Abstract
Purpose
Fuchs dystrophy is the leading indication for corneal transplantation in the United States. A CTG18.1 trinucleotide repeat in TCF4 correlates with increased severity in Fuchs dystrophy; however, quantitative estimates of increased transplantation risk, including effects of age and gender, are unclear.
Methods
In a tertiary institution clinical practice, 574 participants were enrolled in a longitudinal study of Fuchs dystrophy after slit-lamp biomicroscopy confirmed significant central guttae and/or corneal transplantation in both eyes. We documented clinical history, examination findings, and demographic information. We acquired blood samples, extracted DNA and sequenced the CTG18.1 trinucleotide repeat in TCF4. In this retrospective case-control study, the number of participants with triplet expansion, defined as greater than 40 CTG repeats, and transplantation status were assessed. Kaplan-Meier estimates of timing and transplantation events were produced. Cox proportional hazard regression model was utilized to assess for the relationship between age, gender, triplet expansion, and surgery.
Results
A total of 106 participants (18.5%) previously underwent corneal transplantation in at least one eye at the time of initial evaluation. A higher proportion of individuals harboring allele expansion had undergone transplantation (78/357, 21.8%) compared to those without the expanded allele (28/217, 12.9%), a significant association (p=0.007). Log-rank test demonstrates a significant difference in survival function over time (p=0.027), with hazard ratio of 1.64 (95% CI, 1.05 to 2.55).
Conclusions
Expansion of the TCF4 CTG trinucleotide repeat was associated with 1.64 times higher likelihood of corneal transplantation at a given age in patients with Fuchs dystrophy.
Keywords: Fuchs dystrophy, TCF4, corneal transplantation, endothelial keratoplasty
INTRODUCTION
Fuchs corneal dystrophy (FCD) is a hereditary, progressive condition associated with corneal endothelial cell loss and the development of guttae, excrescences of Descemet membrane. The leading cause for corneal transplantation in the United States,1 it affects approximately one out of every 25 Americans above age 40.2
Symptoms begin as blurry morning vision in middle age and loss of contrast sensitivity, eventually resulting in vision loss secondary to corneal edema and development of painful epithelial bullae. Endothelial keratoplasty is curative, and has increased yearly over the past decade to 30,710 cases in 2015 in the United States.1
Previously, Wieben and colleagues demonstrated a strong association between intronic trinucleotide repeat expansion in TCF4 and Fuchs dystrophy,3 following association with the single nucleotide polymorphism (SNP) rs613872, a variant also identified within the same gene.4 Data from a population sample in Tangier island suggested higher severity among affected individuals with the rs613872 SNP,5 and we and others have found in large case-control studies that expansion of the trinucleotide repeat in TCF4 correlates strongly with both disease status and severity of Fuchs dystrophy.6,7
Given the social and economic costs of corneal transplantation, understanding its associated risk factors and their impact would be beneficial to patient counseling and management. Here, we describe the association of the TCF4 trinucleotide repeat with corneal transplantation in a cross-sectional analysis of 574 cases of Fuchs dystrophy, and explore associated time to surgery.
MATERIALS AND METHODS
Recruitment
A total of 574 participants were enrolled in a longitudinal study of Fuchs dystrophy after slit-lamp biomicroscopy by an anterior segment specialist revealed clinically significant disease, or if the patient reported a history of Fuchs dystrophy and had undergone corneal transplantation, in the form of either penetrating or endothelial keratoplasty. Clinically significant disease was defined as a minimum of 5 central guttae in both eyes.
All study participants consented to the study protocol approved by the Joint Committee on Clinical Investigation at the Johns Hopkins University School of Medicine and in accordance with the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. Written informed consent was obtained from all individuals prior to enrollment.
All participants provided a blood sample and genomic DNA was extracted from leukocytes using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA).
Sequencing
Sequencing was carried out as previously described.6 Briefly, the 5’ forward primer (P1) was labeled with 6-FAM (5’ CAGATGAGTTTGGTGTAAGATG 3’) and unlabeled reverse primer (P2) (5’ TTGCAGCCCCTTTCTGCTTGT 3’) used in PCR amplification which was carried out using Platinum PCR Supermix High Fidelity (Life Technologies, Carlsbad, CA). Cycling conditions were applied as described by Wieben, et al.3
Triplet repeat primed PCR (TP-PCR) reactions was carried out with 1 microliter of genomic DNA, mixed with 14 microliters of Platinum PCR Supermix High Fidelity (Life Technologies) and 250nM final concentrations of P1 and reverse primer (P4) (5’ TACGCATCCCAGTTTGAGACGCAGCAGCAGCAGCAG 3’) at 95°C: 5min; (95°C: 30sec; 58°C: 1min; 68°C: 4min) X 20 cycles; 68°C for 10min. An additional 15sec was introduced in each of the 8th to 20th cycles. 2 microliters of PCR product was used as a template for a second PCR reaction by mixing with 13 microliters of the Platinum PCR Supermix with 250nM final concentration of 6-FAM labeled forward primer and unlabeled reverse primer (P3) (5’ TACGCATCCCAGTTTGAGACG 3’). The reverse primer of the second PCR step binds to the adapter sequence on P4 that is non-homologous to human genomic DNA. The second PCR was cycled at 95°C: 5min; (95°C: 30sec; 62°C: 1min; 68°C: 6min) X 25 cycles; 68°C for 10min.
PCR products were resolved using the ABI3730XL DNA Analyzer (Applied Biosystems Inc, Foster City, CA) with GeneScan 500 LIZ dye size standard mix. The results were analyzed using Gene Mapper (Applied Biosystems Inc.).
Statistical Analyses
We employed a cross-sectional approach upon a cohort of 574 patients for which we had previously identified a strong association between the CTG repeat in TCF4 and affection with Fuchs Dystrophy. Demographic information was provided by participants, which included race, age, and gender.
To test for association with age, Student’s t-test were conducted to compare mean age for participants with and without surgery, and chi-square tests were conducted to assess for association between gender and surgery. To test for effect of laterality, a mixed-effects logistic regression model with a random intercept for patients was used. The random intercept was employed to account for the correlation between eyes for bilateral patients.
We utilized logistic regression to assess the relation of age, gender, and expanded repeat status with surgery status. Odds ratios and 95% confidence intervals were calculated.
To explore timing of surgery with respect to age between the expanded and non-expanded repeat in patients undergoing surgery, Kaplan-Meier survival curves were estimated and Cox proportional hazard regression modeling was performed to assess for equality in the survival functions.
All analyses were performed using STATA version 14.0 (Stata Statistical Software; College Station, TX, USA). A p-value less than or equal to 0.05 is treated as statistical significance.
RESULTS
Of 574 participants affected by Fuchs dystrophy, 106 individuals (18.5%) had undergone corneal transplantation in at least one eye at the time of initial visit. There was no difference in probability of having surgeries between right (85 eyes, 14.8%) and left (71 eyes, 12.4%) eyes (OR=0.64; p=0.082, mixed-effects logistic regression). A total of 51 participants had surgery in both eyes.
A total of 26 African-American participants with Fuchs dystrophy participated in the study (4.5% of total sample), of which 3 (11.5%) had undergone corneal transplantation at time of enrollment. Two participants self-identified as Asian (0/2, 0% post-transplant), and 546 as Caucasian (103/546, 18.9% post-transplant). The difference in proportions of African-American and Caucasian patients undergoing surgery was not significant (p=0.45).
Mean age of participants who had undergone surgery (68.4 years old) and those who had not at the time of initial visit (67.7 years old) was not significantly different (p=0.29), nor was the difference in ages between those without and with repeat expansion (67.0 vs. 68.3, respectively, p=0.21).
While the participants were predominantly female (64.8% vs. 35.2%), there was no association between gender and surgery (19.8% of males vs. 17.7% of females had undergone corneal transplantation, p = 0.54).
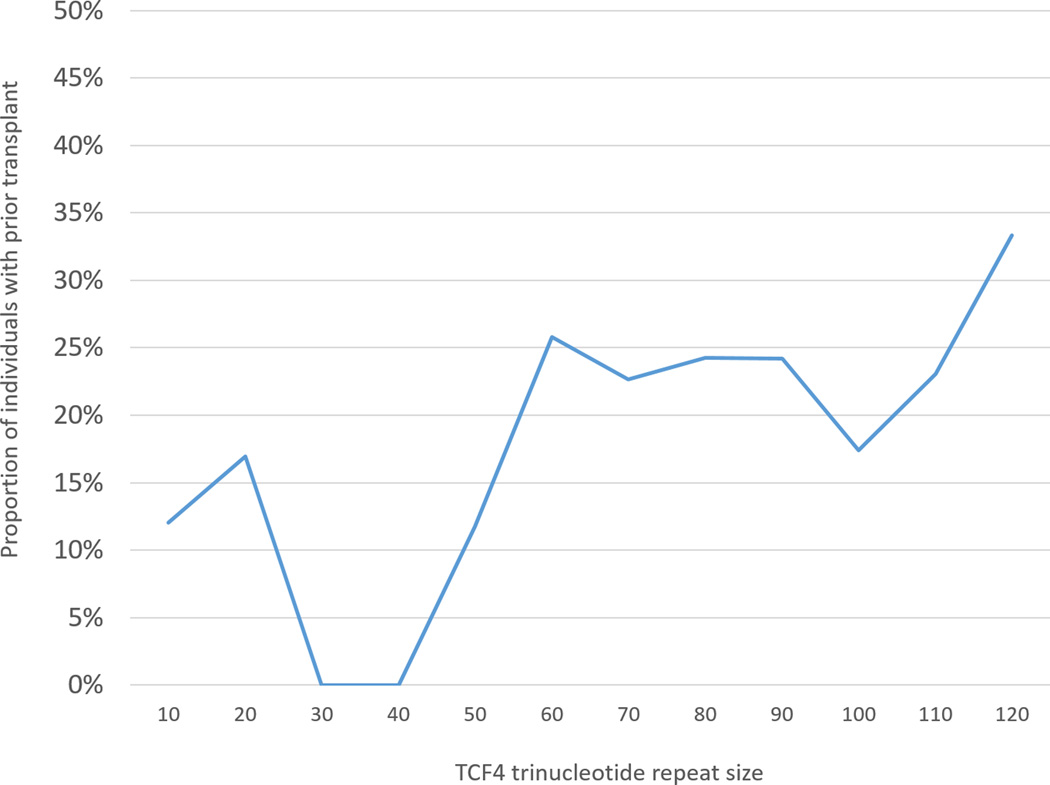
As demonstrated in Table 1, a higher proportion of participants who had undergone surgery at the time of enrollment demonstrated an expanded allele (78/106, 73.6%) compared to those who had not undergone surgery (279/468, 59.6%), a significant difference (p=0.007). Relative proportions of repeat size in surgical and non-surgical patients are illustrated in Figure 1.
Table 1.
Participants and surgery status. Data indicate a significant association between CTG expansion and cornea transplant status (p=0.007, Pearson chi squared). NN: Repeat size of less than 40. NX/XX: An expansion of at least 40 repeats on either (NX) or both (XX) alleles.
| History of cornea transplant |
||||
|---|---|---|---|---|
| No | Yes | |||
| CTG Expansion |
NN | 189 | 28 | 217 |
| NX/XX | 279 | 78 | 357 | |
| 468 | 106 | 574 | ||
Figure 1.
Proportion of subjects who had undergone corneal transplantation at time of enrollment in a longitudinal study of Fuchs Corneal Dystrophy, by repeat expansion status. The number of trinucleotide repeats, listed on the x-axis, represents the designated number of repeats up to the following interval (e.g. “30” includes participants with repeat sizes from 30 to 39).
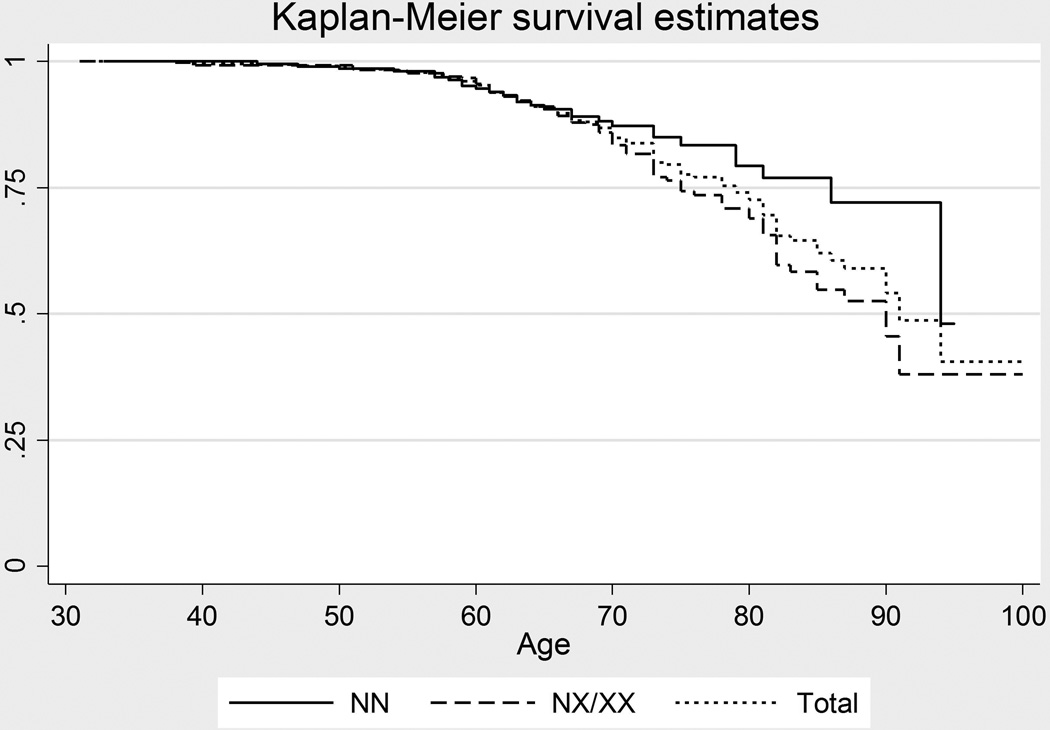
Kaplan-Meier survival estimates were produced (Figure 2), and proportional hazard Cox regression model was performed to explore the progression of transplantation with respect to age for patients with and without the expanded repeat while accounting for gender. The data demonstrated a significant difference in survivor functions related to repeat status (p=0.027) with hazard ratio of 1.64 (95% CI, 1.06 to 2.55) but gender did not show significant effect on progression (p=0.747). Overall, the proportion of individuals with repeat expansion who had undergone surgery was 21.8% (78/357) compared to 12.9% (28/217) of those without repeat expansion.
Figure 2.
Estimated survival curves depicting relative differences in age of surgery between patients with less than 40 repeats (NN) vs. greater than 40 repeats in either allele (NX/XX), constructed from clinical data. Cox proportional hazard regression model demonstrates a significant difference in survival function (p=0.027), with hazard ratio of 1.64 (95% CI, 1.05 to 2.55) but without significant association with gender (p=0.747).
DISCUSSION
Here, we demonstrate that expansion of the CTG18.1 trinucleotide repeat in TCF4 is associated with higher risk of corneal transplantation at a younger age, assessed for the first time in a multiracial population sample from the United States.
The increased likelihood of corneal transplantation at a given age seen with the expanded TCF4 repeat allele are consistent with previous findings demonstrating increased severity of disease from this genetic lesion among affected individuals studied by both our group6 and others.7 An association found between the expanded allele and corneal transplantation in a Caucasian sample7 raises questions about the impact of an expanded repeat and gender on timing of transplantation.8 Here, the association is significant using statistical methodology that accounts for both gender and age.
Understanding the increased likelihood of transplantation among those harboring the expanded TCF4 repeat may be particularly relevant for patient counseling in the future. The position of Fuchs dystrophy as the leading indication for corneal transplantation in the United States1 adds particular relevance to the possible benefits of acquiring such information for patients. While the decision to pursue surgery is a complex one that is based on multiple factors associated with visual function, potential applications in the future for genetic status include individualized tailoring of routine visit intervals prior to surgery and counseling regarding prognosis.
Although the odds of an expanded TCF4 repeat were higher in patients who underwent transplantation, not all post-transplant patients harbored the expanded allele. This may reflect the fact that classic Fuchs dystrophy is a disease of late onset, and the pathway causative for disease has multiple inputs. We have previously demonstrated a family in which individuals with both the FCD4 disease allele and a p.Q840P mutation in TCF8 underwent corneal transplantation, while those with only a single disease allele demonstrated mild disease.9 Families associated with the FCD110 and FCD211 loci, and causative mutations in SLC4A11,12 LOXHD113 and ABGL114 have also had members who underwent corneal transplantation, suggesting a role for additional genetic factors associated with progression to transplantation.
In this study, the proportion of patients who underwent corneal transplantation from Caucasian and African-American backgrounds was not found to be significantly different; further analyses in a larger cohort of African-American patients will provide additional insight into whether true disparities exist in clinical practice.
In summary, we demonstrate that, in comparison to patients without the expanded allele, expansion of the trinucleotide repeat in TCF4 is associated with 1.64 times greater likelihood of corneal transplantation at a given age in a large cohort of patients with Fuchs dystrophy.
Acknowledgments
This research was supported by National Institutes of Health Grants R01 EY016835 (JDG) and K12 EY015025-10 (AOE).
Footnotes
The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Eye Bank Association of America. [Accessed August 28, 2016];2015 Eye Banking Statistical Report. August 28. 2016 Available at: http://restoresight.org/wp-content/uploads/2016/03/2015-Statistical-Report.pdf.
- 2.Goar EL. Dystrophy of the Corneal Endothelium (Cornea Guttata), with Report of a Histologic Examination. Trans Am Ophthalmol Soc. 1933;31:48–59. [PMC free article] [PubMed] [Google Scholar]
- 3.Wieben ED, Aleff RA, Tosakulwong N, et al. A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS One. 2012;7:e49083. doi: 10.1371/journal.pone.0049083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasanth S, Eghrari AO, Gapsis BC, et al. Expansion of CTG18.1 Trinucleotide Repeat in TCF4 Is a Potent Driver of Fuchs' Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2015;56:4531–4536. doi: 10.1167/iovs.14-16122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baratz KH, Tosakulwong N, Ryu E, et al. E2-2 protein and Fuchs's corneal dystrophy. N Engl J Med. 2010;363:1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- 6.Eghrari AO, McGlumphy EJ, Iliff BW, et al. Prevalence and severity of fuchs corneal dystrophy in Tangier Island. Am J Ophthalmol. 2012;153:1067–1072. doi: 10.1016/j.ajo.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman AZ, Xing C, Radwan SH, et al. Correlation of Severity of Fuchs Endothelial Corneal Dystrophy With Triplet Repeat Expansion in TCF4. JAMA Ophthalmol. 2015;133:1386–1391. doi: 10.1001/jamaophthalmol.2015.3430. [DOI] [PubMed] [Google Scholar]
- 8.Sundin OH. Genetics of Fuchs Corneal Dystrophy Comes of Age: Sweet Repeats. JAMA Ophthalmol. 2015;133:1392. doi: 10.1001/jamaophthalmol.2015.3445. [DOI] [PubMed] [Google Scholar]
- 9.Riazuddin SA, Zaghloul NA, Al-Saif A, et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am J Hum Genet. 2010;86:45–53. doi: 10.1016/j.ajhg.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundin OH, Jun AS, Broman KW, et al. Linkage of late-onset Fuchs corneal dystrophy to a novel locus at 13pTel-13q12.13. Invest Ophthalmol Vis Sci. 2006;47:140–145. doi: 10.1167/iovs.05-0578. [DOI] [PubMed] [Google Scholar]
- 11.Sundin OH, Broman KW, Chang HH, et al. A common locus for late-onset Fuchs corneal dystrophy maps to 18q21.2-q21.32. Invest Ophthalmol Vis Sci. 2006;47:3919–3926. doi: 10.1167/iovs.05-1619. [DOI] [PubMed] [Google Scholar]
- 12.Riazuddin SA, Vithana EN, Seet LF, et al. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum Mutat. 2010;31:1261–1268. doi: 10.1002/humu.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riazuddin SA, Parker DS, McGlumphy EJ, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012;90:533–539. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riazuddin SA, Vasanth S, Katsanis N, et al. Mutations in AGBL1 cause dominant late-onset Fuchs corneal dystrophy and alter protein-protein interaction with TCF4. Am J Hum Genet. 2013;93:758–764. doi: 10.1016/j.ajhg.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]