Abstract
Oncolytic adenoviruses (Onc.Ads) selectively replicate in and lyse cancer cells and are therefore commonly used vectors in clinical trials for cancer gene therapy. Building upon the well-characterized adenoviral natural tropism, genetic modification of Onc.Ad can enhance/regulate their transduction and replication within specific cancer cell types. However, Onc.Ad-mediated tumor cell lysis cannot fully eliminate tumors. The hostile tumor microenvironment provides many barriers to efficient oncolytic virotherapy, as tumors develop structure and immune-evasion mechanisms in order to grow and ultimately spread. For these reasons, Onc.Ads modified to deliver structural or immune modulatory molecules (Armed Onc.Ads) have been developed to overcome the physical and immunological barriers of solid tumors. The combination of oncolysis with tumor microenvironment modulation/destruction may provide a promising platform for Ad-based cancer gene therapy.
Introduction
Oncolytic viruses (OVs) are promising cancer gene therapy agents, as they have the unique ability to selectively replicate in malignant cells, causing cancer cell lysis and inflammation, which in turn can stimulate host immune responses to cancer cells [1]. OV-induced necrosis results in the release of damage-associated molecular patterns (DAMPs), which stimulate tumor-infiltrating antigen presenting cells (e.g. dendritic cells) and subsequent adaptive immune responses [2]. However, solid tumors are complex, heterogeneous structures that impede OV-dependent oncolysis. OVs can be modified to increase their lytic potency by delivering modulatory molecule(s) (“Armed” OVs) that target the structure of the tumor microenvironment, thereby destroying malignant cells and also cells providing support for the growing tumor. Additionally, OVs can be armed with immunostimulatory molecules to further increase the development of anti-tumor immune responses. Recent clinical studies have demonstrated that Armed OVs such as herpes simplex virus (T-VEC), poxvirus (Pexa-vec), and adenovirus (ONCOS-102) can mediate clinical responses with few severe side effects [3]. The US FDA recently approved T-VEC expressing granulocyte macrophage colony-stimulating factor (GM-CSF) to treat melanoma [4]. Treatment of advanced melanoma with T-VEC was safe and resulted in a 10.8% complete response rate, significantly higher than systemic administration of GM-CSF alone [5]. Thus, oncolytic virotherapy represents a new class of promising cancer immunotherapy agents. In this review, we will specifically discuss the application of adenoviral-based oncolytic viruses.
Specific Transduction and Replication of Oncolytic Adenovirus in Cancer Cells
Oncolytic adenoviruses (Onc.Ads) have long been studied and tested in patients with malignancies without severe side effects and several clinical trials are ongoing (Table 1) [3]. For effective oncolytic activity, Onc.Ads must specifically infect and efficiently replicate within cancer cells; however, many cancer cells do not express or downregulate the coxsackie and adenovirus receptor (CAR), resulting in decreased transduction of serotype 5 Ad (Ad5), which is commonly used for Ad-based vectors [6]. Therefore, Ad5 fibers, the capsid moiety responsible for virus-cell surface receptor interaction, have been modified to increase their transduction to cancer cells. An RGD-motif inserted into the fiber knob increases viral interaction with integrins, which are highly expressed on some cancer cells, including prostate [7] and ovarian cancers [8]. Ad5 fiber has also been replaced by other serotype fibers to redirect to different receptors. For instance, serotype 35 fibers bind CD46 [9], which is upregulated in breast and colorectal cancers, among others [10], while serotype 3 utilizes desmoglein 2, a component of the epithelial cell adhesion structure known to be overexpressed in multiple epithelial malignancies [11]. These fiber modifications have been utilized in both preclinical studies and clinical trials to enhance Onc.Ad transduction to malignant cells.
Table 1.
Recently completed and current clinical trials using Oncolytic Adenoviruses
| OncAd | Modification | Transgene | Target | Administration | Phase | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| ICOVIR-5 | E2F-E1A Δ24 RGD |
Solid Tumors | Mesenchymal stem cell delivery IV | I,II | NCT0184461 | |
| LOAd703 | 5/3 Δ24 | CD40L & 4-1BBL | Pancreatic Cancer | IT | I/IIa | NCT02705196 |
| Ad5-yCD/mutTKSR39rep-hIL12 | E1B-55K | CD/TK hIL12 |
Prostate Cancer | Intraprostatic | I | NCT02555397 |
| ONCOS-102 w/ cyclophosphamide | 5/3 Δ24 | GM-CSF | Advanced neoplasms | IV | I | NCT01598129 |
| VCN-01 w/ or w/o Abraxane® and Gemcitabine | DM-1-E2F-E1A Δ24 RGD |
Hyaluronidase | Advanced solid tumors | IV | I | NCT02045602 |
| VCN-01 w/Abraxane® and Gemcitabine | DM-1-E2F- E1A Δ24 RGD |
Hyaluronidase | Advanced Pancreatic Cancer | IV | I | NCT02045589 |
| CG0070 | E2F-E1A | GM-CSF | Bladder Cancer | IV | III | NCT02365818 |
| CG0070 | E2F-E1A | GM-CSF | Bladder Cancer | IV | II/III | NCT01438112 |
| Colo-Ad1 | Ad11p/Ad3 | Colon, NSCLC, Bladder, Renal Cancer | IT, IV | I | NCT02053220 | |
| DNX-2401 w/Temozolomide | E1A Δ24 RGD |
Glioblastoma Multiforme | IT | I | NCT01956734 | |
| DNX-2401 w/ IFNγ | E1A Δ24 RGD |
Brain Tumors | IT | I | NCT02197169 | |
| Ad5-yCD/mutTKSR39rep-ADP w/IMRT | E1B-55K | CD/TK | Prostate Carcinoma | Intraprostatic | II/III | NCT00583492 |
| OBP-301 | hTERT | Hepatocellular Carcinoma | IT | I/II | NCT02293850 |
Abbreviations: CD, cytosine deaminase; GM-CSF, granulocyte macrophage colony-stimulating factor; IFNγ, interferon γ;IMRT, intensity-modulated radiation therapy; IT, intratumoral; IV, intravenous; NSCLC, non-small cell lung cancer; TK, tyrosine kinase; w/, with; w/o, without
Onc.Ads have been genetically modified to allow for selective replication in cancer cells with abnormal protein expression patterns compared to normal cells. Adenovirus replication is initiated by E1A, the first adenoviral transcription unit. E1A gene products are responsible for dissociation of the retinoblastoma (Rb)/E2F complex, resulting in free transcription factor E2F activation of the remaining early transcription units, E1B, E2, E3, and E4 genes [12]. A commonly used Ad5-based Onc.Ad contains a 24bp mutation in the E1A gene (E1AΔ24), which disrupts the retinoblastoma (Rb) binding domain and releases free E2F from Rb/E2F complex, resulting in an E1AΔ24 protein that cannot promote virus replication without free E2F [13]. Cancer cells, on the other hand, typically have high levels of free E2F resulting in the preferential replication of Onc.AdΔ24 in cancer cells. The Onc.Ads ICOVIR-5, -7, and -15 were developed to take advantage of excessive free E2F by regulating E1A transcription via the insertion E2F binding sites or E2F promoters [14], and these ICOVIRs still harbor E1AΔ24 as an additional safety switch. Onc.Ads have also been generated by replacing the native E1A promoter with a tissue- or cancer cell-specific promoter such as the tCCN1 promoter, which is active in prostate cancer [15]. Although E1 gene regulation has improved cancer cell specific lysis and the safety of Onc.Ads in vitro and in preclinical xenograft models, human solid tumors are more complex, as discussed below.
Physical Barriers to Oncolytic Adenovirus Dissemination within Solid Tumors
Solid tumors are heterogeneous structures made up of malignant cells that recruit normal cells such as immune cells, fibroblasts, and endothelial cells to promote tumor growth (Figure 1). Additionally, the presence of dense stromal tissue and high amounts of extracellular matrix (ECM) results in high fluid pressure in tumors, which inhibits the ability of Onc.Ads to spread throughout the tumor, thus limiting their effectiveness. Therefore, to enhance viral spread Armed Onc.Ads have been generated to target the ECM and angiogenesis. Onc.Ads expressing molecules such as relaxin [16] and hyaluronidase [17] to disrupt the ECM have shown promising preclinical results. Onc.Ad expressing relaxin, known to upregulate matrix metalloproteinases (MMPs), successfully increased viral spread in multiple tumor models [18]. VCN-01, an Onc.Ad armed with hyaluronidase, was able to decrease the presence of hyaluronic acid, a component of the ECM, and prolong survival in two orthotopic murine glioma models [17]. Contrastingly, Onc.Ads have also been armed with inhibitors of metalloproteinases (TIMPs), as these molecules inhibit the degradation of the ECM to affect tumor cell proliferation, migration, and angiogenesis. An Onc.Ad expressing TIMP2 had increased viral replication in primary ovarian tumor tissue; however, viral distribution was not evaluated [19]. Lucas et al recently reported that a modification of the Onc.Ad capsid to insert targeting peptide (CKS17) into the Ad hexon hypervariable region 5 could alter the tropism of the virus to infect and lyse not only human pancreatic carcinoma cells, but also human pancreatic stellate cells, a type of stromal cell. This hexon-modified Onc.Ad led to transforming growth factor beta receptor (TGFBRII)-dependent transduction to CAR-negative pancreatic cancer cells and primary stellate cells, while decreasing Ad uptake by the liver due to reducing Factor X/hexon binding [20].
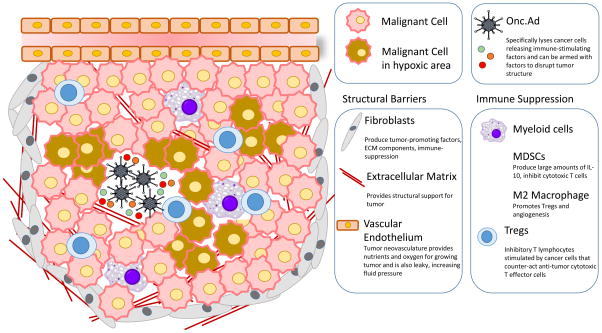
Figure 1. The inhibitory tumor microenvironment.
The tumor microenvironment is heterogeneous, consisting not only of malignant cells but also a wide range of cellular and non-cellular components that contribute to cancer progression. Although Onc.Ads can cause cancer cell lysis resulting in some anti-tumor effects, in order to eliminate the tumor Onc.Ads need to target a variety of other tumor-associated factors within the microenvironment. Accumulation of cancer associated fibroblasts, dense extracellular matrix, and formation of neovasculature promote the growth of the tumor and also impede the dissemination of Onc.Ads throughout the entire tumor mass. Areas of the tumor are often hypoxic and this also inhibits viral replication. While Onc.Ad-mediated cancer cell lysis can lead to immune activation by releasing damage-associated molecular patterns (DAMPs) and tumor neoantigens, the tumor microenvironment often contains inhibitory immune cells such as Tregs, MDSCs, and M2 macrophages, which counteract the immune stimulatory advantages of Onc.Ads.
Many solid tumors have other common characteristics such as abnormal vascularization and increased pressure, which restrict the distribution of intratumoral administered Onc.Ads. The presence of dense vasculature and collagen bundles can impede virus dissemination within the tumor. One group has recently increased Onc.Ad distribution within glioblastomas by combining the virus with a blocking antibody for vascular endothelial growth factor (VEGF), which is overexpressed in the vast majority of malignancies. This combination increased MMP2-mediated collagen degradation and doubled Onc.Ad distribution in an orthotopic glioma model [21].
Angiogenesis is critical to promote the growth of tumors and metastasis. VEGF, the best-characterized proangiogenic regulator, is a promising target for anti-angiogenic therapy. A potent method for inhibiting VEGF stimulation of endothelial cells is the use of a soluble decoy receptor with high affinity for VEGF, which inhibits VEGF interaction with VEGF receptors. An Armed Onc.Ad has been developed expressing FP3 (soluble VEGF receptor 3), which reduces cancer cell VEGF expression and HUVEC proliferation, and has increased oncolytic effect. This Armed Onc.Ad demonstrated enhanced anti-tumor effect in two lung cancer xenograft models [22]. When combined, two breast cancer-selective Onc.Ads engineered to express soluble Flt1 (a portion of VEGF receptor 1) or soluble DII4 (Delta-like 4), which negatively regulate Notch signaling to inhibit maturation of endothelium, provided a significant reduction in vascular formation and doubled animal survival in ER-negative breast cancer xenograft models [23]. Endostatin is a potent inhibitor of angiogenesis and has been safely used in replication-deficient Ad vector (Ad-Endo) in clinical trials for patients with advanced solid tumors. These Ad-Endo trials resulted in one objective response in a patient with nasopharyngeal carcinoma, 12 patients had stable disease, and two had disease progression [24] [25]. Ad-Endo has been used in combination with an Onc.Ad to amplify the endostatin expression in murine xenograft models, and this combination demonstrated more potent anti-tumor and antiangiogenic effects than treatments of either agent alone [26].
Solid tumors are often hypoxic environments in which adenoviral replication is reduced due to inhibition of E1A expression. To combat this, Onc.Ad replication can be placed under the control of a hypoxia response element (HRE) to enhance replication and therefore oncolysis. While such Onc.Ads can replicate in hypoxic conditions, they cannot eradicate tumors in vivo due to their patchy replication pattern, as only portions of tumors are hypoxic [27][28].
Immunologic Barriers to Effective Onc.Ad Anti-Tumor Effect in Solid Tumors
1) Modulation of Tumor Immune Status
Tumor cells and stroma produce inhibitory factors such as TGF-β and IL-10, which promote myeloid-derived suppressor cells (MDSCs), regulatory T-cells (Tregs) and M2 macrophages, all of which contribute to the immunosuppressive tumor environment [29]. M2 macrophages, which express VEGF, TGF-β, and PD-L1 [30], create an environment that skews cancer-targeting T-cells to T-regulatory cells. Many Onc.Ads have been generated to combat these inhibitory factors. Arming Onc.Ads with molecules that induce immune cell infiltration, proliferation, or activation, increases their effectiveness as cancer immunotherapy agents. Granulocyte macrophage colony stimulating factor (GM-CSF) is a cytokine expressed by immune cells such as macrophages, T cells, and NK cells, among others. GM-CSF can recruit and activate antigen presenting cells and NK cells within the tumor. A chimeric Onc.Ad5/3 has been generated to express GM-CSF. The treatment, called ONCOS-102, has been clinically evaluated in a phase I studies [31] [32]. Repeated intratumoral administration of ONCOS-102, in combination with cyclophosphamide, mediated infiltration of CD8+ and CD4+ T-cells to the treated tumor site, concomitant with an increase in tumor-specific activation of CD8+ cells in the peripheral blood [33]. Evaluation of patients receiving ONCOS-102 revealed that tumor cells up-regulated PD-L1 expression, leading to the possibility that combining ONCOS-102 with PD-L1 checkpoint blockade could further enhance the anti-tumor effects of this immunotherapy [32].
Many other cytokine-armed Onc.Ads are being investigated. Tumor necrosis factor alpha (TNFα) can induce cancer cell apoptosis and necrosis, as well as activate immune cells and stimulate the release of other cytokines. An armed Onc.Ad expressing TNFα was shown to demonstrate greater lytic effect than unarmed Onc.Ad in multiple human cancer cell lines in vitro and anti-tumor effect in vivo; this Armed Onc.Ad increased CD8+ T cell infiltration at the tumor site as well as a reduced tumor growth in a syngeneic mouse model [34]. An armed Onc.Ad expressing IFNα resulted in significant tumor suppression and survival advantage in a Syrian hamster model of pancreatic cancer compared to replication deficient Ad or a control Onc.Ad, although all of the animals eventually succumbed to the disease. Immune cell infiltration was not evaluated in this model [35]. The transient anti-tumor response may reflect transcriptional silencing of Ad replication and/or elimination of Ad infected cells, which can occur as a result of type I IFN-induced anti-viral responses [36].
Interleukins (ILs) have also been extensively used in combination with OVs to enhance immune responses. IL-12 can stimulate immune cell proliferation and polarize T cells to Th1 cells, as well as stimulate IFNγ and TNFα and counteract the immunosuppressive effects of IL-4 [37]. Intratumoral administration of an armed Onc.Ad expressing murine IL-12p40 (mIL-12p40) resulted in anti-tumor effects in both primary and metastasized tumors in a syngeneic mouse model of prostate cancer [38]. Although systemic administration of recombinant IL-12 results in high toxicity, a large preclinical study with approximately 100 mice receiving the Onc.Ad expressing mIL-12p40 resulted in no significant toxicity when administered intratumorally [39]. IL-24 is also known as a tumor suppressive protein, causing apoptosis and activation of the caspase cascade by cleaving caspases 3 and 9 [40]. An armed Onc.Ad expressing IL-24 reduced tumor formation and tumor progression, and increased T-cell responses and IFN-γ and IL-6 production in a syngeneic pancreatic cancer mouse model [41].
2) Immune co-stimulators
CD40 ligand (CD40L) is a co-stimulatory molecule expressed on activated T cells, and other immune cells, which binds to CD40 on antigen presenting cells. CD40 is also expressed on many advanced cancers. The CD40/CD40L interaction can elicit a helper T cell response by stimulating dendritic cells. CD40L has been used in a non-replicating Ad vector in patients with bladder carcinoma, and increased CD4+ and CD8+ T-cell infiltration to bladder tissue. In the phase I cohort, no detectable malignant cells remained in the bladder in 3 of 5 patients, although all patients in the phase IIa study had residual malignancy [42]. Subsequently, an oncolytic adenovirus armed with CD40L, Ad5/3-hTERT-CD40L (CGTG-401) was tested in patients with advanced solid tumors to combine oncolysis with CD40L immune stimulation. The patient cohort receiving CGTG-401 had significantly prolonged median survival compared to patients receiving a control Onc.Ad [43]. A recently developed Onc.Ad armed with CD40L and 4-1BBL, a molecule known to enhance proliferation and survival of activated T-cells, named LOAd703, has been shown to elicit a Th1 immune response and significant anti-tumor effects in xenograft and immunocompetent mouse models (E Svensson et al., abstract nr297, 106th Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, April 2015). LOAd703 is currently being tested in a clinical trial for patients with pancreatic cancer (NCT02705196).
Onc.Ads can be armed with a variety of structure- or immune-modulatory molecules. However, due to the packaging limitations of Onc.Ads, it is difficult to generate an Onc.Ads containing multiple immunomodulatory molecules, if full activation of host immune system is required. Our group has overcome this issue by developing a combinatorial adenoviral vector (CAd-VEC) approach in which Onc.Ad is combined with a helper-dependent Ad (HDAd), which has cargo capacity up to 34 kb, to overcome the inherent limitation of each agent (Onc.Ad: small transgene capacity, HDAd: no lytic effect) [44]. Therefore, this CAd-VEC system could be used to deliver multiple modulatory molecules including structural- and immune- modulators at once.
Conclusion
Oncolytic adenoviruses provide a promising platform for cancer-immunotherapy agents. Since adenoviral structure and gene function have been well characterized, Onc.Ads can be easily modified and adapted to increase the selectivity and potency of vector transduction and replication in cancer cells. Onc.Ads have also demonstrated a satisfactory safety profile in previous clinical trials, and several clinical trials are ongoing. Tumors are complex structures that can employ multiple mechanisms to evade anti-tumor immune responses, often in a tumor-specific manner. In order to combat these evasion strategies, we need to assess which modulatory factors, including both immune-modulators and structural modulators, discussed in this review could be curative targets. Since human adenovirus-based Onc.Ads have low replication capacity in mouse cancer cells, the development of immunocompetent animal models susceptible to human-specific adenovirus is also an important goal for the evaluation of newly developed Armed Onc.Ads before clinical translation. The shift from single action Onc.Ads targeting tumors exclusively by oncolysis to Onc.Ad-mediated destruction of the tumor microenvironment and stimulation of immune-mediated anti-tumor responses shows great promise in combating the complexity of human cancer.
Highlights.
Oncolytic Adenoviruses (Onc.Ads) are promising as cancer gene therapy agents
Onc.Ad selectivity and cytolytic potency can be enhanced by genetic modifications
Onc.Ads can be modified to target various aspects of the tumor microenvironment
Onc.Ad delivery of immune-modulatory molecules can enhance anti-tumor immunity
Acknowledgments
The authors would like to thank Catherine Gillespie in the Center for Cell and Gene Therapy at Baylor College of Medicine for her editing of the paper.
Funding: This work was supported by National Institutes of Health (R00HL098692) to M Suzuki, T32HL092332 to A Rosewell Shaw.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Fueyo J. Healing after death: antitumor immunity induced by oncolytic adenoviral therapy. Oncoimmunology. 2014;3:e947872. doi: 10.4161/21624011.2014.947872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pol J, Buqué A, Aranda F, Bloy N, Cremer I, Eggermont A, Erbs P, Fucikova J, Galon J, Limacher J, et al. Trial Watch-Oncolytic viruses and cancer therapy. Oncoimmunology. 2016;3:e1117740. doi: 10.1080/2162402X.2015.1117740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolgin E. Oncolytic viruses get a boost with first FDA-Approval recommendation. Nat Rev Drug Discov. 2015;14:369–371. doi: 10.1038/nrd4643. [DOI] [PubMed] [Google Scholar]
- **5.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. Results from the phase III clinical trial of T-VEC, the first oncolytic virus approved by the FDA, in patients with melanoma showing therapeutic benefit with a median overall survival of 23.3 months with T-VEC compared to 18.9 months in patients receiving only GM-CSF. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Pong RC, Bergelson JM, Hall MC, Sagalowsky AI, Tseng CP, Wang Z, Hsieh JT. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 1999;59:325–330. [PubMed] [Google Scholar]
- 7.Shen YH, Yang F, Wang H, Cai ZJ, Xu YP, Zhao A, Su Y, Zhang G, Zhu SX. Arg-Gly-Asp (RGD)-modified E1A/E1B double mutant adenovirus enhances antitumor activity in prostate cancer cells in vitro and in mice. PLoS One. 2016;11:e0147173. doi: 10.1371/journal.pone.0147173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I, Krasnykh V, Mikheeva GV, Barnes MN, Alvarez RD, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002;62:1271–1274. [PubMed] [Google Scholar]
- 9.Tuve S, Wang H, Ware C, Liu Y, Gagger A, Bernt K, Shayakhmetov D, Li Z, Strauss R, Stone D, et al. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorsteinsson L, O’Dowd GM, Harrington PM, Johnson PM. The complement regulatory proteins CD46 and CD59; but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. APMIS. 1998;106:869–879. doi: 10.1111/j.1699-0463.1998.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11, and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nevins JR. Transcriptional regulation. A closer look at E2F. Nature. 1992;358:375–376. doi: 10.1038/358375a0. [DOI] [PubMed] [Google Scholar]
- 13.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 14.Rojas JJ, Guedan S, Searle PF, Martinez-Quintanilla J, Gil-Hoyos R, Alcayaga-Miranda F, Cascallo M, Alemany R. Minimal RB-responsive E1A promoter modification to attain potency, selectivity, and transgene-arming capacity in oncolytic adenoviruses. Mol Ther. 2010;18:1960–1971. doi: 10.1038/mt.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, Klibanov AL, Wang XY, Pellecchia M, Sarkar D, et al. Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget. 2015;6:10712–10727. doi: 10.18632/oncotarget.3544. A dual regulated oncolytic Ad in which E1A is controlled by a prostate cancer specific promoter, tCCN1, and mda-7/IL-24 transgenes are controlled by the CMV promoter to generate cancer cell specific lysis and bystander anti-tumor effects. An ultrasound guided microbubble approach is used to enhance cancer targeting and avoid virus elimination by immune cells after systemic delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Park HR, Rhee J, Park YM, Kim SH. Therapeutic effect of oncolytic adenovirus expressing relaxin in radioresistant oral squamous cell carcinoma. Oncol Res. 2013;20:419–425. doi: 10.3727/096504013X13657689383139. [DOI] [PubMed] [Google Scholar]
- **17.Vera B, Martinez-Vélez N, Xipell E, Acanda de la Rocha A, Patiño-García A, Saez-Castresana J, Gonzalez-Huarriz M, Cascallo M, Alemany R, Alonso MM. Characterization of the antiglioma effect of the oncolytic adenovirus VCN-01. PLoS One. 2016;11:e0147211. doi: 10.1371/journal.pone.0147211. A single intratumoral administration of VCN-01, an Onc.Ad expressing hyaluronidase, doubled animal survival in two orthotopic glioma models, eradicating tumors in long-term survivors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesh S, Gonzales EM, Idamakanti N, Abramova M, Vanroey M, Robinson M, Yun CO, Jooss K. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67:4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [DOI] [PubMed] [Google Scholar]
- 19.Yang SW, Cody JJ, Rivera AA, Waehler R, Wang M, Kimball KJ, Alvarez RA, Siegal GP, Douglas JT, Ponnazhagan S. Conditionally replicating adenovirus expressing TIMP2 for ovarian cancer therapy. Clin Cancer Res. 2011;17:538–549. doi: 10.1158/1078-0432.CCR-10-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Lucas T, Benihoud K, Vigant F, Schmidt CQ, Wortmann A, Bachem MG, Simmet T, Kochanek S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS One. 2015;18:e0117254. doi: 10.1371/journal.pone.0117254. Insertion of a targeting peptide, CKS17, into the Ad hexon protein enhanced Onc.Ad transduction to both malignant cells and pancreatic stellate cells while decreasing liver uptake and increases cytotoxicity of complex co-cultures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaci B, Ulasov IV, Ahmed AU, Ferguson SD, Han Y, Lesniak MS. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013;20:318–327. doi: 10.1038/gt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi IK, Shin H, Oh E, Yoo JY, Hwang JK, Shin K, Yu DC, Yun CO. Potent and long-term antiangiogenic efficacy mediated by FP3-expressing oncolytic adenovirus. Int J Cancer. 2015;137:2253–2269. doi: 10.1002/ijc.29592. [DOI] [PubMed] [Google Scholar]
- 23.Bazan-Peregrino M, Sainson RC, Carlisle RC, Thoma C, Waters RA, Arvanitis C, Harris AL, Hernandez-Alcoceba R, Seymour LW. Combining virotherapy and angiotherapy for the treatment of breast cancer. Cancer Gene Ther. 2013;20:461–468. doi: 10.1038/cgt.2013.41. [DOI] [PubMed] [Google Scholar]
- 24.Li HL, Li S, Shao JY, Lin XB, Cao Y, Jiang WQ, Liu RY, Zhao P, Zhu XF, Zeng MS, et al. Pharmacokinetic and pharmacodynamics study of intratumoral injection of an adenovirus encoding endostatin in patients with advanced tumors. Gene Ther. 2008;15:247–256. doi: 10.1038/sj.gt.3303038. [DOI] [PubMed] [Google Scholar]
- 25.Lin X, Huang H, Li S, Li H, Li Y, Cao Y, Zhang D, Xia Y, Guo Y, Huang W, et al. A Phase I clinical trial of an adenovirus-mediated endostatin gene (E10A) in patients with solid tumors. Cancer Biol Ther. 2007;6:648–653. doi: 10.4161/cbt.6.5.4004. [DOI] [PubMed] [Google Scholar]
- 26.Li LX, Zhang YL, Zhou L, Ke ML, Chen JM, Fu X, Ye CL, Wu JX, Liu RY, Huang W. Antitumor efficacy of a recombinant adenovirus encoding endostatin combined with an E1B55KD-deficient adenovirus in gastric cancer cells. J Transl Med. 2013;11:257. doi: 10.1186/1479-5876-11-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuevas Y, Hernández-Alcoceba R, Aragones J, Naranjo-Suárez S, Castellanos MC, Esteban MA, Martin-Puig S, Landazuri MO, del Peso L. Specific oncolytic effect of a new hypoxia-inducible factor dependent replicative adenovirus on von Hippel-Lindau-defective renal cell carcinoma. Cancer Res. 2003;63:6877–6884. [PubMed] [Google Scholar]
- 28.Post DE, Devi NS, Li Z, Brat DJ, Kaur B, Nicholson A, Olson JJ, Zhang Z, Van Meir EG. Cancer therapy with a replicating oncolytic adenovirus targeting the hypoxic microenvironment of tumors. Clin Cancer Res. 2004;10:8603–8612. doi: 10.1158/1078-0432.CCR-04-1432. [DOI] [PubMed] [Google Scholar]
- 29.Loskog A. Immunostimulatory gene therapy using oncolytic viruses as vehicles. Viruses. 2015;6:5780–5791. doi: 10.3390/v7112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Ranki T, Pesonen S, Hemminki A, Partanen K, Kairemo K, Alanko T, Lundin J, Linder N, Turkki R, Ristimäki A, et al. Phase I study with ONCOS-102 for the treatment of solid tumors – an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. A GM-CSF expressing Onc.Ad (ONCOS-102) was safely administered to 12 patients with refractory solid tumors, 40% of evaluable patients had stable disease at 3 months with 11 of 12 patients having evidence of TILs post treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vassilev L, Ranki T, Joensuu T, Jäger E, Karbach J, Wahle C, Partanen K, Kairemo K, Alanko T, Turkki R, et al. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8+ T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology. 2015;4:e1017702. doi: 10.1080/2162402X.2015.1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Hirvinen M, Rajecki M, Kapanen M, Parviainen S, Rouvinen-Lagerström N, Diaconu I, Nokisalmi P, Tenhunen M, Hemminki A, Cerullo V. Immunological effects of a tumor necrosis factor alpha-armed oncolytic adenovirus. Hum Gene Ther. 2015;26:134–144. doi: 10.1089/hum.2014.069. An Onc.Ad expressing TNFα provides enhanced anti-tumor effect in a xenograft prostate cancer model and increased CD8+ T cell infiltration to B16-OVA syngeneic mouse model. [DOI] [PubMed] [Google Scholar]
- 35.LaRocca CJ, Han J, Gavrikova T, Armstrong L, Oliveira AR, Shanley R, Vickers Sm, Yamamoto M, Davydova J. Oncolytic adenovirus expressing interferon alpha in a syngeneic Syrian hamster model for the treatment of pancreatic cancer. Surgery. 2015;157:888–898. doi: 10.1016/j.surg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M, Bertin TK, Rogers GL, Cela RG, Zolotukhin I, Palmer D, Ng P, Herzog RW, Lee B. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs adeno-associated viral vectorsin vivo. Mol Ther. 2013;21:796–805. doi: 10.1038/mt.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (TH1)-specific immune responses and inhibits the development of IL-4-producing TH cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freytag SO, Barton KN, Zhang Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013;20:1131–1139. doi: 10.1038/gt.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fretag SO, Zhang Y, Siddiqui F. Preclinical toxicology of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for prostate cancer. Mol Ther Oncolytics. 2015;2:15006. doi: 10.1038/mto.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J, et al. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845–858. doi: 10.1089/hum.2005.16.845. [DOI] [PubMed] [Google Scholar]
- 41.He B, Huang X, Liu X, Xu B. Cancer targeting gene-viro-therapy for pancreatic cancer using oncolytic adenovirus ZD55-IL-24 in immune-competent mice. Mol Biol Rep. 2013;40:5397–5405. doi: 10.1007/s11033-013-2638-8. [DOI] [PubMed] [Google Scholar]
- 42.Malmstrom PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, Gårdmark T, Tötterman TH. AdCD40L immunogene therapy for bladder carcinoma – the first phase I/IIa trial. Clin Cancer Res. 2010;16:3279–3287. doi: 10.1158/1078-0432.CCR-10-0385. [DOI] [PubMed] [Google Scholar]
- 43.Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK, Gerdemann U, Leen AM, Kairemo K, Oksanen M, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72:1621–1631. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 44.Farzad L, Cerullo V, Yagyu S, Bertin T, Hemminki A, Rooney C, Lee B, Suzuki M. Combinatorial treatment with oncolytic adenovirus and helper-dependent adenovirus augments adenoviral cancer gene therapy. Mol Ther Oncolytics. 2014;1:14008. doi: 10.1038/mto.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]