Abstract
Purpose
We explored the influence of FACBC (fluciclovine) positron emission tomography-computed tomography (PET-CT) on the decision to offer radiotherapy and radiotherapy treatment field recommendations in post-prostatectomy patients with recurrent prostate cancer.
Materials and Methods
Following institutional review board approval and informed consent, 87 patients with detectable prostate specific antigen (PSA) were recruited into a prospective clinical trial. Following an initial provider-determined radiotherapy plan based on conventional imaging, 44/87 patients were randomized to additionally undergo fluciclovine PET-CT. Pre- and post-fluciclovine radiotherapy decisions were compared and changes noted. Statistical significance of these decision changes was determined.
Results
2/44 patients in the experimental arm dropped out before fluciclovine scanning. 34/42 (81.0%) had positive results on fluciclovine. Overall radiotherapy decision was changed in 17/42 (40.5%). Mean PSA, original Gleason score (GS), and prostatectomy-PET interval did not differ significantly between patients with and without radiotherapy decision changes.
2/42 (4.8%) had the decision for radiotherapy withdrawn due to positive extra-pelvic findings. Radiotherapy field decision was changed in 15/42 (35.7%). 11/15 (73.3%) had fields changed from prostate bed only to both prostate bed and pelvis, while 4/15 (26.7%) had fields changed from both prostate bed and pelvis to prostate bed only. Changes in overall radiotherapy decision and field were statistically significant (P<0.0001). However, the change in the decision to offer radiotherapy or not was not statistically significant (P=0.15).
Conclusion
Fluciclovine PET-CT significantly changed radiotherapy management decisions in post-prostatectomy patients with recurrent prostate cancer. Further work in determining differences in PSA free survival is ongoing.
Keywords: FACBC, fluciclovine, prostate cancer, CT, PET, radiotherapy
INTRODUCTION
Approximately one-fifth of prostate cancer patients have a recurrence within an average of 5 – 6 years after prostatectomy [1–3]. Salvage radiotherapy, which is often performed for biochemical failure is fraught with its own challenges, having PSA progression-free rates ranging from 18 to 69% within 4 – 7 years of the procedure [4, 5]. Androgen Deprivation Therapy is another treatment modality in the management of prostate cancer recurrence [6, 7]. Although salvage lymph node dissection is sometimes employed, this is not the routine standard of care [6–8].
The high recurrence rate may be due to the unsatisfactory performance of conventional imaging techniques in disease localization and definition of disease extent which may account for suboptimal patient selection for salvage radiotherapy [9, 10]. Thus, other imaging techniques, including multiparametric Magnetic Resonance Imaging (MRI) and PET radiotracers which image molecular pathways and receptors including those of acetate, choline, and prostate specific membrane antigen (PSMA) among others are being explored to better inform patient management [11–14].
Anti-1-amino-3-[18F] fluorocyclobutane-1-carboxylic acid (FACBC or fluciclovine) is a fluorinated synthetic amino acid PET radiotracer transported primarily by the amino acid transporters ASCT2 and LAT1, which are overexpressed in prostate cancer [15–18]. The diagnostic performance of fluciclovine in recurrent prostate cancer has compared favorably to conventional imaging with CT and to radiotracers such as 111In-Capromab Pendetide and 11C-Choline [19–21].
Recent case reports have demonstrated the feasibility of fluciclovine use in planning salvage radiotherapy [22, 23]. Thus we explored this concept more formally via an intention-to-treat clinical trial in which post-prostatectomy patients with biochemical failure are randomized to undergo fluciclovine PET-CT after conventional radiotherapy planning. This report is a planned secondary analysis aimed at determining changes in management, specifically the decisions to offer radiotherapy and design of radiotherapy fields following fluciclovine PET compared to the initial decision based solely on conventional imaging.
MATERIALS AND METHODS
Patient Selection
This is a prospective randomized Health Insurance Portability and Accountability Act compliant clinical trial conducted after institutional review board approval (ClinicalTrials.Gov ID:NCT01666808) with arms A and B both consisting of patients with PSA failure after radical prostatectomy for prostate cancer with no evidence of extra-pelvic metastasis on bone scan, CT or MRI. Inclusion criteria included age ≥ eighteen years, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and post-prostatectomy status with detectable PSA levels. Written informed consent was obtained for all patients. Radiotherapy decision for patients in arm A was based on clinical history, pathology findings, PSA trajectory, and conventional imaging only: CT or MRI and bone scan. Patients in Arm B (experimental) underwent fluciclovine PET-CT scan following an initial radiotherapy decision based on conventional imaging. Radiotherapy decision in this arm was thereafter modified based on fluciclovine scan results.
Fluciclovine PET-CT Imaging
Fluciclovine was prepared as earlier reported [21, 24]. Patients were scanned on a GE Discovery MV690 PET-CT scanner after at least 4 hours of fasting to normalize amino acid levels. An initial abdominopelvic CT scan was conducted with administration of oral contrast only at 80–120 mA and 120 kVp. Following this, 8.6 – 10.78 millicuries (318.2 – 398.86 megabecquerel) of fluciclovine was injected intravenously over 2 minutes, with a 3-minute blood pool clearance window after which 5–15.5 minute and 16–27.5 minute images extending from the pelvis to the diaphragm were acquired and data transferred to a MIMVista workstation (MIM Software; Cleveland, OH) for analysis.
Image Analysis
Image interpretation was done independently by two board certified nuclear medicine physicians. A consensus was reached by both readers on discordant image interpretations. As previously reported, positivity criteria on fluciclovine PET included persistent nonphysiologic moderate (greater than marrow) focal uptake in prostate bed, lymph nodes or bone [17].
Decision Criteria
For the purpose of this study, the prostate bed was defined using the Radiation Therapy Oncology Group (RTOG) contouring guidelines [25]. Pre-fluciclovine radiotherapy decision was based on results of conventional imaging using well recognized clinical criteria [26, 27] and an intention to treat form was populated by the treating radiation oncologist.
Post-Fluciclovine decision regarding offering radiotherapy and field of radiotherapy was based on imaging findings on fluciclovine PET-CT as follows:
a) Patients with no uptake or uptake in the prostate bed only were offered radiotherapy to prostate bed only; b) Patients with uptake in pelvic nodes or pN1 (regional node involvement) were offered standard fields to the prostate bed and pelvic nodes; c) The decision to give radiotherapy was withdrawn in patients with extra-pelvic uptake and these patients offered systemic therapy.
Statistical Analysis
Clopper-Pearson (exact) binomial method was used to calculate the statistical significance of the overall decision changes. These include changes in pre- and post- fluciclovine treatment plans regarding (a) the decision to offer radiotherapy or not b) the field of radiotherapy i.e. treat prostate bed only or include pelvic nodes and c) overall radiotherapy decision were evaluated. Two-sample T-test was used to calculate the statistical significance of differences in PSA levels, Gleason scores and prostatectomy-fluciclovine interval across both the group that had decision changes and the group that did not. P-values less than 0.05 were regarded as statistically significant. Statistical Analysis Software (SAS Version 9.3 SAS Institute Inc. Cary, NC, USA) and Microsoft Excel 2010 were utilized in data analysis.
RESULTS
Demographics
87 patients who met the inclusion criteria were recruited into the study. 44 of these were randomized into arm B but since 2 of these patients did not undergo fluciclovine PET, 42 patients were analyzed in this arm. Full demographic details are provided in Table 1. All patients had undergone prostatectomy an average (±SD, range) of 2.5 (±2.95, 0–11) years prior to fluciclovine. The mean PSA (±SD, range) was 2.09 (±2.96, 0.07 – 11.15) ng/ml.
Table 1.
Demographics Characteristics of Study Participants (n=42)
| Age (years): | |
| Mean ± SD | 62 ± 7.54 |
| Median (range) | 61.5 (42–75) |
| Q1, Q3 | 57.0, 68.0 |
| PSA (ng/ml): | |
| Mean ± SD | 2.1 ± 2.96 |
| Median (range) | 0.55 (0.07 – 11.15) |
| Q1, Q3 | 0.24, 2.05 |
| Original Gleason Score: | |
| Mean ± SD | 7 ± 0.98 |
| Median (range) | 7 (6 – 10) |
| Q1, Q3 | 7, 8 |
| Gleason Score breakdown n (%) | |
| GS ≤ 3+4 (Grade groups 1, 2) | 18 (42.9) |
| GS ≥ 4+3 (Grade groups 3, 4, 5) | 24 (57.1) |
| Prostatectomy-FACBC Interval (years) | |
| Mean ± SD | 2.5 ± 2.95 |
| Median (range) | 1 (0 – 11) |
| Q1, Q3 | 0,4 |
| Fluciclovine Dose (mCi): | |
| Mean ± SD | 10.03 ± 0.32 |
| Median (range) | 10.01 (8.6 – 10.78) |
| Q1, Q3 | 9.90, 10.15 |
| Positive Fluciclovine Scan n(%): | |
| Total | 34/42 (81.0) |
| Prostate bed only | 12/34 (35.29) |
| Extra-pelvic regions | 2/34 (5.88) |
| Prostate + extraprostatic regions | 20/34 (58.82) |
| Fluciclovine Detection Rate n (%) | |
| PSA <1 ng/ml | 18/25 (72.0) |
| PSA 1–<2 ng/ml | 5/6(83.3) |
| PSA ≥2 ng/ml | 11/11(100) |
Fluciclovine positivity rates
On whole body basis, 34/42 (81.0%) patients had positive findings on fluciclovine PET. Detection rates varied with PSA values and improved as PSA increased (Table 1). 12/34 (35.3%) were positive in the prostate bed only and 20/34 (58.8%) in both bed and pelvis. Despite having no evidence of extrapelvic disease on conventional imaging, 2/34 (5.9%) had fluciclovine uptake in extra-pelvic regions (Table 1). Of the two who had extra-pelvic uptake, one had uptake in the iliac crest and prostate bed; while the other had uptake in retroperitoneal, paraaortic, prevertebral and pelvic lymph nodes.
26 of the 42 patients who underwent fluciclovine scans were initially planned for radiotherapy to the prostate bed only (Figure 1). Fluciclovine was positive in the prostate only in 9/26 (34.6%) and in both the prostate and pelvic regions in 11/26 (42.3%). No disease was identified in 6/26 (23.1%) of the patients.
Figure 1.
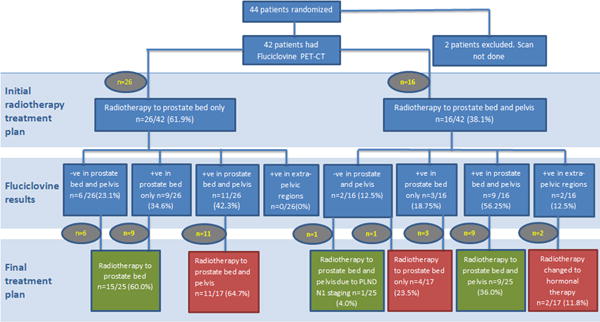
Study Flow Diagram Showing Pre- and Post-Fluciclovine Radiotherapy Decisions
PLND–Pelvic Lymph Node Dissection
Red Boxes–Decision Change; Green–No Decision Change
9 of the 16 patients (56.25%) initially planned for radiotherapy to both the prostate bed and pelvis had positive fluciclovine findings in both prostate and pelvis while 3/16 (18.75%) had positive findings in only the prostate and 2/16 (12.5%) were positive in extra-pelvic regions (Figure 1). No disease was identified in 2/16 (12.5%) of the patients.
Radiotherapy Decision Change
All 42 study participants who underwent fluciclovine PET were initially planned for radiotherapy. As a result of fluciclovine findings, radiotherapy decisions were changed in 17/42 (40.5%) patients. There was no significant difference in prognostic factors between patients with and without pre and post fluciclovine changes in radiotherapy decisions (Table 2).
Table 2.
Test of Significance of Difference in Prognostic Factors Across Categories
| Prognostic Factor | Decision Change (n=17) (Mean±SD) |
No Decision Change (n=25) (Mean±SD) |
P-Value |
|---|---|---|---|
| PSA (ng/ml) | 2.09±3.04 | 2.09±2.92 | 0.9973 |
| <1 | 0.42±0.28 | 0.30±0.22 | 0.2790 |
| 1–<2 | 1.30±0.40 | 1.61±0.22 | 0.3197 |
| ≥2 | 5.56±3.48 | 7.11±1.86 | 0.4051 |
| Gleason Score | 7.24±0.97 | 7.36±1.00 | 0.6882 |
| GS ≤ 3+4 | 6.71±0.49 | 6.90±0.88 | 0.5855 |
| GS ≥ 4+3 | 7.60±1.08 | 7.67±0.98 | 0.8766 |
| Prostatectomy-Fluciclovine Interval (yrs) | 2.68±2.72 | 2.24±3.33 | 0.6509 |
| ≤2 | 0.50±0.80 | 0.80±0.78 | 0.3356 |
| >2 | 5.50±2.01 | 6.40±3.44 | 0.6116 |
2/42 (4.8%) patients had radiotherapy decisions withdrawn as fluciclovine found evidence of extra-pelvic disease. Figure 2 is an example of fluciclovine uptake in small retroperitoneal nodes in a patient earlier planned for radiotherapy to both prostate bed and lymph nodes but whose treatment was changed to hormonal therapy based on fluciclovine results.
Figure 2. Extrapelvic uptake on fluciclovine scan.
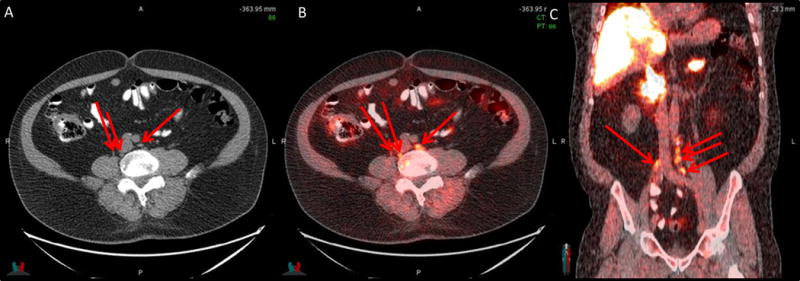
Retroperitoneal lymph node - 0.5 – 1cm (arrows) uptake on CT (A), PET/CT (B) and PET/CT coronal plane (C) in a 72-year-old earlier planned for radiotherapy to prostate bed and pelvis. Radiotherapy decision was withdrawn following FACBC and hormonal therapy offered (PSA 3.46 ng/ml)
Of the remaining 40 patients who were still to undergo radiotherapy, 15/40 (37.5%) had radiotherapy fields changed. 11/15 (73.3%) of these had fields increased from the prostate bed only to both the prostate and pelvis, while 4/15 (26.7%) had fields reduced from both the prostate and pelvis to the prostate bed only. Despite having a negative fluciclovine scan in the prostate bed and pelvis, one patient had radiotherapy to both prostate bed and pelvic lymph nodes per accepted clinical criteria as patient had been staged pN1 based on pelvic nodal dissection which had removed the metastatic node prior to fluciclovine imaging. Figure 3 shows a positive left internal iliac lymph node seen on fluciclovine in a patient earlier planned for radiotherapy to the prostate bed only. Radiotherapy decision was then modified to include both bed and lymph nodes. Fluciclovine PET-CT also demonstrated bilateral seminal vesicle uptake in a patient earlier planned for radiotherapy to the prostate bed and lymph nodes and is an example of radiotherapy field change to prostate bed only which includes the seminal vesicles (Figure 4).
Figure 3. Pelvic uptake on fluciclovine scan.
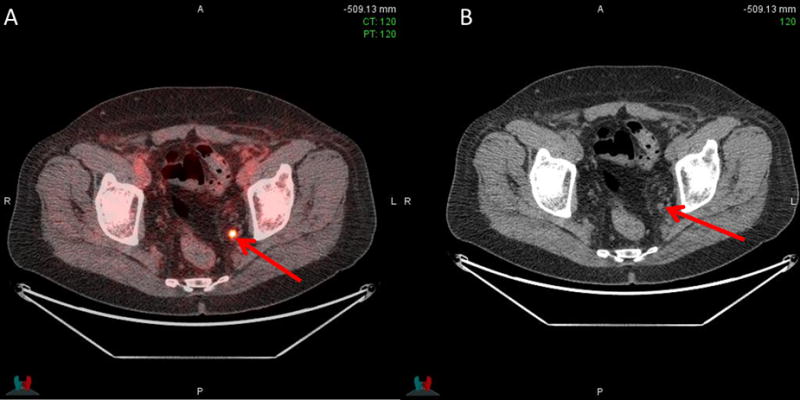
Positive left internal iliac lymph node −0.5 × 0.5 cm (arrows) on PET/CT (A) and CT (B) in a 66-year old earlier planned for radiotherapy to prostate bed only. Radiotherapy decision was thereafter changed to radiotherapy to prostate bed and pelvis (PSA 0.19 ng/ml)
Figure 4. Prostate bed uptake on fluciclovine scan.
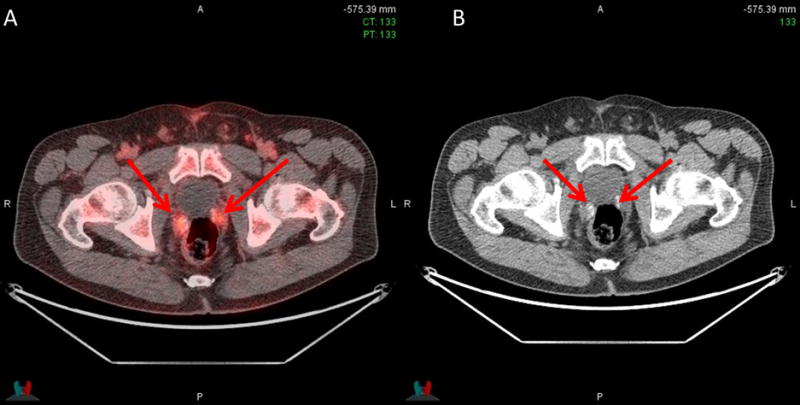
Uptake in right and left seminal vesicles (arrows) on PET/CT (A) and CT (B) in a 60-year-old earlier planned for radiotherapy to prostate bed and pelvis only. Radiotherapy decision was thereafter changed to radiotherapy to prostate bed only (PSA 1.1 ng/ml)
Changes in radiotherapy field and overall radiotherapy decision were both statistically significant (Table 3). However change in the decision to offer radiotherapy or not was not statistically significant as only 2 of the patients had a final decision of no radiotherapy.
Table 3.
Influence of FACBC on Radiotherapy Treatment and Treatment Field Recommendations
| Post-Fluciclovine Decision | Decision Change n (%) | P-Value* | |||
|---|---|---|---|---|---|
| Pre-Fluciclovine Decision | |||||
| Radiotherapy Decision (n=42) | Offer XRT | No XRT | |||
| Offer XRT | 40 | 2** | 2 (4.8) | P = 0.15 | |
| No XRT | 0 | 0 | 0 (0) | ||
| Radiotherapy Field (n=40)*** | Prostate only | Prostate + Pelvis | |||
| Prostate only (n=26) | 15 | 11** | 11 (27.5) | P<0.0001 | |
| Prostate + Pelvis (n=14) | 4** | 10 | 4 (10.0) | ||
| Overall XRT Decision (n=42) | Prostate | Prostate + Pelvis | No XRT | ||
| XRT to prostate | 15 | 11** | 0 | 11(26.2) | P<0.0001 |
| XRT to prostate + pelvis | 4** | 10 | 2** | 6 (14.3) | |
| No XRT | 0 | 0 | 0 | 0 (0) | |
Statistical significance of overall decision changes calculated using Clopper-Pearson (exact) binomial method
Decision Change
2 patients excluded as the final decision was not to give radiotherapy XRT Radiotherapy
DISCUSSION
In this study we set out to determine the role of advanced imaging with fluciclovine in the management of post prostatectomy patients with PSA failure being considered for salvage radiotherapy. Our results show that imaging with fluciclovine significantly altered the radiotherapy treatment decision in these patients. 40.5% (17/42) of our patients had a change in radiotherapy decision following fluciclovine PET-CT. 35.7% (15/42) had their radiotherapy fields changed after fluciclovine PET-CT. 4.8% (2/42) were instead offered hormonal therapy due to systemic disease. Data from previous studies have shown that fluciclovine PET-CT is useful in the restaging of prostate cancer patients [28]. However, this study shows the findings on fluciclovine PET could potentially be useful for salvage therapy planning as well, though this would need to be validated by improvement in PSA free survival which is an ultimate endpoint of the trial.
Our findings are important since a wide range of factors including imaging results, PSA, PSA doubling time (PSADT) and Gleason score are considered before the decision to offer and design salvage radiotherapy is made [29–31]. With poor patient selection being a possible contributor to the high biochemical failure rates following salvage radiotherapy [32–34], accurate imaging to improve patient selection is critical. Although radioimmunoscintigraphy against PSMA has been studied [35–37], current treatment planning mostly relies on conventional imaging which may be unrevealing in the initial stages of prostate cancer recurrence [10, 11, 21, 38, 39]. Therefore, molecular imaging techniques such as fluciclovine which have shown promise in more accurately restaging prostate cancer patients may potentially prove useful in salvage radiotherapy selection and planning by reducing over- or under-treatment of these patients.
Our findings are comparable to other studies utilizing PET techniques in assessing the influence of molecular imaging in treatment decisions regarding recurrent prostate cancer management. In a study on the use of 11C-choline PET/CT in treatment planning in recurrent prostate cancer, 13% of the 37 patients in the study had modifications to the earlier planned radiotherapy field compared to 35.7% in our own study [40]. In another study involving 150 patients, 95 of who were planned for salvage radiotherapy, 11C-choline PET/CT resulted in therapy adjustment in 33.7% of patients earlier planned for salvage radiotherapy and change to another form of therapy in 13.7%. [41]. 18F-fluorocholine PET-CT also influenced treatment decisions in patients with recurrent prostate cancer and resulted in a change in earlier planned therapy in 48% of the 156 patients in the study [42]. Radiotherapy decision management following prostate-specific membrane antigen (PSMA) PET-CT imaging in prostate cancer patients has also been studied with a reported radiotherapy change in 46.3 – 54.8% of patients following PSMA PET-CT [43, 44]. Sterzing et al also found a similar (50.8%) change in radiotherapy approach in 29 of 57 patients in their study, 25 of whom were post-prostatectomy [45].
Overall in our study, fluciclovine demonstrated areas of positivity in 34/42 (81.0%) patients. Detection rates varied with PSA values and generally improved as PSA increased with values of 72.0%, 83.3% and 100% at PSA levels <1, 1–<2 and ≥2 ng/ml respectively. The trend of improved detection rates with increasing PSA is similar to that seen with other radiotracers [46, 47]. Yet further study is required since in a study from the Bologna group with a similar population of post-prostatectomy patients, the reported detection rate for fluciclovine was 21% with PSA <1ng/ml [19]. We have also described a detection rate of 39.0% at PSA values <1ng/ml in an earlier study on a mixed post-therapy population [48], and have more recently reported that detection rate increases with shorter doubling times [20]. Interestingly, post hoc analysis of the PSA <1 ng/ml cohort in each of these studies demonstrates a shorter doubling time mean±SD (4.4±13.0 months) in this study corresponding to the higher detection rate versus a longer doubling time (13.9±19.9 months) in the earlier report corresponding to a lower detection rate.
Studies assessing the detection rate of 11C-choline PET-CT in patients with recurrent prostate cancer have yielded varying range of values with rates of 36%, 43%, 62% and 73% at PSA <1, 1–<2, 2 – <3 and ≥3 ng/ml respectively reported in one study [49]. In another study, rates of 19%, 25%, 41% and 67% at PSA levels of ≤1, >1 – ≤2, >2 – ≤5 and > 5 ng/ml respectively were reported with the same radiotracer [50]. 68Ga-PSMA PET-CT is in an earlier stage of development but has reported high sensitivity with detection rates of 57.9%, 72.7%, 93.0% and 96.8% at PSA levels of 0.2 to <0.5, 0.5 to < 1, 1 to <2 and ≥2 ng/ml respectively [51].
The current study has certain limitations: 1) The results are those of a planned analysis of a secondary endpoint of the study. As such, the trial has not reached its final accrual goal (which would involve 81 patients each on the control and fluciclovine arms). However, the strong statistical significance of the decision changes on the current/interim sample is encouraging; 2) Though pre- and post-fluciclovine decisions were captured, the pre-fluciclovine radiotherapy decisions were based on a constellation of items including patient history, prostatectomy pathology findings and PSA trajectory. However, the study does span several radiotherapy providers so the pre-fluciclovine decisions are likely to be a representative cross section of those made in the prostate radiotherapy community.
In conclusion, the decision to offer radiotherapy in recurrent prostate cancer can be challenging. Careful consideration and proper patient selection is essential to ensure favorable outcomes [5, 32, 34, 52]. Use of fluciclovine PET-CT in patients being considered for salvage radiotherapy has demonstrated in this interim analysis a 40.5% overall change in therapy decision, with 4.8% having radiotherapy withdrawn and 35.7% having a change in radiotherapy fields. Further study is ongoing including a determination of whether fluciclovine guided treatment modulation will result in improved outcomes.
Footnotes
Conflicts of Interest Disclosure Statement: Sponsored by the National Institutes of Health (R01CA169188). Blue Earth Diagnostics Ltd provided fluciclovine cassettes to Emory University
References
- 1.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Zahurak M, et al. Biochemical (Prostate Specific Antigen) Recurrence Probability Following Radical Prostatectomy for Clinically Localized Prostate Cancer. The Journal of Urology. 2003;169:517–523. doi: 10.1097/01.ju.0000045749.90353.c7. [DOI] [PubMed] [Google Scholar]
- 3.Caire AA, Sun L, Ode O, et al. Delayed Prostate-specific Antigen Recurrence After Radical Prostatectomy: How to Identify and What Are Their Clinical Outcomes? Urology. 2009;74:643–647. doi: 10.1016/j.urology.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Sia M, Pickles T, Morton G, et al. Salvage radiotherapy following biochemical relapse after radical prostatectomy: proceedings of the Genito-Urinary Radiation Oncologists of Canada consensus meeting. Canadian Urological Association Journal. 2008;2:500–507. doi: 10.5489/cuaj.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geinitz H, Riegel MG, Thamm R, et al. Outcome After Conformal Salvage Radiotherapy in Patients With Rising Prostate-Specific Antigen Levels After Radical Prostatectomy. International Journal of Radiation Oncology, Biology, Physics. 2012;82:1930–1937. doi: 10.1016/j.ijrobp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Antonarakis ES, Zahurak ML, Lin J, et al. Changes in PSA Kinetics Predict Metastasis-Free Survival in Men with PSA-Recurrent Prostate Cancer Treated with Non-Hormonal Agents: Combined Analysis of 4 Phase II Trials. Cancer. 2012;118:1533–1542. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suardi N, Gandaglia G, Gallina A, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015;67:299–309. doi: 10.1016/j.eururo.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Karnes RJ, Murphy CR, Bergstralh EJ, et al. Salvage Lymph Node Dissection for Prostate Cancer Nodal Recurrence Detected by 11C-Choline Positron Emission Tomography/Computerized Tomography. The Journal of Urology. 2015;193:111–116. doi: 10.1016/j.juro.2014.08.082. [DOI] [PubMed] [Google Scholar]
- 9.Zukotynski K, Haider MA. Imaging in Prostate Carcinoma. Hematology/Oncology Clinics of North America. 2013;27:1163–1187. doi: 10.1016/j.hoc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Jackson ASN, Reinsberg SA, Sohaib SA, et al. Dynamic contrast-enhanced MRI for prostate cancer localization. The British Journal of Radiology. 2009;82:148–156. doi: 10.1259/bjr/89518905. [DOI] [PubMed] [Google Scholar]
- 11.Hong H, Zhang Y, Sun J, et al. Positron emission tomography imaging of prostate cancer. Amino acids. 2010;39:11–27. doi: 10.1007/s00726-009-0394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husarik DB, Miralbell R, Dubs M, et al. Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253–263. doi: 10.1007/s00259-007-0552-9. [DOI] [PubMed] [Google Scholar]
- 13.Schuster DM, Votaw JR, Nieh PT, et al. Initial Experience with the Radiotracer Anti-1-Amino-3–18F-Fluorocyclobutane-1-Carboxylic Acid with PET/CT in Prostate Carcinoma. Journal of Nuclear Medicine. 2007;48:56–63. [PubMed] [Google Scholar]
- 14.Johnson LM, Turkbey B, Figg WD, et al. Multiparametric MRI in prostate cancer management. Nat Rev Clin Oncol. 2014;11:346–353. doi: 10.1038/nrclinonc.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka S, Okudaira H, Yoshida Y, et al. Transport mechanisms of trans-1-amino-3-fluoro[1–14C]cyclobutanecarboxylic acid in prostate cancer cells. Nuclear Medicine and Biology. 2012;39:109–119. doi: 10.1016/j.nucmedbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Okudaira H, Shikano N, Nishii R, et al. Putative transport mechanism and intracellular fate of trans-1-amino-3–18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med. 2011;52:822–829. doi: 10.2967/jnumed.110.086074. [DOI] [PubMed] [Google Scholar]
- 17.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Tiffen J, Bailey CG, et al. Targeting Amino Acid Transport in Metastatic Castration-Resistant Prostate Cancer: Effects on Cell Cycle, Cell Growth, and Tumor Development. Journal of the National Cancer Institute. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 19.Nanni C, Zanoni L, Pultrone C. 18F-FACBC (anti1-amino-3-F-fluorocyclobutane-1-carboxylic acid) versus C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3329-1. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016 doi: 10.1007/s00259-016-3383-8. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[18F]FACBC Positron Emission Tomography-Computerized Tomography and 111In-Capromab Pendetide Single Photon Emission Computerized Tomography-Computerized Tomography for Recurrent Prostate Carcinoma: Results of a Prospective Clinical Trial. The Journal of Urology. 2014;191:1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunocilla E, Schiavina R, Nanni C, et al. First case of 18F-FACBC PET/CT-guided salvage radiotherapy for local relapse after radical prostatectomy with negative 11C-Choline PET/CT and multiparametric MRI: New imaging techniques may improve patient selection. Arch Ital Urol Androl. 2014;86:239–240. doi: 10.4081/aiua.2014.3.239. [DOI] [PubMed] [Google Scholar]
- 23.Jani AB, Fox TH, Whitaker D, et al. Case study of anti-1-amino-3-F-18 fluorocyclobutane-1-carboxylic acid (anti-[F-18] FACBC) to guide prostate cancer radiotherapy target design. Clin Nucl Med. 2009;34:279–284. doi: 10.1097/RLU.0b013e31819e51e3. [DOI] [PubMed] [Google Scholar]
- 24.McConathy J, Voll RJ, Yu W, et al. Improved synthesis of anti-[18F]FACBC: improved preparation of labeling precursor and automated radiosynthesis. Applied Radiation and Isotopes. 2003;58:657–666. doi: 10.1016/s0969-8043(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 25.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic Normal Tissue Contouring Guidelines for Radiation Therapy: A Radiation Therapy Oncology Group Consensus Panel Atlas. International journal of radiation oncology, biology, physics. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Wahab M, Mahmoud O, Merrick G, et al. ACR Appropriateness Criteria(R) external-beam radiation therapy treatment planning for clinically localized prostate cancer. J Am Coll Radiol. 2012;9:233–238. doi: 10.1016/j.jacr.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Kairemo K, Rasulova N, Partanen K, et al. Preliminary Clinical Experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT Imaging in Prostate Cancer Patients. BioMed Research International. 2014;2014:305182. doi: 10.1155/2014/305182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choo R. Salvage Radiotherapy for Patients with PSA Relapse Following Radical Prostatectomy: Issues and Challenges. Cancer Research and Treatment: Official Journal of Korean Cancer Association. 2010;42:1–11. doi: 10.4143/crt.2010.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paller CJ, Antonarakis ES. Management of Biochemically Recurrent Prostate Cancer After Local Therapy: Evolving Standards of Care and New Directions. Clinical advances in hematology & oncology: H&O. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 31.Cotter SE, Chen MH, Moul JW, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011;117:3925–3932. doi: 10.1002/cncr.25993. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward JF, Moul JW. Rising prostate-specific antigen after primary prostate cancer therapy. Nat Clin Pract Urol. 2005;2:174–182. doi: 10.1038/ncpuro0145. [DOI] [PubMed] [Google Scholar]
- 34.Moreira DM, Jayachandran J, Presti JC, Jr, et al. Validation of a nomogram to predict disease progression following salvage radiotherapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2009;104:1452–1456. doi: 10.1111/j.1464-410X.2009.08623.x. [DOI] [PubMed] [Google Scholar]
- 35.Jani AB, Blend MJ, Hamilton R, et al. Radioimmunoscintigraphy for postprostatectomy radiotherapy: analysis of toxicity and biochemical control. J Nucl Med. 2004;45:1315–1322. [PubMed] [Google Scholar]
- 36.Jani AB, Blend MJ, Hamilton R, et al. Influence of radioimmunoscintigraphy on postprostatectomy radiotherapy treatment decision making. J Nucl Med. 2004;45:571–578. [PubMed] [Google Scholar]
- 37.Jani AB, Spelbring D, Hamilton R, et al. Impact of radioimmunoscintigraphy on definition of clinical target volume for radiotherapy after prostatectomy. J Nucl Med. 2004;45:238–246. [PubMed] [Google Scholar]
- 38.Izawa JI. Salvage radiotherapy after radical prostatectomy. Canadian Urological Association Journal. 2009;3:245–250. [PMC free article] [PubMed] [Google Scholar]
- 39.Pound CR, Brawer MK, Partin AW. Evaluation and Treatment of Men with Biochemical Prostate-Specific Antigen Recurrence Following Definitive Therapy for Clinically Localized Prostate Cancer. Reviews in Urology. 2001;3:72–84. [PMC free article] [PubMed] [Google Scholar]
- 40.Souvatzoglou M, Krause BJ, Pürschel A, et al. Influence of 11C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiotherapy and Oncology. 2011;99:193–200. doi: 10.1016/j.radonc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Ceci F, Herrmann K, Castellucci P, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41:2222–2231. doi: 10.1007/s00259-014-2872-x. [DOI] [PubMed] [Google Scholar]
- 42.Soyka JD, Muster MA, Schmid DT, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39:936–943. doi: 10.1007/s00259-012-2083-2. [DOI] [PubMed] [Google Scholar]
- 43.Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiation Oncology. 2015;10:1–4. doi: 10.1186/s13014-015-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giesel F, Kratochwil C, Fiedler H, et al. Impact of 68Ga-PSMA-PET-CT to the radiotherapeutic management of prostate cancer patients. Journal of Nuclear Medicine. 2015;56:214. doi: 10.1007/s00259-015-3188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterzing F, Kratochwil C, Fiedler H, et al. (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. doi: 10.1007/s00259-015-3188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzola MC, Chondrogiannis S, Ferretti A, et al. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med. 2013;38:e26–32. doi: 10.1097/RLU.0b013e318266cc38. [DOI] [PubMed] [Google Scholar]
- 47.Bluemel C, Krebs M, Polat B, et al. 68Ga-PSMA-PET/CT in Patients With Biochemical Prostate Cancer Recurrence and Negative 18F-Choline-PET/CT. Clin Nucl Med. 2016;41:515–521. doi: 10.1097/RLU.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odewole O, Taleghani P, Jani AB, et al. Radiological Society of North America 2013 Scientific Assembly and Annual Meeting. Chicago, IL: 2013. Diagnostic Performance of Synthetic Amino Acid Anti-3-[18F] FACBC PET in Recurrent Prostate Carcinoma Detection. Abstract. [Google Scholar]
- 49.Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:18–23. doi: 10.1007/s00259-007-0581-4. [DOI] [PubMed] [Google Scholar]
- 50.Castellucci P, Fuccio C, Nanni C, et al. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–1400. doi: 10.2967/jnumed.108.061507. [DOI] [PubMed] [Google Scholar]
- 51.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 52.Gebhardt BJ, McDonald AM, Bae S, et al. Prognostic factors for salvage radiotherapy with an analysis of post-prostatectomy PSA kinetics. Journal of Solid Tumors. 2013;3:32–43. [Google Scholar]


