Abstract
There is an urgent need for new clinically applicable drug-delivery methods to enhance accumulation of immune-activating drugs in tumors. We synthesized a poly(L-glutamic acid)-CpG ODN2216 conjugate (PG-CpG) and injected it intratumorally into C57BL/6 mice bearing subcutaneous B16-ovalbumin melanoma. PG-CpG elicited the same potent antitumoral activity as CpG with respect to reducing tumor growth and triggering antigen-specific CD8+ T-cell responses in this well-established solid tumor model. Moreover, PG-CpG was retained significantly longer in both tumor and draining lymph nodes than was free CpG after intratumoral injection. Specifically, 48 h after injection, 26.5±16.9% of the injected PG-CpG dose versus 4.72±2.61% of free CpG remained at the tumor, and 1.53±1.22% of the injected PG-CpG versus 0.37±0.33% of free CpG was retained in the draining inguinal lymph nodes. These findings indicate that PG is an effective synthetic polymeric carrier for delivery of immunostimulatory agents to tumors and lymph nodes.
Keywords: CpG, Poly-L-glutamic acid, B16-OVA melanoma, macrophage, tetramer-specific CD8+ T cells
Introduction
The Toll-like receptor (TLR) family consists of 13 different receptors that recognize microbial DNA and RNA structures.1, 2 TLR ligands can be used as immunomodulators in tumors, where they elicit a potent immune response similar to the one that would be triggered if a foreign pathogen were present. It was previously reported that intratumoral injection of TLR9 agonist CpG enhanced the efficacy of CD8+ T-cell-mediated killing in a mouse model of melanoma.3 Specifically, A-class CpG oligonucleotides, such as ODN2216, induce robust production of type I interferon by plasmacytoid dendritic cells (pDCs) and highly stimulate natural killer (NK) cells but have little stimulatory effect on B cells.
The route of administration of CpG affects its antitumor efficacy and its toxicity. Free CpG and other stable phosphorothioate oligonucleotides administered by intravenous injection are cleared rapidly and have a broad tissue distribution.4, 5 These properties are thought to contribute to the failure of systemically administered free CpG in human volunteers.6 Furthermore, systemically administered free CpG can induce nonspecific immune activation leading to severe side effects, including immune cell exhaustion, destruction of lymphoid follicles, liver damage, and exacerbation of autoimmune diseases.7-9 Studies have shown that intratumoral injection of CpG significantly enhances its antitumor effect, through “focusing” the immune stimulation in tumors and draining lymph nodes.3 However, it is difficult to control the retention time of CpG injected into tumors and lymph nodes; free CpG can still be absorbed to the circulation through diffusion.
To overcome these problems, we developed a biocompatible, biodegradable polymer platform based on poly(L-glutamic acid) (PG) to direct and control the release of CpG at tumor sites. PG is a unique synthetic polymer with naturally occurring L-glutamic acid linked together through an amide bond backbone. The polymer is water-soluble owing to the pendent free γ-carboxyl groups, which also enable chemical conjugation of drug molecules. A PG-paclitaxel conjugate developed in our laboratory, in which paclitaxel is covalently linked at the 2′-hydroxyl group by an ester bond to L-PG, has shown significant antitumor activity in a variety of preclinical animal tumor models and in early phase I trials.10-13 PG is biodegradable, and versatile chemistry is available for the synthesis of PG-based polymeric drugs with controlled molecular weight and degradability.14-17 These features make PG a promising candidate for a carrier of CpG. PG conjugated with a near-infrared dye and DTPA-Gd has previously been shown to be localized to sentinel lymph nodes after subcutaneous injection in both normal mice and tumor-bearing mice with lymph node metastasis.15 The pattern of contrast enhancement of sentinel lymph nodes visualized on near-infrared fluorescence imaging showed distribution of the polymer to the subcapsular sinus and cortex/paracortex zone of lymph nodes.15
In the study reported here, we investigated the use of PG-CpG for activation of antitumor immunity in a mouse model of melanoma. Our data showed that PG-CpG triggered both innate and antigen-specific CD8+ T-cell responses against an established B16-ovalbumin (B16-OVA) subcutaneous tumor, suppressing tumor growth. Moreover, PG-CpG had better tumor retention than CpG and accumulated in tumor-draining lymph nodes.
Materials and Methods
Synthesis of PG-CpG and PG-CpG-NIR813
PG (weight-average molecular weight, ∼56 KDa; number-average molecular weight, ∼33.1 KDa; degree of polymerization, 219; Sigma, St. Louis, MO) was first reacted with N-(2-aminoethyl)maleimide (Sigma) in the presence of N-hydroxysulfosuccinimide (Sigma) and N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDC; Sigma). CpG oligonucleotide (ODC 2216) with 3′ terminal-amino group (5′-EEGGGACGATCGTCEEEEG-3′-NH2, CpG-3′-NH2; Invitrogen, Chicago, IL) was reacted with N-succinimidyl S-acetylthioacetate (SATA; Pierce, Philadelphia, PA) to introduce a thio group on the 3′- terminus. The 3′-acetylthio group was deprotected by hydroxylamine (50 mM), and then CpG was added to maleimide-modified PG solution. The reaction mixture of CpG and maleimido-PG was incubated at 4°C overnight. The purity of PG-CpG was examined by gel electrophoresis. Free CpG (CpG-3′-NH2), CpG-3′-acetylthioacetamide (CpG-3′-SATA), and purified PG-CpG were loaded onto 15% polyacrylamide-urea gel and stained with SYBR Gold stain. The CpG concentration was measured by using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). PG alone had negligible absorption at the wavelength used in measurement. To validate the measurement, CpG with known concentrations was used as a standard. Taking the molecular weight of CpG as 6,088, molecular weight of PG as 33,100, and the weight percentage of CpG in PG-CpG as “n”, the number of CpG molecules per polymer chain “x” was calculated using the formula: x = 33100*n ∕ 6088(1-n).
NIR813 dye was synthesized according to previously described procedures 15. For conjugation to PG-CpG, NIR813 (0.5 mg) in 0.1 mL of DMSO was added to a solution of PG-CpG (10 mg) in 0.1 M MES buffer (1 mL) in the presence of EDC. The reaction mixture was stirred at 4°C overnight while protected from light. The crude product was filtered through a 0.2-μm filter and purified by dialysis against deionized water. PG-CpG-NIR813 was obtained as a dark blue power after lyophilization. The conjugate contained about 4% (wt/wt) near-infrared (NIR) dye.
Radiolabeling of CpG and PG-CpG
For 111In labeling of CpG, the 3′-amino terminus of CpG was modified with radiometal chelator by reaction with 2-(4-isothiocyanatobenzyl)-diethylenetriaminepentaacetic acid (p-SCN-Bn-DTPA; Macrocyclics, Dallas, TX). The unreacted p-SCN-Bn-DTPA was removed by using a PD-10 column (GE Healthcare, Wauwatosa, WI). 111InCl3 (1 mCi) (Perkin Elmer, Waltham, MA) in 0.1 M sodium acetate was added into CpG-3′-DTPA (50 μg), and the mixture was incubated for 30 min at room temperature. Free 111In was removed by using a PD-10 column, which yielded 0.8 mCi of 111In-DTPA-CpG. Radiochemical purity was monitored by high-performance liquid chromatography (HPLC) with a poroshell 300SB C-18 column (Agilent, Santa Clara, CA) and a radiodetector.
For 111In labeling of PG-CpG, PG was attached to DTPA as previously described.18 The loading ratio of DTPA to PG was 1 mole of DTPA to 20 moles of carboxylic units in the polymer. For coupling of CpG to PG, N-(2-aminoethyl)maleimide was conjugated to the polymer side chain through the carboxylic group. Then CpG-3′-thio obtained from reaction of CpG-3′-NH2 with SATA was conjugated to PG polymer. DTPA-PG-CpG (54 μg of conjugate, 27 μg equivalent of CpG) was labeled with 1 mCi of 111InCl3. Radiochemical purity was monitored by HPLC.
Mice
Female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), approximately 2-3 months old at time of use, were housed in pathogen-free animal facilities at The University of Texas MD Anderson Cancer Center. All procedures were performed according to Institutional Animal Care and Use Committee-approved protocols.
Evaluation of ex vivo immunostimulatory activity
To ensure that conjugation of CpG to PG polymer did not adversely affect CpG's immunostimulatory ability, we stimulated mouse splenocytes with various concentrations of PG-CpG, CpG, and PG in vitro. Spleens were removed from euthanized mice and mashed through 70-μm filters. The single-cell suspensions of splenocytes were washed with PBS, and 106 cells were combined with various concentrations of PG-CpG, CpG, and PG (1.56-100 μg/ml) in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin-streptomycin (Mediatech, Inc., Manassas, VA) for 24 h at 37°C. Phorbol 12-myristate 13-acetate (PMA)/ionomycin were used as a positive control. Activation of NK cells and macrophages was measured by flow cytometry with the following gating strategy: leukocytes were gated from forward scatter versus side scatter plots, and then live cells were gated as Aqua-negative cells (Live/Dead Aqua stain; Invitrogen, Eugene, OR) and NK1.1+ cells (NK cells) and CD11b+ cells (macrophages) were plotted versus the lymphocyte activation marker CD69 (antibodies from eBiosciences, San Diego, CA). The mean fluorescence intensity (MFI) of CD69 expression was used to represent the immunostimulatory activity of PG-CpG and CpG.
Evaluation of antitumor activity and melanoma-specific CD8+ T-cell response
C57BL/6 mice bearing subcutaneous B16-OVA melanoma tumors (average diameter, 4-6 mm) were randomly assigned to different treatment groups (5 mice each). Mice in each group underwent intratumoral injection of PBS, CpG, PG-CpG, or PG on days 1, 7, and 10 at a dose of 50 μg equivalent CpG per injection (150 μg equivalent PG) in 100-μl volume. Tumor size was measured starting on day 8 (day 7 after first injection) and then every 2-3 days until day 16. The longest length (a) and the length perpendicular to the longest length (b) were used in the formula V = ½a(b)2 to obtain the tumor volume in mm3. Peripheral blood was obtained from each mouse on days 3, 7, 9, 12, and 16 for assessment of OVA-specific CD8+ T cells by flow cytometry. Lymphocyte and live/dead gating were performed as described in the preceding section, and then tumor-specific T-cells were gated as OVA-specific tetramer (OVA257-264 peptide-loaded tetramers, Pharmingen, San Jose, CA) versus CD8+ cell populations.
Biodistribution
C57BL/6 mice were inoculated subcutaneously with 5 × 105 B16-OVA melanoma cells. When tumors reached 6-8 mm in diameter, mice were divided into 2 groups with 6 mice each. 111In-CpG (3 μg, 50 μCi) or 111In-PG-CpG (3 μg equivalent CpG, 50 μCi) was injected directly into the tumor (10 μL). At 24 and 48 h after injection, mice were sacrificed. Tumors, blood, and organs, including heart, liver, spleen, kidney, and lymph nodes, were removed from each mouse. The tissues were weighed, and radioactivity of each tissue was measured by using a Cobra auto-gamma counter (Perkin Elmer). The data are expressed as percentage of the injected dose in each organ tissue.
Micro-SPECT/CT imaging
C57BL/6 mice bearing subcutaneous B16-OVA tumors underwent intratumoral injection of 111In-CpG (6 μg, 100 μCi) or 111In-PG-CpG (3.5 μg equivalent of CpG, 100 μCi). At 24 and 48 h after radiotracer injection, single-photon emission computed tomography/computed tomography (SPECT/CT) images were acquired with a FLEX X-O.X micro-SPECT imaging system (Gamma Medica-IDEAS, Northridge, CA). The SPECT and CT images were reconstructed and merged by using X SPECT Fusion 2 software (Gamma Medica-IDEAS).
Fluorescence imaging
B16/F10 melanoma cells (5×105) were subcutaneously inoculated in the front right leg of C57BL/6 mice. PG-CpG-NIR813 was intratumorally injected at 10 days after inoculation. At 4 h after injection, the mice were sacrificed, and tumors were dissected for frozen sectioning. For macrophage staining, the tissues were stained with rat anti-mouse CD68 polyclonal antibody (1:100) followed by Texas red-conjugated goat anti-rat IgG (1:700). For pDC staining, the tissues were stained with biotin-conjugated anti-BDCA-2 monoclonal antibody (1:100) followed by Alexa Fluor 594-conjugated streptavidin (1:700). The cell nuclei were counterstained with DAPI. The slices were examined under a fluorescent microscope with Cy7 filter for PG-CpG-NIR813 (pseudo-green), rhodamine filter for Texas red or Alexa Fluor (red), and UV filter for DAPI (blue).
Statistical analysis
A mixed-effects linear model that accounts for intramouse correlation was fit to model tumor growth over time, with effects for time, treatment, and the interaction between time and treatment. Holm's method was used to adjust for multiple comparisons. Analysis of variance (ANOVA) was used to assess differences among groups separately by time point for NK cells in tumor and spleen. Dunnett's procedure was used to adjust for multiple comparisons against the PBS control group.
Results
Structure of PG-CpG
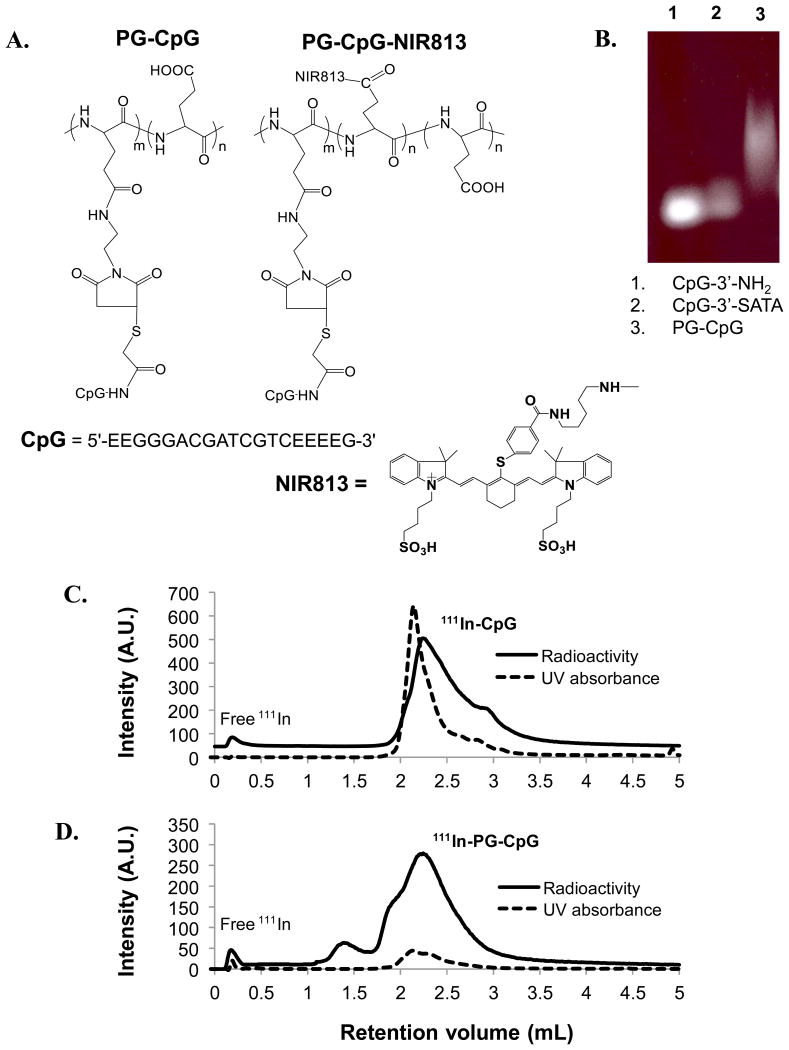
The structure of maleimide-modified PG conjugated to CpG via the 3′-acetylthio group of CpG (CpG-3′-SATA) is shown in Figure 1A. The conjugation and purity of PG-CpG were analyzed by agarose gel electrophoresis, which showed absence of free CpG (CpG-3′-NH2) or CpG-3′-SATA in PG-CpG (Fig. 1B). The smearing of PG-CpG on the gel was due to the broad distribution of molecular weight and charge of PG, not to aggregation of the conjugates. CpG content in the conjugate was 25-35% (w/w). Each polymer chain was estimated to contain 2-3 CpG molecules. The structure of near-infrared dye–conjugated PG-CpG, PG-CpG-NIR813, is shown in Figure 1A. HPLC analysis of 111In-labeled CpG (Fig. 1C) and 111In-labeled PG-CpG (Fig. 1D) showed 97% and 95% radiochemical purity, respectively.
Figure 1. Design of polymer-conjugated CpG ODN2216 (PG-CpG).
(A) Structures of PG-CpG and PG-CpG-NIR813. (B) Purity of PG-CpG. Free CpG (CpG-3′-NH2), CpG-3′-acetylthioacetamide (CpG-3′-SATA), and purified PG-CpG were loaded onto 15% polyacrylamide-urea gel and stained with SYBR Gold stain. (C and D) HPLC analysis of 111In-labeled CpG (C) and 111In-labeled PG-CpG (D). The overlay of UV absorbance peak and radioactivity peak confirmed radiolabeling of 111In onto CpG and PG-CpG. The radiochemical purities of 111In-CpG and 111In-PG-CpG were 97% and 95%, respectively. A.U., arbitrary units.
PG-CpG has immunostimulatory activity in vitro
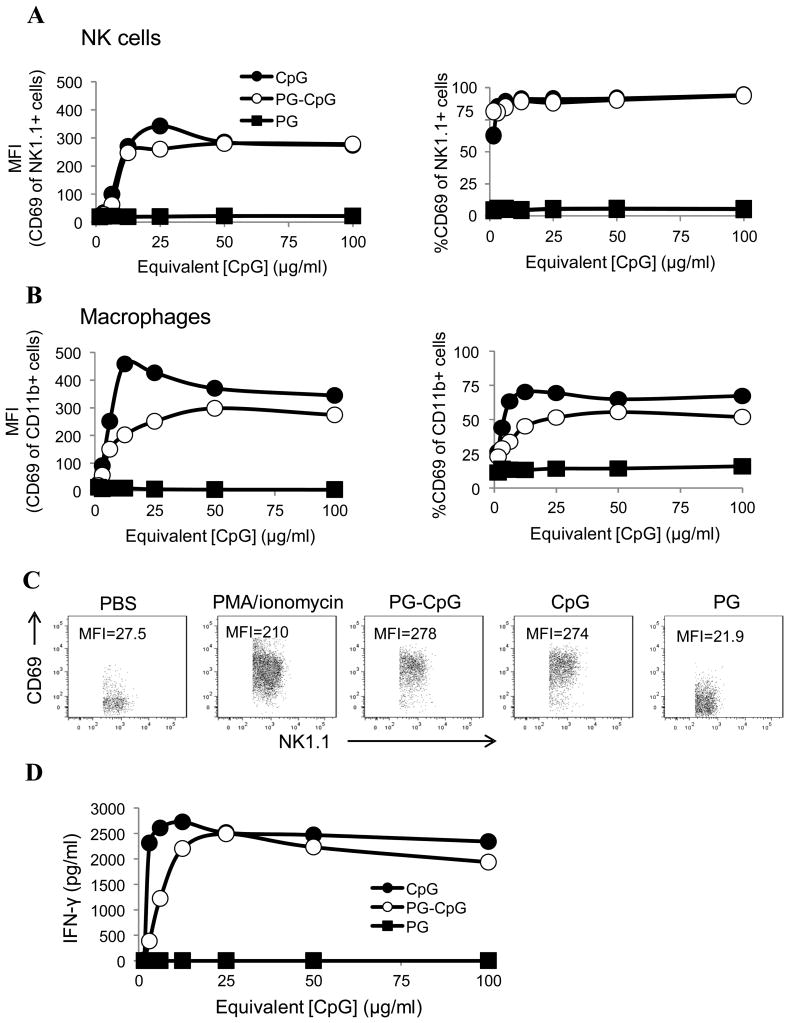
As shown in Figure 2A, the activation status of NK cells (NK1.1+), determined by MFI of CD69 expression, was similar between CpG and PG-CpG pulsed at various concentrations. Representative flow cytometry plots of PG-CpG-, CpG-, and PG-treated cells and PBS- and Phorbol 12-myristate 13-acetate (PMA)/ionomycin-treated controls are shown in Figure 2C. At 100 μg/ml, the MFI of CD69 expression on NK1.1+ cells was 278 for PG-CpG and 274 for CpG, both of which values were similar to the value for the PMA/ionomycin positive control (MFI=210). The value for PG-treated cells (MFI=21.9) was similar to the value for PBS background (MFI=27.5). These data demonstrated that both CpG and PG-CpG potently stimulated and activated NK cells in vitro.
Figure 2. PG-CpG activates splenic NK cells and macrophages in vitro.
PG-CpG, CpG, and PG (with equivalent CpG concentrations from 1.56 to 100 μg/ml) were cultured with mouse splenocytes for 24 h. (A) NK cell activation. MFI of CD69 expression (left) and the percentage of CD69+ NK cells (NK1.1+) (right) were measured by flow cytometry after stimulation with various concentrations of reagents. (B) Macrophage activation. MFI of CD69 expression (left) and the percentage of CD69+ macrophages (CD11b+) (right) were measured by flow cytometry after stimulation with various concentrations of reagents. (C) Representative dot plots of the MFI of CD69 expression on CpG- and PG-CpG-stimulated NK cells versus PG-treated cells. PMA/ionomycin stimulation was used as a positive control for NK cell activation. (D) Interferon-γ production. The supernatant of activated splenocyte culture was collected, and the concentration of interferon-γ was measured by ELISA. Data are representative of 5 independent experiments (C) and summarized in A, B, and D. The concentrations labeled on X axis are equivalent CpG concentrations for PG-CpG and PG.
PG-CpG also triggered potent activation of macrophages (CD11b+), albeit slightly less than CpG did. PG alone did not activate macrophages above the background level (Fig. 2B).
Finally, measurement of interferon-γ levels in the supernatant of activated splenocytes showed that PG-CpG induced a robust interferon-γ response similar to the one induced by CpG (Fig. 2D).
PG-CpG has potent antitumor efficacy in vivo
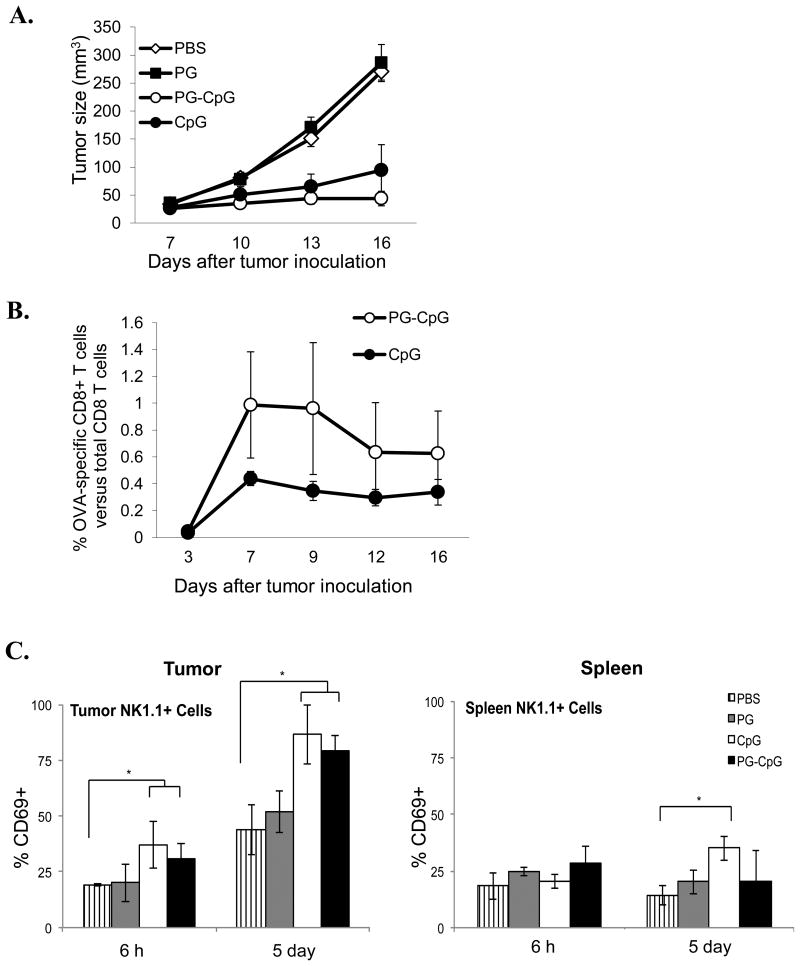
Using the subcutaneous mouse B16-OVA melanoma model, we found that intratumoral injection of PG-CpG as a single agent triggered significant antitumor activity against established tumors, resulting in significant inhibition of tumor growth compared to PBS or PG control groups (Fig. 3A). Using a mixed-effects linear model for the overall comparison of the 4 groups, we found a highly significant group difference between the treatment (PG-CpG and CpG) and control (PBS and PG) groups (p<0.0001). Although the tumor growth curve for PG-CpG treatment was below the curve for CpG treatment, tumor growth did not differ significantly between these 2 groups (p = 0.30). However, at day 16, tumor volumes were smaller in PG-CpG-treated mice (10.4, 21.8, 40.3, 67.9, and 79.1 mm3) than in CpG-treated mice (24.6, 33.3, 42.6, 101, 269 mm3) (Table 1). Furthermore, tumor volume shrank over time in 2 of the 5 mice treated with PG-CpG (mice #313 and 315) but none of the mice treated with CpG. In fact, 2 of 5 mice treated with CpG (mice #318 and 320) had tumor growth similar to that in control groups.
Figure 3. Antitumor efficacy and immunostimulatory potency of PG-CpG in vivo.
Seven days after subcutaneous inoculation of B16-OVA tumor cells in C57BL/6 mice, PG-CpG (50 μg equivalent CpG), CpG (50 μg), or PG (500 μg) was injected into the tumor in 100 μL volume. (A) Tumor growth curves. The mixed-effects linear model was used for the overall comparison of the 4 groups over the entire growth curve. Tumor growth (p<0.0001) was significantly inhibited with PG-CpG or CpG treatment compared to control PBS or PG. (B) Tumor antigen-specific CD8+ T-cell responses. OVA-specific CD8+ T cells from peripheral blood were measured by OVA257-264 peptide-loaded tetramers with flow cytometry. The difference between PG-CpG and CpG was not significant (p = 0.65). (C) NK cell activation. After intratumoral injection of each reagent, cells were collected from tumor (left) and spleen (right) and analyzed for CD69 expression on NK cells. ANOVA was used to assess differences among groups separately by time point (6 h and 5 days) (*p<0.05). Data are from 2 independent experiments with 5 mice/group.
Table 1. Volumes of individual tumors at the indicated days after PG-CpG or CpG treatment (n = 5/group).
| PG-CpG | ||||
|---|---|---|---|---|
| Mouse# | Days | |||
| 7 | 10 | 13 | 16 | |
| 311 | 28.1 | 36.7 | 64.2 | 67.9 |
| 312 | 45.6 | 44.7 | 70.2 | 79.1 |
| 313 | 22.1 | 17.8 | 16.8 | 10.4 |
| 314 | 22.1 | 35.0 | 35.9 | 40.3 |
| 315 | 13.7 | 39.1 | 31.6 | 21.8 |
| CpG | ||||
| Mouse# | Days | |||
| 7 | 10 | 13 | 16 | |
| 316 | 22.1 | 30.8 | 47.6 | 42.6 |
| 317 | 27.7 | 30.2 | 32.0 | 33.3 |
| 318 | 49.2 | 94.3 | 148 | 269 |
| 319 | 12.3 | 26.7 | 25.4 | 24.6 |
| 320 | 26.7 | 72.5 | 72.0 | 101 |
As shown in Figure 3B, although PG-CpG appeared to induce greater OVA-specific response than CpG did (indicated by tetramer-specific CD8+ T cells as a percentage of total CD8+ T cells in peripheral blood), neither the difference between the 2 groups at day 16 after tumor inoculation (p = 0.65) nor the difference between the 2 groups over the entire duration (p = 0.29) was significant. PG-CpG and CpG treatments induced similar numbers of tumor antigen-specific CD8+ T cells, while PBS and PG did not generate any tetramer-positive CD8+ T cells (data not shown). Representative flow cytometry data are presented in Supplemental Figure S1A.
We also measured NK cell activation in tumor and spleen. As shown in Figure 3C (left), intratumorally injected CpG and PG-CpG significantly increased the percentage of CD69+ NK cells in the tumor at 6 h (p = 0.007) and 5 days (p = 0.0003) after treatment compared to PBS and PG controls. However, whereas free CpG induced significant nonspecific activation of NK cells in the spleen at 5 days after treatment compared to PBS (p = 0.04), PG-CpG did not induce nonspecific NK cell activation in the spleen (Fig. 3C, right). Representative flow cytometry data are presented in Supplemental Figure S1B. PG-CpG did not induce any change in spleen cellularity, and there was no significant difference in the weight of spleen at 7 days between the CpG and PG-CpG treatment groups (data not shown).
PG delivery system enhances intratumoral retention after intratumoral injection
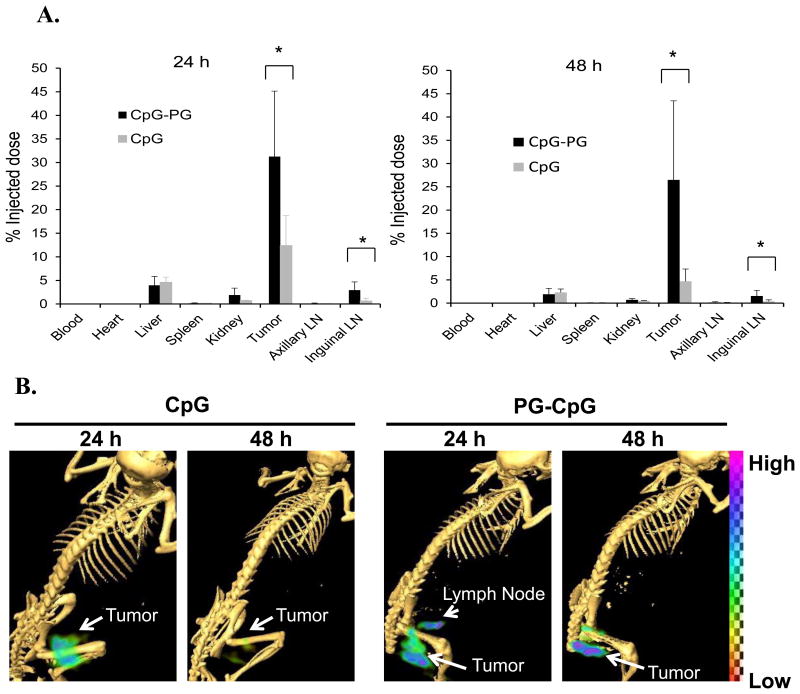
To investigate mechanisms for the observed in vivo effects, we assessed the distribution of 111In-labeled PG-CpG and CpG following intratumoral injection. Significantly more 111In-PG-CpG than 111In-CpG was retained at the tumor site at 24 h (31.2±13.9% vs 12.4±6.3%; p = 0.025) and 48 h (26.5±17.0% vs 4.7% 4.7±2.6%; p = 0.024) after injection (Fig. 4A). Moreover, significantly more of the injected dose was retained in the draining inguinal lymph nodes after 111In-PG-CpG injection than after 111In-CpG injection at both 24 h (2.9±1.7% vs 0.6±0.6%; p = 0.01) and 48 h (1.5±1.2% vs 0.37±0.33%; p = 0.03) (Fig. 4A). In comparison, uptake of both agents in the distant axillary lymph nodes was extremely low, and no difference was found between 111In-PG-CpG and 111In-CpG uptake in distant axillary nodes. μSPECT/CT imaging of tumor-bearing mice confirmed prolonged retention of PG-CpG in the tumor after injection (Fig. 4B). Draining lymph nodes could be clearly visualized by μSPECT/CT at 24 h after 111In-PG-CpG injection but not at 24 h after 111In-CpG injection (Fig. 4B).
Figure 4. Biodistribution and imaging of CpG-PG and CpG in tumor and major organs.
C57BL/6 mice bearing B16-OVA melanoma tumors 6-8 mm in diameter were divided into 2 groups with 6 mice each. 111In-CpG or 111In-PG-CpG was injected directly into the tumor. (A) Biodistribution of CpG-PG and CpG. Data are expressed as percentage of injected dose (%ID), represented as mean ± standard deviation. Significantly more 111In-CpG-PG than 111In-CpG was retained in the tumor and the draining lymph nodes (LNs). *p<0.05. (B) Representative SPECT/CT images of mice (n = 3) 24 h and 48 h after intratumoral injection of 111In-PG-CpG and 111In-CpG, showing the site and amount of the radiotracer retained in tumor and tissue.
PG-CpG is efficiently taken up by macrophages and pDC in tumors
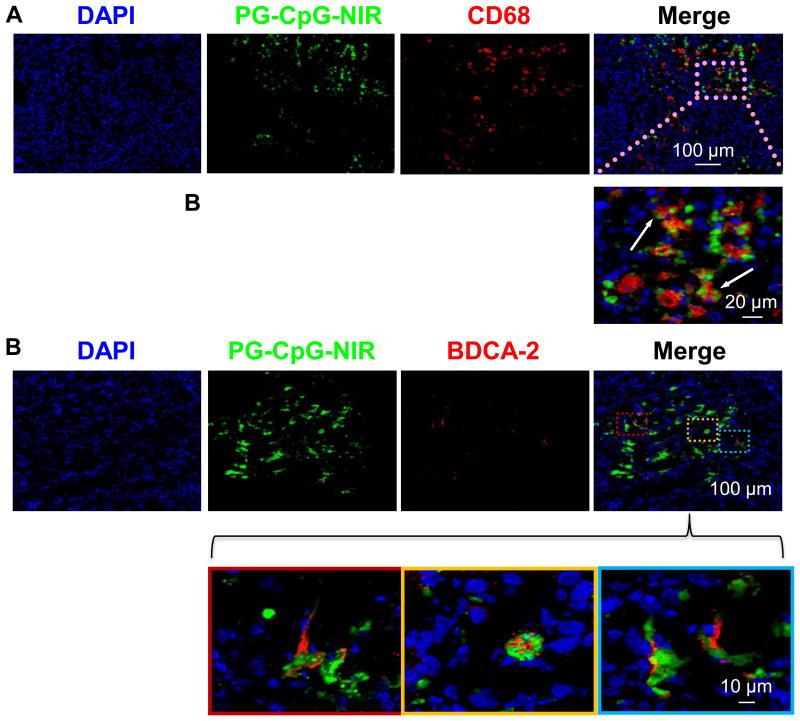
Four hours after intratumoral injection, PG-CpG-NIR813 (fluorescently labeled PG-CpG) was broadly distributed throughout the tumor. There were numerous tumor-associated macrophages (CD68-positive cells) in B16/F10 melanoma (Fig. 5A). The polymeric conjugate co-localized to macrophages and was engulfed into the vesicular compartment (arrows) as seen at higher magnification (Fig. 5A). We found that pDCs (BDCA-2-positive cells) were sparsely distributed in the B16/F10 tumors. However, at 4 h after intratumoral injection, most pDCs had taken up PG-CpG-NIR813 (Fig. 5B). These data suggest that both tumor-associated macrophages and pDCs were directly involved in the processing of PG-CpG-NIR813 (and PG-CpG).
Figure 5. Intratumoral distribution of PG-CpG-NIR813.
PG-CpG was conjugated to NIR dye as described and injected directly into tumors. Mice (n = 3) were sacrificed at 4 h after injection, and tumor sections were prepared for immunohistochemical analysis. CD68 (red) was used to stain for tumor-associated macrophages, and BDCA-2 (red) was used to stain for pDCs. PG-CpG-NIR813 was peudocolored green and used in combination with DAPI to identify PG-CpG uptake by macrophages (A) or pDCs (B) in tumors.
Discussion
Our in vitro and in vivo experiments demonstrated that conjugation of CpG to PG did not change the bioactivity of CpG and that PG-CpG elicited potent antitumor responses with respect to delaying tumor growth and triggering antigen-specific CD8+ T-cell responses in subcutaneously transplanted B16-OVA melanoma in C57BL/6 mice. Moreover, PG-CpG was retained in tumors and tumor-draining lymph nodes after intratumoral injection. Taken together, our data support the use of PG-CpG as an immunostimulatory TLR9 agonist.
Our previous studies suggested that PG is primarily taken up by tumor-associated macrophages,19 and in the current study, we observed similar results with NIR-conjugated PG-CpG. Several mechanisms may contribute to PG-CpG's observed antitumor activity, including (i) a depot effect, whereby PG-CpG is retained in the tumor for a prolonged period and CpG is slowly released from the site of its injection, (ii) and enhanced delivery of CpG to dendritic cells and other antigen-presenting cells, such as macrophages, in the tumor-draining lymph nodes. Indeed, other studies of nanoparticles containing CpG have shown better immunotherapeutic activity than with free CpG following systemic administration, owing to the 2 aforementioned effects.20-23 For example, Liu et al. 23 reported a diacyl lipid-conjugated CpG with strong binding to albumin. They observed significant retention of lipo-CpG in both inguinal and axillary lymph nodes after subcutaneous injection. Compared to CpG, lipid-CpG was associated with a greatly reduced systemic inflammatory response related to free CpG after repeated injection. We observed significant activation of NK cells in tumors at 6 h and 5 days after intratumoral injection of either CpG or PG-CpG. The treatment-induced changes in percentage of CD69+ NK cells could reflect in situ activation in short-term (6 h), new recruitment in long-term (5 days), or both. Further studies are needed to clarify this issue. The observed increase of percentage of CD69+ NK cells in PBS- and PG-treated tumors on day 5 compared to 6 h time point could well be caused by the slow recruitment of cells into solid tumor mass. It is noted that the changes in percentage of CD69+ NK cells after PBS or PG treatments were not observed in the circulation as there was no change in the proportion of CD69+ NK cells on day 5 in the spleen. Only CpG induced systemic activation of NK cells in the spleen at 5 days after injection (Fig. 3C).
So far, several CpG-containing nanoparticles delivered by the subcutaneous route has been tested and shown to result in significantly enhanced immunostimulatory and antitumor activities compared to free CpG in animal models of melanoma.8, 24-26 We used noninvasive imaging to evaluate mechanisms underlying enhanced immunostimulatory and antitumor activities of PG-CpG and found both increased and prolonged accumulation of PG-CpG in the tumor and draining lymph nodes. When CpG nanoparticles are delivered subcutaneously, co-incorporation of tumor-associated antigens into the CpG nanoparticles is often required in order to induce tumor-specific cytotoxic T-lymphocyte response.8 Intratumoral administration may allow for the TLR9 activation and signaling to occur in the presence of or close to the source of tumor-associated antigens (the tumor itself). In our previous study, direct intratumoral injection of free CpG showed promise in an animal model of melanoma.3 Moreover, intratumoral injection of a PG-CpG conjugate should restrict immune activation in the tumor, which may avoid systemic exhaustion of pDCs observed in previous studies.4-7 Intratumoral injection of PG-CpG, therefore, may enhance antigen-specific antitumor responses without the deleterious effects of free CpG.
PG is a biocompatible, biodegradable synthetic polymer composed of naturally occurring amino acid, L-glutamic acid. A drug delivery system based on PG, PG-paclitaxel, has advanced into clinical phase III studies. Advancing to such trials means that rigorous safety studies have been performed, including studies of long-term toxicity. Other nanoparticle carriers, such as carbon nanotubes, have been the subject of intense investigation including for the delivery of CpG but have not undergone such clinical evaluation.27 Our current study shows efficacy of PG-CpG conjugate against a well-established solid tumor, which has not been observed with some other nanoparticle-CpG cancer models.8, 28 It is thought that nanoparticles with a hydrodynamic diameter of 5-40 nm are suitable for sentinel lymph node targeting after subcutaneous injection.29, 30 The hydrodynamic volume of PG-conjugated contrast agents is 40-50 nm.15 Taking together, PG polymer appears to be a suitable carrier for the delivery of CpG to tumor and draining lymph nodes and for localized T-cell priming. Future work on the use of PG-CpG in combination with tumor antigen for local delivery as an adjuvant vaccination strategy or to potentiate the abscopal effect of other localized antitumor therapies is warranted.
Supplementary Material
Acknowledgments
We thank Stephanie P. Deming for editing this manuscript and Qizhen Cao for helping flow cytometry analysis. This work was supported in part by the John S. Dunn, Sr. Distinguished Chair in Diagnostic Imaging, National Institutes of Health grant AI079232 (DZ), and Natural Science Foundation of China grant 81570007 (DZ). ESD was supported by National Cancer Institute training grant T32CA009598. The Small Animal Imaging Facility is supported by a Cancer Center Support Grant from the National Institutes of Health to The University of Texas MD Anderson Cancer Center (P30CA016672).
Sources of funding: This work was supported in part by the John S. Dunn, Sr. Distinguished Chair in Diagnostic Imaging, National Institutes of Health grant AI079232 (DZ), and Natural Science Foundation of China grant 81570007 (DZ). ESD was supported by National Cancer Institute training grant T32CA009598. The Small Animal Imaging Facility is supported by a Cancer Center Support Grant from the National Institutes of Health to The University of Texas MD Anderson Cancer Center (P30CA016672).
Footnotes
Conflicts of interest: D.Z. is President of NanoCruise Pharmaceutical Suzhou Ltd., a consultant for BioTex, Houston, Texas, and an inventor involved in patents related to technologies mentioned in this study, issued or in application. C.L is a co-inventor involved in patents related to technologies mentioned in this study, issued or in application.
References
- 1.Colonna M. TLR pathways and IFN-regulatory factors: to each its own. Eur J Immunol. 2007;37:306–309. doi: 10.1002/eji.200637009. [DOI] [PubMed] [Google Scholar]
- 2.Vollmer J. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin Biol Ther. 2005;5:673–682. doi: 10.1517/14712598.5.5.673. [DOI] [PubMed] [Google Scholar]
- 3.Lou Y, Liu C, Lizee G, et al. Antitumor activity mediated by CpG: the route of administration is critical. J Immunother. 2011;34:279–288. doi: 10.1097/CJI.0b013e31820d2a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 5.Yu RZ, Geary RS, Leeds JM, et al. Comparison of pharmacokinetics and tissue disposition of an antisense phosphorothioate oligonucleotide targeting human Ha-ras mRNA in mouse and monkey. J Pharm Sci. 2001;90:182–193. doi: 10.1002/1520-6017(200102)90:2<182::aid-jps9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 8.Bourquin C, Anz D, Zwiorek K, et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol. 2008;181:2990–2998. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 9.Heikenwalder M, Polymenidou M, Junt T, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Newman RA, Wu QP, et al. Biodistribution of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in mice with ovarian OCa-1 tumor. Cancer Chemother Pharmacol. 2000;46:416–422. doi: 10.1007/s002800000168. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Price JE, Milas L, et al. Antitumor activity of poly(L-glutamic acid)-paclitaxel on syngeneic and xenografted tumors. Clin Cancer Res. 1999;5:891–897. [PubMed] [Google Scholar]
- 12.Li C, Yu DF, Newman RA, et al. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58:2404–2409. [PubMed] [Google Scholar]
- 13.Boddy AV, Plummer ER, Todd R, et al. A phase I and pharmacokinetic study of paclitaxel poliglumex (XYOTAX), investigating both 3-weekly and 2-weekly schedules. Clin Cancer Res. 2005;11:7834–7840. doi: 10.1158/1078-0432.CCR-05-0803. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev. 2008;60:886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melancon MP, Wang Y, Wen X, et al. Development of a macromolecular dual-modality MR-optical imaging for sentinel lymph node mapping. Invest Radiol. 2007;42:569–578. doi: 10.1097/RLI.0b013e31804f5a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melancon MP, Wang W, Wang Y, et al. A novel method for imaging in vivo degradation of poly(L-glutamic acid), a biodegradable drug carrier. Pharm Res. 2007;24:1217–1224. doi: 10.1007/s11095-007-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaffer SA, Baker-Lee C, Kennedy J, et al. In vitro and in vivo metabolism of paclitaxel poliglumex: identification of metabolites and active proteases. Cancer Chemother Pharmacol. 2007;59:537–548. doi: 10.1007/s00280-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 18.Wen X, Jackson EF, Price RE, et al. Synthesis and characterization of poly(L-glutamic acid) gadolinium chelate: a new biodegradable MRI contrast agent. Bioconjug Chem. 2004;15:1408–1415. doi: 10.1021/bc049910m. [DOI] [PubMed] [Google Scholar]
- 19.Melancon MP, Lu W, Huang Q, et al. Targeted imaging of tumor-associated M2 macrophages using a macromolecular contrast agent PG-Gd-NIR813. Biomaterials. 2010;31:6567–6573. doi: 10.1016/j.biomaterials.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmore MM, Li S, Falo L, Jr, Huang L. Systemic administration of LPD prepared with CpG oligonucleotides inhibits the growth of established pulmonary metastases by stimulating innate and acquired antitumor immune responses. Cancer Immunol Immunother. 2001;50:503–514. doi: 10.1007/s002620100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai F, Terada T, Maruyama M, et al. Therapeutic effect of intravenous delivery of lipoplexes containing the interferon-[beta] gene and poly I: poly C in a murine lung metastasis model. Cancer Gene Ther. 10:661–668. doi: 10.1038/sj.cgt.7700617. [DOI] [PubMed] [Google Scholar]
- 22.Higgins R, McKisic M, Dickinson P, et al. Growth inhibition of an orthotopic glioblastoma in immunocompetent mice by cationic lipid-DNA complexes. Cancer Immunol Immunother. 2004;53:338–344. doi: 10.1007/s00262-003-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Moynihan KD, Zheng Y, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Jong S, Chikh G, Sekirov L, et al. Encapsulation in liposomal nanoparticles enhances the immunostimulatory, adjuvant and anti-tumor activity of subcutaneously administered CpG ODN. Cancer Immunol Immunother. 2007;56:1251–1264. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Standley SM, Mende I, Goh SL, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18:77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 26.Li WM, Bally MB, Schutze-Redelmeier MP. Enhanced immune response to T-independent antigen by using CpG oligodeoxynucleotides encapsulated in liposomes. Vaccine. 2001;20:148–157. doi: 10.1016/s0264-410x(01)00277-8. [DOI] [PubMed] [Google Scholar]
- 27.Fan H, Zhang I, Chen X, et al. Intracerebral CpG immunotherapy with carbon nanotubes abrogates growth of subcutaneous melanomas in mice. Clin Cancer Res. 2012;18:5628–5638. doi: 10.1158/1078-0432.CCR-12-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin AY, Mattos Almeida JP, Bear A, et al. Gold nanoparticle delivery of modified CpG stimulates macrophages and inhibits tumor growth for enhanced immunotherapy. PLoS One. 2013;8:e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghimi SM, Bonnemain B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv Drug Deliv Rev. 1999;37:295–312. doi: 10.1016/s0169-409x(98)00099-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.