082 inhibited IL-6 secretion stimulated by Pg LPS, IL-1β or the combination of Pg LPS and IL-1β. In contrast, the JNK pathway inhibitor SP600125 failed to inhibit IL-6 secretion, suggesting that ERK, p38 MAPK and NFκB pathways, but not JNK cascade, are involved in the stimulation by Pg LPS and IL-1β of IL-6 expression.
Figure 7.
Inhibition by pharmacological inhibitors of IL-6 secretion stimulated by Pg LPS, IL-1β or both. Human gingival fibroblasts were treated with or without 100 pg/ml of IL-1β, 1 µg/ml of Pg LPS or both in the absence or presence of 5 or 10 µM of PD98059 (PD), inhibitor for ERK pathway (A); 5 or 10 µM of SP600126 (SP) (A), inhibitor for JNK pathway; 5 or 10 µM of SB203580 (SB) (B), inhibitor for p38 MAPK pathway; or 1 or 5 µM of Bay11-7082 (Bay) (B), inhibitor for NFκB pathway, for 24 h. After the treatment, IL-6 in culture medium was quantified using ELISA. The data (mean ± SD) presented are from one of three independent experiments with similar results.
4. Discussion
Both IL-1R and TLR belong to the IL-1R/TLR superfamily since they have the same intracellular amino acid sequence region called TIR domain that is crucial for activation of MyD88-dependent signaling pathways [8, 9]. Our current study demonstrated that TLR4 ligand LPS and IL-1R ligand IL-1β exerted a synergy on the upregulation of proinflammatory molecules by a coordinated activation of the MyD88-dependent signaling pathways in human gingival fibroblasts.
In this study, we focused on human gingival fibroblasts since gingival fibroblasts are important cells involved in gingival connective tissue inflammation and periodontitis [10]. In addition to generating structural connective tissue proteins such as collagen and elastin, gingival fibroblasts are also capable of producing large amount of immunoregulatory cytokines and chemical mediators in response to bacteria and bacterial products such as LPS [10]. Our previous study using a co-culture system has shown that IL-6 released from gingival fibroblasts stimulated by LPS upregulates tissue-degrading matrix metalloproteinase-1 expression in mononuclear cells [11], suggesting that the interaction between gingival fibroblasts and mononuclear cells plays a key role in the connective tissue degradation.
One interesting finding from our current study is that while IL-1β stimulated the release of itself from gingival fibroblasts, Pg LPS further increased IL-1β IL-1β secretion by 2-fold (Fig. 1C). The results from the PCR array suggest that the enhancement of IL-1β IL-1β secretion by Pg LPS resulted from the upreulation of IL-1β mRNA expression (Table 1). Consequently, the increased secretion of IL-1β by the combination of IL-1β and Pg LPS further stimulates IL-1R-mediated activation of MyD88-dependent signaling pathways, leading to the release of more proinflammatory cytokines including IL-1β and hence an escalated host inflammatory response.
Another interesting finding from our current study is that Pg LPS and IL-1β synergistically stimulate MyD88 expression. The results from our PCR array study (Table 1) showed that Pg LPS or IL-1β alone did not stimulate MyD88, but the combination of Pg LPS and IL-1β led to a 3-fold increase in MyD88 expression. Our time course study in which MyD88 mRNA was quantified using quantitative real-time PCR (Fig. 3) confirmed the finding from the PCR array. Given that MyD88 is a critical adaptor protein bridging TLR or IL-1R to IRAKs and subsequent NFκB and MAPK signaling pathways [2, 12], it is conceivable that the upregulation of MyD88 expression contributes to the synergistic effect of Pg LPS and IL-1β on proinflammatory gene expression. Indeed, the involvement of MyD88 in the upregulation of proinflammatory cytokine expression by Pg LPS and IL-1β was confirmed by our study in which fibroblasts were transfected with MyD88 siRNA to reduce MyD88 expression before stimulation with Pg LPS or/and IL-1β. Results showed that MyD88 knockdown abolished the synergy by Pg LPS and IL-1β on IL-6 secretion.
It has been reported previously that MyD88 expression can be upregulated beyond its basal level by proinflammatory mediators, including IL-6 [13], phorbo ester PMA [14], LPS and interferon [15]. Our current study showed for the first time that Pg LPS and IL-1β had a synergistic stimulation on MyD88 expression in gingival fibroblasts, suggesting that MyD88 expression can be coordinately upregulated by two mediators. Since MyD88 mediates MAPK and NFκB pathways, it is expected that MyD88 upregulation is associated an increased MAPK and NFκB signaling. Consistently, our current study showed that Pg LPS and IL-1β had cooperative effect on ERK, JNK and p38 MAPK pathways, and NFκB cascade (Figs. 5 and 6). Furthermore, the involvement of MAPK and NFκB pathway in the upregulation of proinflammatory cytokine IL-6 was demonstrated by the studies using pharmacological inhibitors (Fig. 7).
Periodontal disease is a bacterial infection of the supporting structures of the teeth, characterized by tissue inflammation and destruction that eventually lead to tooth loss [16, 17]. Among bacteria involved in periodontal disease, Pg along with Treponema denticola and Tannerella forsythia in the red bacterial complex has been strongly related to advanced periodontal lesions [18]. Therefore, we employed Pg LPS in the current study. Although animal studies have demonstrated that Pg LPS is capable of inducing periodontitis in animal models [19, 20], it was found to be much less potent for inflammatory cytokine expression in vitro than LPS derived from other oral bacteria such as Aggregatibacter actinomycetemcomitans [21]. Later, Darveau and coworkers reported that Pg contains LPS with heterogeneous lipid A that renders LPS different capacities of being agonistic, inert or antagonistic to TLR4 activation [22, 23], which is likely to contribute to the low potency of Pg LPS in the stimulation of proinflammatory gene expression. Interestingly, our current study showed that although Pg LPS alone was not a potent stimulator for the expression and secretion of cytokines such as IL-6, GM-CSF and IL-1β, it remarkably enhanced IL-1β-stimulated cytokine secretion (Table 1 and Fig. 1). Given that inflammatory cytokines such as IL-1β play a crucial role in periodontal tissue inflammation and alveolar bone loss during periodontitis [24–26], our current study suggests that Pg LPS may contribute to periodontitis by exerting its synergistic effect with proinflammatory cytokines such as IL-1β on the expression of proinflammatory mediators.
In conclusion, our current study investigated the interaction between Pg LPS and IL-1β in the upregulation of inflammatory cytokines in human gingival fibroblasts. We demonstrated that Pg LPS and IL-1β coordinated a synergy on the expression of inflammatory molecules by upregulating MyD88 expression, revealing a novel mechanism that may be potentially involved in the pathogenesis of chronic periodontitis.
Highlights.
LPS derived from Porphyromonas gingivalis (Pg LPS) and IL-1β synergistically upregulate proinflammatory cytokines in human gingival fibroblasts.
Pg LPS and IL-1β cooperatively stimulate MyD88 expression, which is involved in the upregulation by Pg LPS and IL-1β of cytokine secretion.
Pg LPS and IL-1β cooperatively stimulate MyD88-dependent MAPK and NFκB signaling pathways.
Acknowledgments
This work was supported by a Merit Review grant from the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs and NIH grant R01 DE016353 (to Y.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
Yan Huang designed the study. Colleen W. Brinson, Zhongyang Lu and Yanchun Li performed the experiments. Colleen W. Brinson, Zhongyang Lu, Yanchun Li, and Yan Huang analyzed data. Yan Huang and Maria F. Lopes-Virella wrote the manuscript. Yan Huang provided the financial support.
Disclosures
The authors declare that they have no conflict of interests.
REFERENCES
- 1.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science (New York, N.Y.) 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 2.Warner N, Nunez G. MyD88: a critical adaptor protein in innate immunity signal transduction. J Immunol. 2013;190:3–4. doi: 10.4049/jimmunol.1203103. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B. LPS in microbial pathogenesis: promise and fulfilment. J Endotoxin Res. 2002;8:329–335. doi: 10.1179/096805102125000650. [DOI] [PubMed] [Google Scholar]
- 4.Kent LW, Rahemtulla F, Hockett RD, Jr, Gilleland RC, Michalek SM. Effect of lipopolysaccharide and inflammatory cytokines on interleukin-6 production by healthy human gingival fibroblasts. Infect Immun. 1998;66:608–614. doi: 10.1128/iai.66.2.608-614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang PL, Ohura K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors. Crit Rev Oral Biol Med. 2002;13:132–142. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- 11.Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J Biol Chem. 2009;284:13714–13724. doi: 10.1074/jbc.M806573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deguine J, Barton GM. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5:1095–1097. [PubMed] [Google Scholar]
- 14.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 15.Sareneva T, Julkunen I, Matikainen S. IFN-alpha and IL-12 induce IL-18 receptor gene expression in human NK and T cells. J Immunol. 2000;165:1933–1938. doi: 10.4049/jimmunol.165.4.1933. [DOI] [PubMed] [Google Scholar]
- 16.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 17.Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 18.Bodet C, Chandad F, Grenier D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathol Biol (Paris) 2007;55:154–162. doi: 10.1016/j.patbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Wang PL, Oido-Mori M, Fujii T, Kowashi Y, Kikuchi M, Suetsugu Y, Tanaka J, Azuma Y, Shinohara M, Ohura K. Effect of anti-CD14 antibody on experimental periodontitis induced by Porphyromonas gingivalis lipopolysaccharide. Jpn J Pharmacol. 2002;89:176–183. doi: 10.1254/jjp.89.176. [DOI] [PubMed] [Google Scholar]
- 20.Kador PF, O'Meara JD, Blessing K, Marx DB, Reinhardt RA. Efficacy of structurally diverse aldose reductase inhibitors on experimental periodontitis in rats. J Periodontol. 2011;82:926–933. doi: 10.1902/jop.2010.100442. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodontal Res. 1997;32:279–286. doi: 10.1111/j.1600-0765.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 22.Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontol 2000. 2010;54:53–70. doi: 10.1111/j.1600-0757.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javed F, Al-Hezaimi K, Salameh Z, Almas K, Romanos GE. Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine. 2011;53:8–12. doi: 10.1016/j.cyto.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 26.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]