Abstract
Recombinant adeno-associated viral vectors (rAAV) are regarded as promising vehicles for therapeutic gene delivery. Continued development and new strategies are essential to improve the potency of AAV vectors and reduce the effective dose needed for clinical efficacy. In this regard, many studies have focused on understanding the cellular transduction mechanisms of rAAV, often with the goal of exploiting this knowledge to increase gene transfer efficiency. Here, we provide an overview of our evolving understanding of rAAV cellular trafficking pathways through the host cell, beginning with cellular entry and ending with transcription of the vector genome. Strategies to exploit this information for improving rAAV transduction are discussed.
Introduction
Gene therapy broadly describes strategies in which genetic material is introduced into a target cell in an effort to treat or cure disease. Of the approaches that are currently being explored, recombinant adeno-associated viral vectors (rAAV) have emerged as one of the most promising candidates. Adeno-associated virus (AAV) is a member of the parvoviridae family that was initially discovered as a contaminant in simian adenovirus preparations (1). A small, icosahedral non-enveloped virus ~25 nm in diameter that contains a single-stranded DNA genome (2), AAV is distinct from other members of the parvoviridae family due to its inability to replicate without the assistance of a helper virus, such as Adenovirus (Ad), Herpes simplex virus (HSV), human papilloma virus (HPV), or vaccinia virus (3). Additionally, AAV can confer long term gene expression, has a range of serotypes that collectively have a range of tissue tropism, and can package any transgenes flanked by AAV inverted terminal repeats (ITRs) and with the total genome size not exceeding ~5 kb (4). All of these properties make AAV an excellent candidate for therapeutic gene delivery.
It is well known that eukaryotic mechanisms to sort and degrade internalized cargo are exploited by several viruses and pathogens for infecting and replicating within host cells. At the same time, several cellular factors act to restrict viral infection within the host. A similar dichotomy is apparent in case of rAAV, with host factors demonstrating the ability to aid or limit transduction efficiency. For instance, one study estimates that following cellular uptake, only ~30% of AAV particles will successfully reach the nucleus (5). Here, we review the cellular transduction mechanisms of rAAV vectors by breaking down the different steps leading to transgene expression. We also discuss strategies to overcome host restriction factors that act as barriers that limit the potential of rAAV-mediated gene therapy in the clinic.
Cellular Uptake of rAAV
The first step in AAV infection requires binding to cell surface glycan receptors(6). This key step mediates cell surface attachment of virions and triggers subsequent cellular internalization and trafficking leading to transduction. Cellular uptake of AAV particles into endocytic vesicles is thought to be mediated by integrins and/or different transmembrane receptors. However, our understanding of AAV receptor usage continues to evolve. Although it is currently unclear how different AAV serotypes exploit specific receptors for cellular uptake, it is well known that mammalian cells are known to internalize extracellular material by numerous endocytic pathways, several of which have been implicated in uptake of rAAV. The first studies to investigate AAV uptake suggested that internalization of rAAV occurred via clathrin-mediated endocytosis. In these studies, transduction of AAV2 was inhibited by expression of a dominant-negative mutant of dynamin, a protein necessary for successful clathrin-mediated endocytosis (7, 8). Additionally, internalized AAV2 colocalized with transferrin, a protein known to be internalized by this mechanism. It is also worth noting that transcytosis of rAAVs has been shown to occur in polarized cells in a serotype-dependent manner, and it has been suggested that this phenomenon is dependent upon caveolin (9).
A more recent study suggests that uptake of AAV2 is dependent on the clathrin-independent carriers and GPI-enriched endocytic compartment (CLIC/GEEC) endocytic pathway (10). This study demonstrated that AAV2 uptake was inhibited by dominant negative versions of Arf1, Cdc42, and GRAF1, three important effectors of the of the CLIC/GEEC pathway. In addition, AAV2 colocalized with cholera toxin B and GPI-anchored GFP, two markers of CLIC vesicles, after internalization. In addition, this study identified EIPA as an inhibitor of CLIC/GEEC endocytosis. However, it is worth noting that EIPA is classically known as an inhibitor of macropinocytosis. Consistently, other studies have suggested a role for macropinocytosis in rAAV uptake. One such study demonstrated that inhibition of Rac1 activation, a key effector of macropinocytosis, inhibits AAV internalization (11). Another study used multiple small molecule inhibitors of macropinocytosis, including EIPA, to demonstrate that inhibition of macropinocytosis decreased transduction in some cell types, while demonstrating enhanced transduction in others (12).
Although diverse endocytic mechanisms have been implicated in AAV cell entry, it is evident that certain uptake pathways lead to successful transduction, while other pathways in the same cells lead to a “dead end” for AAV (10, 11). Further, it is likely that such mechanisms are altered in a cell-type dependent manner (12).
Post-Entry Trafficking of rAAV
After rAAV enters the cell, it must traffic towards the nucleus in order to successfully deliver its genetic cargo. Immediately after uptake, AAV is presumably trafficked to Rab5+ early endosomal compartment, which is a feature conserved amongst many parvoviruses (13). From here, rAAV traffics through a number of different compartments. Studies have demonstrated that rAAV2 traffics through both Rab7+ late endosomes and Rab11+ recycling endosomes (14). As is the case with AAV cell entry, intracellular trafficking pathways likely differ in a cell line-dependent and serotype-dependent fashion. For instance, while rAAV9 was localized to Rab5+, Rab7+, and Rab11+ vesicles in neurons in cell culture, this serotype was shown to only traffic effectively along axons in Rab7+ endosomes (15).
Vesicle-entrapped rAAV particles have been shown by numerous studies to traffic to the Golgi apparatus (16–18). One of the steps known to be required for efficient trafficking is endosome acidification, as the vacuolar H+-ATPase inhibitor bafilomycin A1 effectively blocks transduction (8, 19). Additionally, studies utilizing the small molecules brefeldin A and golgicide A, known to disrupt the Golgi apparatus, have shown that trafficking of rAAV through the Golgi apparatus is also required (19, 20). Recently, we demonstrated that inhibition of endoplasmic reticulum-associated degradation (ERAD) by eeyarestatin I (EerI) in HeLa cells reroutes rAAV to enlarged Lamp1+ lysosomes. This approach increased transduction, indicating that trafficking of rAAV through LAMP1+ vesicles may be an important step in infection (21). It is important to note that, as of now, no studies have observed rAAV localization within the endoplasmic reticulum (ER) or implicated a role for rAAV trafficking through the ER prior to nuclear entry.
At the molecular level, a recent study showed that siRNA-mediated knockdown of syntaxin 5 (STX5), as well as disruption of STX5 by the small molecule Retro2.1, reduced rAAV transduction, suggesting that retrograde transport of rAAV to the trans-Golgi network (TGN) mediated by syntaxin 5 is important for transduction (20). Another recent study utilized a screen based on haploid cells and identified KIAA0319L as a cellular factor required for infection of cells by multiple AAV serotypes, which they termed AAV receptor (AAVR) (22). CRISPR-based technology was utilized to knockout AAVR both in vitro and in vivo to demonstrate that AAV infection is severely inhibited in the absence of AAVR, and can be rescued by subsequent complementation by expression of ectopic AAVR. Interestingly, AAVR largely localizes to the Golgi, and contains a signal in the C-terminus that results in dynamic recycling of AAVR from the cellular surface to the TGN. While the precise mechanisms underlying AAVR-mediated AAV transduction remain to be understood, the data demonstrates that AAVR is an essential cellular factor that mediates AAV transport through the endomembrane system to the TGN for successful infection.
After trafficking of rAAV through the endomembrane system, rAAV escapes the endosome into the cytosol. Endosomal escape is dependent on a phospholipase A2 (PLA2) domain located in the VP1 unique region of AAV (23, 24). Multiple studies have shown that mutation or deletion of the PLA2 domain prevents endosomal escape and subsequent transduction (25). Thus, an absolutely essential outcome of these trafficking steps is the triggering of conformational changes in the capsid (26) leading to exposure of the N-terminal domains of VP1 and VP2, which are buried inside the capsid prior to infection (27). Exposure of these domains for successful transduction is required, as it has been demonstrated that microinjection of both complete virions, as well as VP3-only virions, directly into the cytosol do not properly transduce the cell (28). It has been suggested that the AAV capsid has protease activity that is pH-dependent, which could possibly be triggered by the acidification of the endosome (29). However, it has yet to be determined if the self-cleavage events mediated by this activity also play a role in the exposure of the VP1/VP2 N-termini, or in other events related to transduction.
Nuclear Import of rAAV
After escaping the endosome into the cytosol, rAAV then must enter the nucleus, where it will undergo uncoating and deliver its genetic cargo. Nuclear entry of AAV has been proposed as a major rate-limiting step in the infectious pathway (8). Four basic regions (BR1-4) were identified on AAV2 that were conserved among serotypes 1–11, that were investigated for their potential function as nuclear localization signals (NLS). BR4 is located within VP3 and was shown to have no impact on nuclear import of rAAV virions, but mutation of BR4 did result in virion assembly defects (outside the focus of this review) (30). However, BR3, located within both VP1 and VP2, is essential for AAV transduction (28). In addition, BR1 and BR2, to a lesser extent, are also important for AAV transduction. However, confocal microscopy studies of BR-negative mutants demonstrated that BR2 and BR3 are important for nuclear translocation (17).
A recent study demonstrated that rAAV2 enters the nucleus through the nuclear pore complex (NPC) by blocking nuclear entry of rAAV2 with wheat germ agglutinin, a lectin that binds the NPC and blocks and cargo from traversing through (31). This study also demonstrated that importin-β1 is the host protein responsible for import of rAAV2 particles through the NPC. Capsid interaction with members of the importin-α family of proteins was also shown by co-IP, but their involvement in nuclear import of rAAV2 remains unclear. This route of nuclear translocation was further supported by live cell imaging technology that witnessed labeled rAAV2 particles traverse the nuclear envelope through labeled NPCs (32).
After nuclear entry, rAAV has been shown to traffic to the nucleolus. AAV capsids have been shown to interact with nucleophosmin and nucleoin, both of which localize to the nucleolus (33, 34). Interestingly, rAAV particles remain intact and infectious in the nucleolus, thereby indicating that the nucleolar compartment is not where the capsids uncoat and deliver their genetic payload. Instead, it appears that the capsids must egress out of the nucleolus and move into the nucleoplasm in order to successfully transduce the cell. These observations are indirectly supported by small molecule and siRNA knockdown studies, which seem to suggest that nucleolar accumulation of rAAV is unfavorable for transduction (35). More investigation into possible differences in nucleolar accumulation between cell types and serotypes could help understand the role of the nucleolus in rAAV transduction.
Second-strand synthesis and transgene expression
After AAV reaches the nucleus, the viral capsid must uncoat/disassemble to release the packaged genome. The mechanisms underlying uncoating of AAV remain unclear, though it is known that genome composition may play an important role in uncoating and genome release (36). After uncoating, the virus must undergo conversion of the single-stranded DNA genome into its double-stranded form, a process involving second-strand DNA synthesis. This process has been shown to be a rate limiting step in infection in the absence of a helper virus (37), since second strand DNA synthesis is blocked by numerous host cellular factors critical for cellular DNA quality control. For instance, FKBP52 has been shown to bind the ITRs and blocks second-strand synthesis (38, 39). Additionally, the MRN complex, a cellular DNA damage-sensing complex consisting of the three cellular proteins Mre11, Rad50, and Nbs1, severely blocks AAV infection at the genome level (40–42). Furthermore, other proteins involved in the DNA damage response have been shown to interact with AAV genomes, such as Ku86 and Rad52, but the specifics underlying these interactions are currently unclear (43). The AAV genomes are then circularized and concatemerized (44) by DNA recombination events.
Recently, it has been shown that AAV capsid proteins might play a role in both second-strand DNA synthesis and subsequent transcription after capsid uncoating (45). These observations are corroborated in part by the observation that the AAV capsid might not disassemble completely during uncoating and remains associated with the genome during this critical event (36). While, the exact mechanism by which the capsid might play a role in these steps remains unclear, it has recently been demonstrated that splicing factors can bind the exposed genome and the capsid cooperatively to block transcription (46). Specifically, knockdown of the splicing factors PHF5A or SF3B1, components of the U2 snRNP greatly augmented AAV transduction. Furthermore, both of these factors were shown to interact with AAV capsids, indicating a direct interaction between the cellular splicing machinery and AAV.
Strategies to Augment rAAV Transduction
Growing insight into how the AAV capsid and genome interact with different cellular host factors has led to development of diverse strategies to enhance the potency of rAAV vectors. The ultimate goal of these strategies is to reduce the effective vector dose needed for efficient transgene expression. These approaches to enhance AAV transduction range from agents that amplify/inhibit the function of host factors that have been implicated in AAV trafficking to capsid modifications that can augment transduction.
It has been hypothesized that the proteasome plays a strong inhibitory role in rAAV transduction by ubiquitin-dependent degradation of capsids before they can traffic to the nucleus. This hypothesis is supported by several studies, the first of which demonstrated a large increase in transduction of rAAV2 when cells were treated with the proteasome inhibitor MG132 (19). Since then, several more studies have shown an increase with other proteasome inhibitors, namely LLnL, bortezomib (Velcade®), and carfilzomib (47–50). Additionally, it has been shown that rAAV2 and rAAV5 capsids are a substrate for ubiquitin conjugation (47), which is often a tag for proteasome-dependent degradation. Further, we have shown that inhibition of all cellular ubiquitination events with the UBEA1 inhibitor PYR-41 increases transduction (21). However, it should be noted that inhibition of ubiquitin-dependent capsid degradation by the proteasome might only account in part for the entire transduction increase witnessed. For instance, it is known that bortezomib can have non-proteasomal targets, such as serine proteases (51).
Several studies indicate the involvement of both proteotoxic stress and ER stress as cellular responses that occur during proteasome inhibition. Indeed, several other approaches that induce these stress responses also increase rAAV transduction. Cellular heat shock (52), chemical inhibition of Hsp90 (a molecular chaperone) (18), and increased oxygen free radicals (53), all induce an ER or proteotoxic stress response, and have been shown to increase rAAV transduction to a similar extent. Additionally, as mentioned earlier, we have recently demonstrated that modification of the intracellular trafficking of rAAV2 by the ERAD inhibitor EerI increases transduction (21). Interestingly, EerI has been shown to induce the ER stress response, but it is currently unknown, whether the trafficking changes observed are a direct result of this cellular stress (54, 55).
Another set of strategies being investigated involve modification of the AAV capsid and/or genome to increase transduction. One successful example of this approach is mutagenesis of tyrosine residues on the capsid surface to phenylalanine residues. It has been postulated that tyrosine residues on the capsid surface act as sites for phosphorylation and subsequent signaling motifs for ubiquitination, therefore leading to capsid degradation. The resulting Try-Phe (Y-F) mutations resulted in rAAV capsids that showed marked increase in transduction (56–59). Another strategy used to increase AAV transduction is modification of the viral genome. As previously mentioned, second-strand synthesis has been shown to be a major rate-limiting step in AAV transduction. Knowledge of this bottleneck led to the development of self-complementary AAV (scAAV) vectors, which are discussed in detail elsewhere (60).
In conclusion, studies aimed at understanding the intracellular trafficking pathways of rAAVs have provided a wealth of information that has been able to inform clinical and experimental approaches to gene delivery. However, our knowledge of AAV transduction mechanisms continues to evolve and there is a need to increase the percentage of infectious AAV particles that enter the nucleus and mediate robust transgene expression. Leveraging our understanding of rAAV cellular trafficking and structure could yield improved vectors with higher potency and/or lead to the development of adjuvant-based strategies to enhance AAV transduction.
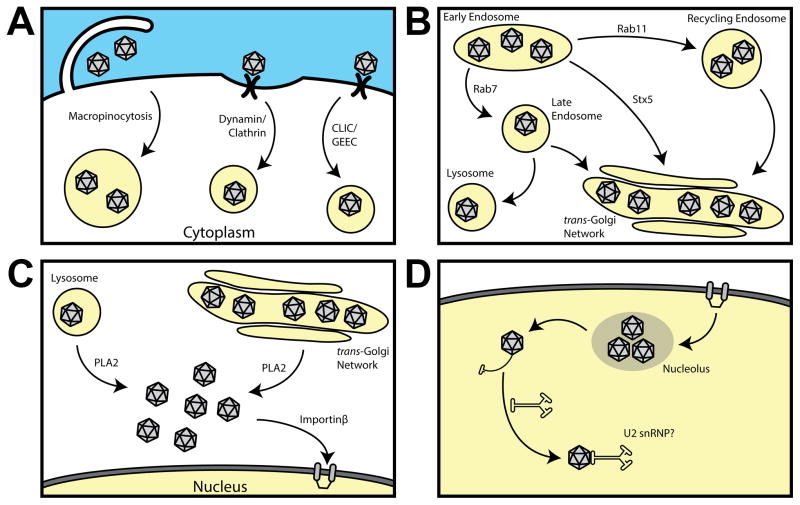
Figure 1.
Summary of different intracellular trafficking events leading to successful AAV transduction. (A) Cellular internalization of AAV vectors can occur through different endocytic cargo uptake mechanisms. (B) Following uptake, AAV traffics through endolysosomal vesicles and the trans-Golgi network. (C) AAV capsid processing within vesicular and Golgi compartments are thought to prime the AAV capsid for nuclear entry. (D) Nuclear entry of AAV capsids mediated by importin is followed by capsid uncoating, genome release and interaction with the cellular transcriptional machinery.
Highlights.
AAV exploits a spectrum of endocytic pathways after cellular uptake.
AAV trafficking through the trans-Golgi network precedes nuclear entry.
Second strand synthesis is a rate limiting step in AAV transduction.
Strategies to circumvent AAV interactions with host restriction factors can help augment transduction.
Acknowledgments
We would like to acknowledge funding support from the NIH (R01HL089221; P01HL112761 awarded to AA and training grant T32GM007092 to GB).
Footnotes
Conflict of Interest
Aravind Asokan is a co-founder at Stridebio LLC and an inventor on patents owned by UNC-CH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ATCHISON RW, CASTO BC, HAMMON WM. Adenovirus-Associated Defective Virus Particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 2.Bowles DE, Rabinowitz JE, Samulski RJ. The genus Dependovirus. In: Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR, editors. Parvoviruses. Edward Arnold Ltd; New York: 2006. pp. 15–24. [Google Scholar]
- 3.Geoffroy MC, Salvetti A. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr Gene Ther. 2005;5:265–271. doi: 10.2174/1566523054064977. [DOI] [PubMed] [Google Scholar]
- 4.Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–254. doi: 10.1016/B978-0-12-386509-0.00012-0. [DOI] [PubMed] [Google Scholar]
- 5.Xiao PJ, Li C, Neumann A, Samulski RJ. Quantitative 3D tracing of gene-delivery viral vectors in human cells and animal tissues. Mol Ther. 2012;20:317–328. doi: 10.1038/mt.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang LY, Halder S, Agbandje-McKenna M. Parvovirus glycan interactions. Curr Opin Virol. 2014;7C:108–118. doi: 10.1016/j.coviro.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan D, Li Q, Kao AW, Yue Y, Pessin JE, Engelhardt JF. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol Ther. 2006;13:506–516. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 10*.Nonnenmacher M, Weber T. Adeno-associated virus 2 infection requires endocytosis through the CLIC/GEEC pathway. Cell Host Microbe. 2011;10:563–576. doi: 10.1016/j.chom.2011.10.014. This study identified the CLIC/GEEC pathway as a route of productive AAV cellular uptake. This is the first study to implicate this pathway in AAV trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Weinberg MS, Nicolson S, Bhatt AP, McLendon M, Li C, Samulski RJ. Recombinant adeno-associated virus utilizes cell-specific infectious entry mechanisms. J Virol. 2014;88:12472–12484. doi: 10.1128/JVI.01971-14. This study demonstrated that inhibition of macropinocytosis has cell-specific effects on AAV transduction, enhancing transduction in some cell types while inhibiting transduction in others. This study highlights the existence of host cell-specific entry pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbison CE, Lyi SM, Weichert WS, Parrish CR. Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking. J Virol. 2009;83:10504–10514. doi: 10.1128/JVI.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding W, Zhang LN, Yeaman C, Engelhardt JF. rAAV2 traffics through both the late and the recycling endosomes in a dose-dependent fashion. Mol Ther. 2006;13:671–682. doi: 10.1016/j.ymthe.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castle MJ, Perlson E, Holzbaur EL, Wolfe JH. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol Ther. 2014;22:554–566. doi: 10.1038/mt.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bantel-Schaal U, Hub B, Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson JS, Li C, DiPrimio N, Weinberg MS, McCown TJ, Samulski RJ. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol. 2010;84:8888–8902. doi: 10.1128/JVI.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JS, Gentzsch M, Zhang L, Ribeiro CM, Kantor B, Kafri T, Pickles RJ, Samulski RJ. AAV exploits subcellular stress associated with inflammation, endoplasmic reticulum expansion, and misfolded proteins in models of cystic fibrosis. PLoS Pathog. 2011;7:e1002053. doi: 10.1371/journal.ppat.1002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–1687. doi: 10.1128/JVI.02520-14. This study identified the protein syntaxin 5 as an indispensable protein for the trafficking of AAV to the trans-Golgi network. This study demonstrated that knockdown and chemical inhibition of syntaxin 5 strongly inhibited AAV transduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry GE, Asokan A. Chemical Modulation of Endocytic Sorting Augments Adeno-associated Viral Transduction. J Biol Chem. 2016;291:939–947. doi: 10.1074/jbc.M115.687657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. This is the first study to identify a universal receptor for AAV, termed AAVR. This landmark study used both in vitro and in vivo studies to confirm the role of AAVR in the transduction of several AAV serotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girod A, Wobus CE, Zadori Z, Ried M, Leike K, Tijssen P, Kleinschmidt JA, Hallek M. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol. 2002;83:973–978. doi: 10.1099/0022-1317-83-5-973. [DOI] [PubMed] [Google Scholar]
- 24.Stahnke S, Lux K, Uhrig S, Kreppel F, Hosel M, Coutelle O, Ogris M, Hallek M, Buning H. Intrinsic phospholipase A2 activity of adeno-associated virus is involved in endosomal escape of incoming particles. Virology. 2011;409:77–83. doi: 10.1016/j.virol.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Grieger JC, Johnson JS, Gurda-Whitaker B, Agbandje-McKenna M, Samulski RJ. Surface-exposed adeno-associated virus Vp1-NLS capsid fusion protein rescues infectivity of noninfectious wild-type Vp2/Vp3 and Vp3-only capsids but not that of fivefold pore mutant virions. J Virol. 2007;81:7833–7843. doi: 10.1128/JVI.00580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, Salganik M, Muzyczka N, Agbandje-McKenna M. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol. 2011;85:11791–11799. doi: 10.1128/JVI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kronenberg S, Bottcher B, von der Lieth CW, Bleker S, Kleinschmidt JA. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J Virol. 2005;79:5296–5303. doi: 10.1128/JVI.79.9.5296-5303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salganik M, Venkatakrishnan B, Bennett A, Lins B, Yarbrough J, Muzyczka N, Agbandje-McKenna M, McKenna R. Evidence for pH-dependent protease activity in the adeno-associated virus capsid. J Virol. 2012;86:11877–11885. doi: 10.1128/JVI.01717-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grieger JC, Snowdy S, Samulski RJ. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J Virol. 2006;80:5199–5210. doi: 10.1128/JVI.02723-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Nicolson SC, Samulski RJ. Recombinant adeno-associated virus utilizes host cell nuclear import machinery to enter the nucleus. J Virol. 2014;88:4132–4144. doi: 10.1128/JVI.02660-13. This is the first study to definitively demonstrate the mechanism of AAV transport into the nucleus is through the nuclear pore complex. The authors demonstrated that the importinβ family of proteins are important for this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelich JM, Ma J, Dong B, Wang Q, Chin M, Magura CM, Xiao W, Yang W. Super-resolution imaging of nuclear import of adeno-associated virus in live cells. Mol Ther Methods Clin Dev. 2015;2:15047. doi: 10.1038/mtm.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu J, Brown KE. A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid. Virology. 1999;257:373–382. doi: 10.1006/viro.1999.9664. [DOI] [PubMed] [Google Scholar]
- 34.Bevington JM, Needham PG, Verrill KC, Collaco RF, Basrur V, Trempe JP. Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions. Virology. 2007;357:102–113. doi: 10.1016/j.virol.2006.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JS, Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horowitz ED, Rahman KS, Bower BD, Dismuke DJ, Falvo MR, Griffith JD, Harvey SC, Asokan A. Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release. J Virol. 2013;87:2994–3002. doi: 10.1128/JVI.03017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qing K, Wang XS, Kube DM, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci U S A. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qing K, Khuntirat B, Mah C, Kube DM, Wang XS, Ponnazhagan S, Zhou S, Dwarki VJ, Yoder MC, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi VW, McCarty DM, Samulski RJ. Host cell DNA repair pathways in adeno-associated viral genome processing. J Virol. 2006;80:10346–10356. doi: 10.1128/JVI.00841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz RA, Palacios JA, Cassell GD, Adam S, Giacca M, Weitzman MD. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol. 2007;81:12936–12945. doi: 10.1128/JVI.01523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lentz TB, Samulski RJ. Insight into the mechanism of inhibition of adeno-associated virus by the Mre11/Rad50/Nbs1 complex. J Virol. 2015;89:181–194. doi: 10.1128/JVI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zentilin L, Marcello A, Giacca M. Involvement of cellular double-stranded DNA break binding proteins in processing of the recombinant adeno-associated virus genome. J Virol. 2001;75:12279–12287. doi: 10.1128/JVI.75.24.12279-12287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi VW, Samulski RJ, McCarty DM. Effects of adeno-associated virus DNA hairpin structure on recombination. J Virol. 2005;79:6801–6807. doi: 10.1128/JVI.79.11.6801-6807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Salganik M, Aydemir F, Nam HJ, McKenna R, Agbandje-McKenna M, Muzyczka N. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J Virol. 2014;88:1071–1079. doi: 10.1128/JVI.02093-13. This study identified AAV capsid mutants that trafficked normally but were defective for either RNA transcription or second-strand synthesis. This is the first study to demonstrate a possible role for the AAV capsid in transduction after uncoating and genome release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Schreiber CA, Sakuma T, Izumiya Y, Holditch SJ, Hickey RD, Bressin RK, Basu U, Koide K, Asokan A, Ikeda Y. An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses. PLoS Pathog. 2015;11:e1005082. doi: 10.1371/journal.ppat.1005082. This study reports the interaction of AAV with the U2 snRNP spliceosome and identifies the complex as an AAV restriction factor. Further, the authors demonstrated a pharmacological approach to increase AAV transduction through inhibition U2 snRNP-related proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, Peluso R, Engelhardt JF. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nathwani AC, Cochrane M, McIntosh J, Ng CY, Zhou J, Gray JT, Davidoff AM. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16:60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell AM, Samulski RJ. Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors. J Virol. 2013;87:13035–13041. doi: 10.1128/JVI.01826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, Muchamuel T, Bennett MK, Driessen C, Ball AJ, Kirk CJ. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17:2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- 52.Zhong L, Qing K, Si Y, Chen L, Tan M, Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. J Biol Chem. 2004;279:12714–12723. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell AM, Li C, Samulski RJ. Arsenic trioxide stabilizes accumulations of adeno-associated virus virions at the perinuclear region, increasing transduction in vitro and in vivo. J Virol. 2013;87:4571–4583. doi: 10.1128/JVI.03443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brem GJ, Mylonas I, Bruning A. Eeyarestatin causes cervical cancer cell sensitization to bortezomib treatment by augmenting ER stress and CHOP expression. Gynecol Oncol. 2013;128:383–390. doi: 10.1016/j.ygyno.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling C, Li B, Ma W, Srivastava A. Development of Optimized AAV Serotype Vectors for High-Efficiency Transduction at Further Reduced Doses. Hum Gene Ther Methods. 2016 doi: 10.1089/hgtb.2016.054. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]