Abstract
Background
Sarcoma accounts for 20% of solid tumors in children. Surgery has significant morbidity. We hypothesized that delivering chemotherapy directly into tumors through sustained release silk systems could slow tumor growth.
Methods
Human Ewing sarcoma cells A673 were cultured with vincristine and doxorubicin to determine half maximal inhibitory concentration (IC50). Cells were injected into mouse hind leg to create orthotopic tumors. Tumor volumes were measured using ultrasound. When volume reached >250mm3, interventions included: implantation of drug-free silk foam (Control-F), doxorubicin 400μg foam (Dox400-F), vincristine 50μg foam (Vin50-F), drug-free silk gel (Control-G), vincristine 50μg gel (Vin50-G), or single dose intravenous vincristine 50μg (Vin50-IV). End-point was volume >1,000mm3. Kaplan Meier and ANOVA were used.
Results
IC50 for vincristine and doxorubicin were 0.5ng/mL and 200ng/mL, respectively. There was no difference between Dox400-F [6±1 days to end point (DTEP)] and Control-F (5±1.3 DTEP). Vin50-F (12.4±3.5 DTEP) had slower growth compared to Control-F (p<0.001), and no difference between Vin50-F and Vin50-IV (14±0 DTEP). Growth was slowest with Vin50-G, 28±10.3 DTEP compared to all other treatment groups (p<0.05).
Conclusion
Sustained delivery of vincristine inside the sarcoma tumor with silk gel decreased tumor growth. Applying this intra-tumoral treatment strategy may potentially decrease the extent of surgical excision.
Keywords: Sarcoma, local drug delivery, silk, vincristine
INTRODUCTION
Bone and soft tissue sarcomas comprise about 20% of all extracranial solid tumors in the pediatric population [1]. There are a number of histologic subtypes of sarcoma, including rhabdomyosarcoma, the most common soft tissue sarcoma, and Ewing’s Sarcoma and osteosarcoma, which are the most common malignant bone tumors [2]. A recent review of the Surveillance Epidemiology and End Results Database (SEER) found that on average 26–28% have metastatic disease at the time of diagnosis [3]. While overall survival rates have improved over the past decades, 10-year survival of primary Ewing’s sarcoma is 63% and only 32% for metastatic disease [3].
Current sarcoma treatment utilizes a multimodal approach and consists of local and systemic treatment. Local treatment includes surgical resection with adjuvant radiation for tumors with positive margins or aggressive histologic subtypes [4–7]. In order to achieve a complete tumor excision for cure, many of the surgical resections are extremely morbid, including hip disarticulations and amputations [8]. Patient that have undergone limb sparing surgery when compared to amputation have shown to have better daily competence and were less likely to need a walking aid [9]. A local treatment method that can decrease the tumor size to minimize the extent of the surgical resection would significantly improve the quality of life for these patients.
Sarcoma tumor cells have been responsive to many systemic chemotherapy agents such as vincristine, doxorubicin, cyclophosphamide or etoposide [10]. Despite being effective, delivering these chemotherapeutic agents systemically can result in short term complications such as neuropathies and respiratory insufficiency and long-term complications such as cardiac dysfunction, limb length discrepancies, and secondary malignancies [11–14].
Responding to the need for decreasing the tumor size while minimizing the side effects of systemic chemotherapy, many investigators have utilized the local drug delivery approach. For example, Gliadel®, an FDA approved carmustine wafer is implanted directly over the tumor resection bed of patients with malignant gliomas [15]. Local delivery of chemotherapy using a doxorubicin-loaded silk film applied to neuroblastoma has also shown promise in decreasing tumor growth in animal models [16, 17]. These studies demonstrated that tumor growth could be slowed by directly delivering chemotherapy to the tumor resection bed or around the tumor body. Given the success of this treatment strategy, we hypothesized that delivering chemotherapy directly into an orthotopic sarcoma tumor using sustained release silk platforms could slow tumor growth.
2. MATERIALS AND METHODS
2.1 Cell culture
Human Ewing Sarcoma cell line, A673 (ATCC, Manassas, VA), was cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100-μg/ml streptomycin. All cells were maintained in a 5% CO2 atmosphere at 37°C and trypsin-passaged at 80% confluence.
2.2 In vitro cytotoxicity
A673 cells were plated in 96-well plates at 10,000 cells per well and allowed to attach and adhere overnight. Cells were then exposed to varying concentrations of doxorubicin or vincristine for two days. AlamarBlue® assay (Invitrogen, Carlsbad, CA) was used to evaluate cell viability following the manufacturer’s protocol.
2.3 Silk fibroin extraction
Silk fibroin from Bombyx mori cocoons was isolated as previously described [18]. Briefly, cocoons were cut into approximately 1 cm2 pieces and boiled in 0.02 M NaCO3 for 30 or 60 minutes to remove the sericin protein. Silk fibroin fibers were rinse in deionized water and allowed to air dry. Silk fibroin fibers were dissolved in 9.3 M LiBr at 60°C for 4-hours followed by dialysis (Pierce 3.4 kDa MWCO dialysis cassette; Fisher Scientific, Pittsburg, PA) for 2 days against deionized water (PicoPure® water purification system; Hydro Service and Supplies, Durham, NC). The aqueous silk fibroin (hereafter referred to as silk) solution was stored at 4°C until use.
2.4 Vincristine-loaded silk gels
A 6% (w/v) and 8% (w/v) solution of 60 minutes and 30 minutes extracted silk was filter sterilized. Silk solutions were handled aseptically for the remaining sample preparation steps. The silk solutions were sonicated using a Branson Digital Sonifier 450 (Branson Ultrasonics, Danbury, CT, USA) following previously published protocols [19]. After sonication, the silk solutions were placed on ice to slow gelation. Twenty microliters of the 60 minutes extract pre-gel was aspirated into a 0.5 mL insulin syringe and allowed to gel at 60°C. Vincristine (5 mg/mL in deionized water) was added to the 30 minutes extracted pre-gel to a final concentration of 312.5 μg/mL and 625 μg/mL. The final silk concentration was brought to 6% (w/v) using sterile deionized water. Eighty microliters of the vincristine-loaded pre-gel was aspirated into the previously prepared syringes and allowed to completely gel at room temperature overnight. The final vincristine amount was 25 μg (Vin25-G) or 50 μg (Vin50-G) per syringe.
2.5 Doxorubicin-loaded silk foams
A 6% (w/v) solution of 30 minutes extracted silk was prepared. One hundred and fifty microliters of the silk solution was pipetted into wells of a 96-well plate, frozen at −80°C and lyophilized. The lyophilized silk foams were autoclaved to render the silk foams insoluble and sterile. Under aseptic conditions, silk foams were immersed in an aqueous solution of 400 μg/mL doxorubicin hydrochloride. Doxorubicin was allowed to bind to the silk foam for one week [20, 21].
2.6 Animal model
All mouse procedures were performed in accordance with University of Illinois’ recommendations for the care and use of animals and were maintained and handled under protocols approved by the Institutional Animal Care and Use Committee. All procedures were performed with female NCr nude mice (Harlan, Indianapolis, IN) at 7 weeks of age. Procedures and ultrasound measurements were performed under general anesthesia using isoflurane inhalation.
106 human Ewing sarcoma A673 cells were concentrated in 2μL of phosphate buffered saline (PBS). These cells were then injected into the right hind leg muscle of the mouse using a 30G needle. Tumor formation was followed by non-invasive ultrasound measurements, and the animals were euthanized when the tumor volume exceeded 1,000 mm3. The tumors and organs were removed and processed as detailed below.
2.7 Implantation of silk foam into sarcoma tumor
Implantation of all silk foams was the same for foams loaded with doxorubicin 400 μg (Dox400-F), vincristine 50 μg (Vin50–F), or drug free, control (Control–F). There were between five to eight animals per group. When the tumor volume reached 300 mm3, animals were randomly selected for foam implantation. General anesthesia was induced with xylazine and ketamine, and an incision was made over the tumor of the mouse right hind leg. Dissection was carried down to the tumor capsule. Using electrocautery, the tumor capsule was incised, and a pocket just large enough to fit the foam was created within the tumor. The foam was then placed within the tumor, and the tumor capsule was closed using vicryl suture. The fascia and skin were closed in two layers.
2.8 Injection of silk gel into sarcoma tumor
Once tumor volume reached 300mm3 based on ultrasound measurements, animals were randomly selected for injection of silk gels loaded with either no drug (Control–G) or 50μg vincristine (Vin50–G). There were seven and eight mice in each group, respectively. With ultrasound visualization, a 29-gauge needle was inserted into the middle of the tumor and a gel load was injected. Each injected load had 20 μL of drug free silk gel to act as a cap at the end of the injection to prevent leakage of drug-loaded gel.
2.9 Intravenous chemotherapy administration
To serve as a control group, five animals received intravenous administration of a single equivalent dose of vincristine. When tumor volumes reached 300mm3, 50 μg of vincristine was injected via the tail vein.
2.10 High frequency ultrasound
After securing the mouse in a prone position, a VisualSonics Vevo 2100 sonographic probe (Toronto, Ontario, Canada) was applied to the right hind leg to locate the tumor. Serial cross-sectional images (0.076 mm between images) were taken. The tumor volume was measured using the 3-D reconstruction tool (Vevo Software v1.6.0, Toronto, Ontario, Canada). Tumor volume was measured weekly until end-points were met.
2.11 Histologic evaluation
When the animals met their end points, the tumors were excised and fixed in formalin. These were then serially dehydrated and embedded in paraffin. Five-micron slices were made and placed on glass slides. Hematoxylin and eosin (H&E) staining was performed and the slides were examined under light microscopy.
2.12 Statistical analysis
Data analysis was performed using Graph Pad Prism 4 (La Jolla, CA). Kaplan Meier survival curves were used to analyze survival differences among the various treatment groups. Additionally, ANOVA with Tukey’s Multiple Comparison test was used to analyze difference among days for tumor volume to reach greater than 1000mm3. A p value of <0.05 was considered significant.
3 RESULTS
3.1 In vitro cytotoxicity of vincristine and doxorubicin
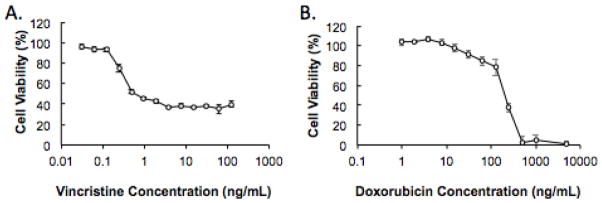
Ewing sarcoma A673 cells were exposed to varying doses of vincristine and doxorubicin for two days to determine the drug cytotoxicity. The half maximal inhibitory concentration (IC50), or the amount of drug needed to kill 50% of the cells, for vincristine and doxorubicin was 0.5 ng/mL and 200 ng/mL, respectively (Figure 1). Based on these findings and our previous drug delivery studies with a neuroblastoma model, 400 μg of doxorubicin and 50 μg of vincristine were loaded into the silk delivery systems [16].
Figure 1. In vitro cellular cytotoxicity induced by vincristine and doxorubicin treatment.
A673 cells were exposed to increasing concentrations of (A) vincristine and (B) doxorubicin for two days.
3.2 In vivo tumor growth
Tumors were successfully established in all but one animal injected with A673 cells. Ultrasound was able to detect tumors prior to being visible on physical examination (Figure 2a). On ultrasound, tumors were well circumscribed and embedded within the normal muscle. The tumor did not appear to be invading the surrounding structures (Figure 2b). On average, tumors were identified 11.5 ± 1.7 days post injection, and the tumors took 22.6 ± 1.9 days to reach >1000mm3 without any intervention. At the time of necropsy no animal had evidence of gross metastatic disease.
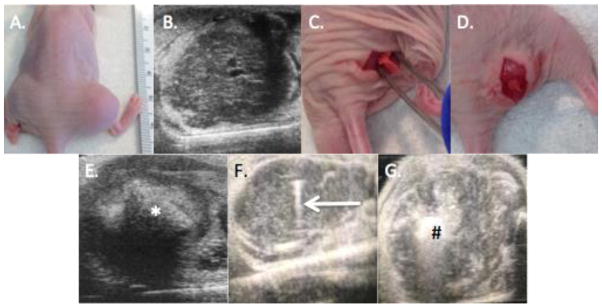
Figure 2. Implantation of silk platforms.
A. Gross appearance of right hind leg tumor B. Representative ultrasound image showing a well circumscribe tumor with normal surrounding muscle. C. A pocket was created within the tumor prior to foam implantation. D. The foam was inserted into the pocket. E. After foam implantation, the foam (*) was detected within the tumor using ultrasound. F. Ultrasound guided injection of silk gel, with arrow demonstrating the needle within the tumor. G. After injection, the gel [denoted with (#)] was identified within the tumor.
3.3 Tumor response to silk foam treatment
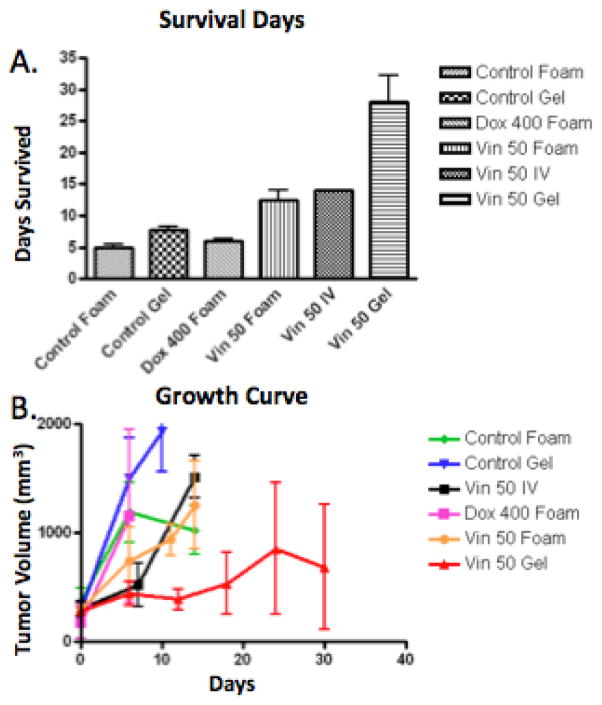
The silk foam was readily identifiable within the tumors (Figure 2e). Tumors implanted with Control-F took 5 ± 1.3 days to reach >1,000 mm3. Tumors implanted with Dox400-F took 6 ± 1 day for tumor volume to reach >1,000 mm3, which was no different than Control-F (p = 0.13). Tumors implanted with Vin50-F took 12.4 ± 3.5 days to reach >1,000 mm3, significantly slower than Control-F treated tumors (p = 0.01). Vin50-IV treated tumors took 14 ± 0 days to reach >1,000 mm3, which was not significantly different compared to those treated with Vin50-F (p > 0.05) (Figure 3a). The tumor size of each treatment group was plotted against days after treatment (Figure 3b).
Figure 3. Tumor growth analysis.
A. Days for tumor to reach >1000mm3 was slowest with animals treated with Vin50-G. B. The tumor size of different treatment groups over time was plotted. Tumors treated with Vin50-G had the slowest tumor growth compared to all other treatment groups.
3.4 Tumor response to silk gel treatment
Given that Vin50-F had successfully slowed tumor grow, we next aimed to improve the delivery procedure and eliminate the open procedure associated with foam implantation. Under ultrasound guidance, silk gel was percutaneously injected into the center of the tumor (Figure 2f and 2g). Tumors treated with Control-G reach >1,000 mm3 7.8 ± 1.4 days post injection, not significantly different than Control-F treated tumors. Tumors treated with Vin50-G took 28 ± 10.3 days to reach 1,000 mm3. This was significantly longer than that of Control-G (p<0.001), Control-F (p<0.001), Vin50-IV (p<0.01), and Vin50-F (p<0.001) (Figure 3a). Vin100-G was evaluated; however, within the first week post administration, the mice lost an average of 26.5% of their initial weight. This treatment was considered too toxic and experiments were not continued (data not shown).
3.5 Survival among all treatment groups
The overall survival of all animals was analyzed. We found that treatment with Vin50-G produced significantly longer survival compared to all other groups (p<0.001). (Figure 3a)
3.6 Histology
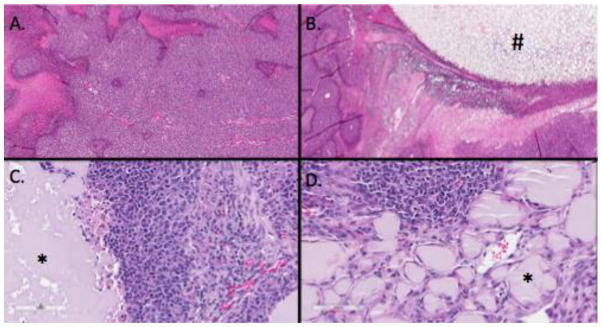
H&E staining of untreated tumors demonstrated the classic small round blue cell features seen with Ewing Sarcoma. This morphology was maintained in tumors treated with Control-G (Figure 4c). The gel was identifiable within the tumor. The tumor abutting the control gel was still viable. Vin50-G treated tumors also demonstrated the small round blue cells. The gel could be identified within the tumor, with macrophages forming around the gel. Additionally, the necrosis rate in this tumor was 5–10%. There were no clear mitotic figures identified within the vincristine-treated tumor samples (Figure 4d).
Figure 4. Histology.
A. Untreated sarcoma tumor that demonstrated small round blue cells, with some areas of necrosis within the tumor. B. Sarcoma treated with foam (#), demonstrating some necrosis adjacent to the foam. C. Pleomorphic tumor cells adjacent to control gel (*). D. Vin50-G (*) with inflammatory infiltration and tumor beyond gel.
4 DISCUSSION
We have demonstrated that development of a mouse orthotopic model of Ewing sarcoma using cell line A673. Ultrasound monitoring was a reliable method to track tumor growth, with detection prior to being appreciable on gross examination. While doxorubicin foam did not alter tumor growth, we found that 50 μg of vincristine in either silk foam or gel systems decreased tumor growth compared to control. The gel delivery of vincristine achieved the slowest tumor growth.
We found that treatment with doxorubicin-loaded foam was not effective in decreasing tumor growth. A possible explanation could be due to the high IC50 concentration of doxorubicin and insufficient amount of free-drug being delivered for tumor kill. Additionally, since doxorubicin is a frequent drug of choice for patients with sarcoma, this A673 cell lines may have previously been treated with doxorubicin prior to being cultured.
Barriers to delivering chemotherapy to solid tumors include the robust extracellular matrix architecture of the tumor as well as the variable distance of the vessels throughout the tumor [22]. Waite et al. described that higher concentrations of systemic chemotherapy to achieve appropriate intratumoral levels were needed due to the dense extracellular matrix surrounding solid tumors[22]. Additionally, the vascular bed becomes less evenly distributed as tumor cells proliferate [23]. The use of intratumoral drug delivery overcomes the extracellular matrix and fibrous capsule by directly penetrating into the tumor. Given the challenges with drug permeability to solid tumors, local delivery can be used in many different aspects of the treatment, such as during control of the primary disease. When feasible, primary resection has been shown to have superior outcomes compared to tumors treated solely with radiation for local control [24]. Another drawback to surgical resection is the potential limb morbidity resulting in the need for limb amputation. While, there have been many advances in prosthetics and grafting, long term risks of infection and graft failure still remain [7, 25]. Treatment of the tumor, initially or post-resection, with local drug therapy could decrease the surgical burden, sparing the need for extensive high dosing radiation therapy and potentially reducing the prevalence of limb amputation.
The most frequent site of metastatic disease in sarcoma is the lungs. It has been well demonstrated that pulmonary metastasectomy is associated with improved overall survival for patients with sarcoma [26]. However, metastasectomy often requires a thoracotomy, which is associated with significant perioperative pain, and induce long-term chronic pain among some patients [27]. Long term follow up after bilateral pulmonary metastasectomy has demonstrated that there is also impaired pulmonary function, with a reduction as high as 40% in forced vital capacity and forced expiratory volume at one minute [28]. Application of local drug delivery to these lesions with intra-tumoral sustained release gel is another potential treatment option that could limit the need for thoracotomy. Image guidance would allow for a significantly less invasive approach and reduce the morbidity of a thoracotomy. The ability to use percutaneous administration of gel could also minimize general anesthetics needed, which has been shown in young children to be associated with moderate risk of neurodevelopmental disorders [29].
Local drug delivery in sarcoma has been previously reported. Monterrubio et al. described implanting a matrix loaded with SN-38 after subtotal sarcoma tumor resection in a mouse xenograft model and achieved significantly delayed tumor growth [30]. SN-38 was loaded into a nano-fiber matrix and delivered to the resection bed of sarcomas. The matrix released almost the entire concentration of SN-38 over the first 24 hours. This was contrasted with our model where the drug delivery was maintained over a longer period of time, resulting in sustained tumor kill. Ren et al also treated sarcoma tumors by delivering 5-FU using a biodegradable micro-device made out of poly-(lactic-co-glycolic) acid with some success [31]. While these studies and our current report employ different delivery systems, all have shown that intra-tumoral delivery of chemotherapy can successfully decrease tumor growth, demonstrating that this is a viable treatment strategy.
Treatment with local therapy will not eliminate the need for systemic chemotherapy. The ideal combination of local and systemic therapy needs to be investigated for optimal therapeutic benefit. The possibility of reducing the overall systemic dose needed could minimize system toxicity and lifelong morbidity associated with chemotherapy.
In conclusion, we found that delivering vincristine-loaded sustained release silk materials into the center of a sarcoma tumor slowed tumor growth. Loading target cancer therapeutics or a combination of drugs into the silk delivery system could be a new mode of treatment for patients diagnosed with sarcoma. Studies on the pharmacokinetics of the drug delivered locally using the silk delivery system will be needed to validate this approach for clinical use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burningham Z, Hashibe M, Spector L, et al. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HaDuong JH, Martin AA, Skapek SX, et al. Sarcomas. Pediatr Clin North Am. 2015;62:179–200. doi: 10.1016/j.pcl.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 4.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 5.La TH, Meyers PA, Wexler LH, et al. Radiation therapy for Ewing's sarcoma: results from Memorial Sloan-Kettering in the modern era. Int J Radiat Oncol Biol Phys. 2006;64:544–550. doi: 10.1016/j.ijrobp.2005.07.299. [DOI] [PubMed] [Google Scholar]
- 6.Krasin MJ, Rodriguez-Galindo C, Davidoff AM, et al. Efficacy of combined surgery and irradiation for localized Ewings sarcoma family of tumors. Pediatric blood & cancer. 2004;43:229–236. doi: 10.1002/pbc.20095. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 8.Paulino AC, Nguyen TX, Mai WY. An analysis of primary site control and late effects according to local control modality in non-metastatic Ewing sarcoma. Pediatric blood & cancer. 2007;48:423–429. doi: 10.1002/pbc.20754. [DOI] [PubMed] [Google Scholar]
- 9.Eiser C, Darlington AS, Stride CB, et al. Quality of life implications as a consequence of surgery: limb salvage, primary and secondary amputation. Sarcoma. 2001;5:189–195. doi: 10.1080/13577140120099173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children's Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dalen EC, van der Pal HJ, Caron HN, et al. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2009:CD005008. doi: 10.1002/14651858.CD005008.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs B, Valenzuela RG, Inwards C, et al. Complications in long-term survivors of Ewing sarcoma. Cancer. 2003;98:2687–2692. doi: 10.1002/cncr.11891. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton SN, Carlson R, Hasan H, et al. Long-term Outcomes and Complications in Pediatric Ewing Sarcoma. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 14.Chauncey TR, Showel JL, Fox JH. Vincristine neurotoxicity. JAMA. 1985;254:507. [PubMed] [Google Scholar]
- 15.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu B, Coburn J, Pilichowska M, et al. Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model. British journal of cancer. 2014;111:708–715. doi: 10.1038/bjc.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seib FP, Coburn J, Konrad I, et al. Focal therapy of neuroblastoma using silk films to deliver kinase and chemotherapeutic agents in vivo. Acta biomaterialia. 2015;20:32–38. doi: 10.1016/j.actbio.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood DN, Preda RC, Yucel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Kluge JA, Leisk GG, et al. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn JM, Na E, Kaplan DL. Modulation of vincristine and doxorubicin binding and release from silk films. Journal of controlled release : official journal of the Controlled Release Society. 2015;220:229–238. doi: 10.1016/j.jconrel.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seib FP, Kaplan DL. Doxorubicin-loaded silk films: drug-silk interactions and in vivo performance in human orthotopic breast cancer. Biomaterials. 2012;33:8442–8450. doi: 10.1016/j.biomaterials.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng. 2012;40:21–41. doi: 10.1615/critrevbiomedeng.v40.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson SS, Torrey M, Link MP, et al. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG #8346. Pediatric Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:125–135. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 25.Hardes J, von Eiff C, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–395. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 26.Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg. 2015;149:85–92. doi: 10.1016/j.jtcvs.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen AD, Pedersen TA, Hjortdal VE, et al. Chronic pain in adults after thoracotomy in childhood or youth. Br J Anaesth. 2010;104:75–79. doi: 10.1093/bja/aep317. [DOI] [PubMed] [Google Scholar]
- 28.Welter S, Cheufou D, Zahin M, et al. Short- and Mid-Term Changes in Lung Function after Bilateral Pulmonary Metastasectomy. Thorac Cardiovasc Surg. 2014 doi: 10.1055/s-0034-1383828. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Du L, Du Z, et al. Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth. 2015;29:749–757. doi: 10.1007/s00540-015-2030-z. [DOI] [PubMed] [Google Scholar]
- 30.Monterrubio C, Pascual-Pasto G, Cano F, et al. SN-38-loaded nanofiber matrices for local control of pediatric solid tumors after subtotal resection surgery. Biomaterials. 2016;79:69–78. doi: 10.1016/j.biomaterials.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 31.Ren X, Zheng N, Gao Y, et al. Biodegradable three-dimension micro-device delivering 5-fluorouracil in tumor bearing mice. Drug Deliv. 2012;19:36–44. doi: 10.3109/10717544.2011.635720. [DOI] [PubMed] [Google Scholar]