Abstract
Brain white matter damage is frequently detected in patients infected with human immunodeficiency virus type 1 (HIV-1). White matter is composed of neuronal axons sheathed by oligodendrocytes (Ols), the myelin-forming cells in central nervous system. Ols are susceptible to HIV-1 viral trans-activator of transcription (Tat) and injury of Ols results in myelin sheath damage. It has been demonstrated that activation of voltage-gated K+ (KV) channels induces cell apoptosis and Ols predominantly express K+ channel KV1.3. It is our hypothesis that Tat injures Ols via activation of KV1.3. To test this hypothesis, we studied the involvement of KV1.3 in Tat-induced Ol/myelin injury both in vitro and ex vivo. Application of Tat to primary rat Ol cultures enhanced whole-cell KV1.3 current recorded under voltage clamp configuration and confirmed by specific KV1.3 antagonists Margatoxin (MgTx) and 5-(4-phenoxybutoxy) psoralen (PAP). The Tat enhancement of KV1.3 current was associated with Tat-induced Ol apoptosis, which was blocked by MgTx and PAP or by siRNA knockdown of KV1.3 gene. The Tat-induced Ol injury was validated in cultured rat brain slices, particularly in corpus callosum and striatum, that incubation of the slices with Tat resulted in myelin damage and reduction of myelin basic protein which were also blocked by aforementioned KV1.3 antagonists. Further studies revealed that Tat interacts with KV1.3 as determined by protein pull-down of recombinant GST-Tat with KV1.3 expressed in rat brains and HEK293 cells. Such protein-protein interaction may alter channel protein phosphorylation, resultant channel activity and consequent Ol/myelin injury. Taken together, these results demonstrate an involvement of KV1.3 in Tat- induced Ol/myelin injury, a potential mechanism for the pathogenesis of HIV-1-associated white matter damage.
Keywords: HIV-1, Tat, brain white matter, myelin, oligodendrocyte, KV1.3, neurodegeneration
1. Introduction
Neurologic complications of human immunodeficiency virus type 1 (HIV-1) infection remain common in the era of effective combination antiretroviral therapy (cART). Up to half of infected individuals develop HIV-1-associated neurocognitive disorders (HAND), the cause(s) remain obscure. Many studies have revealed a preferential damage to cerebral white matter in HIV-1-infected brain (Gongvatana et al., 2009; Hoare et al., 2011; Sarma et al., 2014; Wohlschlaeger et al., 2009), and such damage is prevalent even in the era of cART and more severe in patients with HAND (Chen et al., 2009b; Gosztonyi et al., 1994). Structure magnetic resonance imaging and diffusion tensor imaging studies in HIV-1-infected individuals have revealed a subcortical white matter damage mainly in the regions of the corpus callosum, internal capsule and other brain regions (Chang et al., 2008; Chen et al., 2009b; Gongvatana et al., 2009; Pomara et al., 2001; Sarma et al., 2014; Wu et al., 2006). Moreover, cognitive impairment in HIV-1-infected individuals with AIDS was found to be associated with white matter injury in the corpus callosum, internal capsule, and superior longitudinal fasciculus (Gongvatana et al., 2009; Wu et al., 2006). It appears that subcortical damage in white matter plays a more important role than cortical damage in the mediation of HAND symptoms which are predominantly of the subcortical type (Chen et al., 2009a; Navia et al., 1986; Peavy et al., 1994; Price et al., 1988).
Cerebral white matter consists mostly of myelinated axons and axonal myelination is formed by oligodendrocytes (Ols). The integrity of the myelin sheaths is essential for the propagation of nerve impulses along axons. It has been demonstrated that myelin pallor, an abnormality that could reflect a decrease in myelin components (Glass et al., 1993; Power et al., 1993), is frequently seen in patients with HIV-1 encephalitis and in cART naïve subjects. Myelin sheath damage and changes in numbers of Ols have also been observed in HIV-1-infected individuals (Esiri and Morris, 1996; Esiri et al., 1991). In vitro studies have shown that exposure to recombinant viral envelope protein gp120 resulted in alterations of Ol functional activity and myelin formation in rat Ols maintained in a cell culture system (Bernardo et al., 1997; Kimura-Kuroda et al., 1994), exampling HIV-1 protein impairment of Ol/myelin, which may lead to axonal injury, demyelination and ultimately white matter damage. Nevertheless, how HIV-1 proteins induce Ol/myelin injury is not fully understood.
Increasing evidence indicates that activation of voltage-gated K+ channels (KV) is an essential pathway in programmed cell death (Burg et al., 2006; Remillard and Yuan, 2004) and enhancement of outward K+ current results in neural cell apoptosis (Yu, 2003; Yu et al., 1997). Ols express several subtypes of KV channels including a predominant KV1.3 (Attali et al., 1997; Schmidt et al., 1999). A decrease of KV1.3 expression or outward K+ current in Ols is essential for synthesis of myelin structural proteins and suppression of outward K+ current promotes Ol maturation and survival. These results suggest a role of KV1.3 in the regulation of Ol functionality (Chittajallu et al., 2002; Tegla et al., 2011). Moreover, activation of p53 has been detected in the Ol lineage cells in the postmortem brains of HAND patients, but not in control brains (Jayadev et al., 2007), suggesting Ols undergo apoptosis in HIV-1-infected brains. Thus, it is our hypothesis that continued viral replication and viral proteins induce Ol/myelin injury by activation of Ol KV1.3 channels, leading to myelin/white matter damage and HAND pathogenesis. To test this hypothesis, we studied how HIV-1 protein Tat induces Ol/myelin injury, as infected brain cells continuously express and release Tat protein despite the controlled viral replication (Johnson and Nath, 2014; Johnson et al., 2013). Our results showed that HIV-1 Tat enhances outward K+ current conducted by KV1.3 leading to Ol/myelin injury.
2. Materials and Methods
2.1. Animals
Pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA) and maintained under the ethical guidelines for the care of laboratory animals, and all animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Nebraska Medical Center.
2.2. Ol preparation and culture
OI cell cultures were prepared as described previously (Chen et al., 2007) and all culture materials were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Briefly, cerebral cortical tissues were dissociated from P1-2 neonatal pups and incubated in Hank`s buffered salt solution contained in 0.25% trypsin and 200 U DNAase at 37 °C for 15 min. Collected cells were suspended in DMEM (with L-glutamine and sodium pyruvate, Cellgro, Manassas, VA) supplemented with 20% FBS (Gibco, Grand Island, NY) and 1% penicillin/streptomycin (Gibco). Mixed glia cultures were grown on poly-D-lysine-coated T75 flasks (Thermo, Nazareth, PA) for 10 d and Ols were isolated by shaking overnight at 200 r.p.m. at 37 °C. Cell suspensions were transferred onto non-treated petri dishes for 30 min to further separate Ols by differential adhesion. Ol-contained supernatant was collected by slightly swirling petri dish and passed through 40 µm nylon cell strainers into a sterile 50-ml tube. Ols were collected by centrifugation at 800 r.p.m. for 5 min and suspended in proliferating medium (described below). Ols were plated onto poly-D-lysine-coated coverslips, culture dishes, or plates in different culture media depending on the developmental stage. Basal chemically defined medium (BDM) was made of DMEM containing 0.1% BSA, 1% Insulin-Transferrin-Selenium (Gibco), 10 nM D-biotin, and 10 nM hydrocortisone. Isolated Ols were maintained in proliferating medium (BDM supplemented with 10 ng/ml PDGF-AA and 10 ng/ml bFGF (both from Peprotech, Rocky Hill, NJ) for 7 d. Myelin basic protein (MBP)+ mature Ols were obtained by transferring cells to differentiating medium (BDM, 15 nM triiodothyronine, 10 ng/ml CNTF (Peprotech) and 5 µg/ml N-acetyl-L-cysteine) for 2–3 d.
2.3. Electrophysiology
Cells were seeded onto 35 mm culture dishes for whole-cell recording of ionic currents. Recording electrodes made from borosilicate glass micropipettes (WPI Inc., Sarasota, FL) with a P-97 horizontal micropipette puller (Sutter Instruments, Novato, CA) had a tip resistance of 5–8 MΩ. The electrodes solution contained (in mM): 140 KCl, 2 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES/KOH, pH7.3, and had an osmolarity of 300 mOsm, as measured by a vapor pressure osmometer (WESCOR, Logan, UT). The standard bath solution contained (in mM): 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES/NaOH, pH7.3. D-sucrose was used to adjust the osmolarity of this solution to 305 mOsm prior to experiments. Stock solutions of KV1.3 specific inhibitors, 5-(4-phenoxybutoxy) psoralen (PAP, Sigma) and Margatoxin (MgTx, Sigma), were prepared in deionized water, stored at −20°C freezer and diluted to a working concentration with bath solution immediately before being applied to cells via bath perfusion at a constant flow rate of 1 ml/min. A Burleigh micromanipulator (PC-5000, EXFO, Canada) was used to position the recording electrode. Whole-cell K+ currents were induced by applying voltage steps from −150 mV to +60 mV in increments of 15 mV, and current amplitudes were measured at the peak for each test potential. Current density (pA/pF) was calculated by dividing the whole-cell capacitance (pF), which represents cell membrane surface area, from the peak current amplitude (pA). All experiments were done at room temperature (22–23 °C). Whole cell currents were amplified with an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 1 kHz, digitized at 5 kHz using a Digidata 1440A interface (Molecular Devices), displayed and recorded on a desktop computer. pCLAMP 10.0 (Molecular Devices) was employed for on-line data acquisition and off-line data analyses. All final graphics in the present work were constructed using Origin 8.5 (OriginLab, Northampton, MA).
2.4. siRNA knockdown of KV1.3 gene
Pre-designed ON-target plus SMARTpool siRNA against rat KCNA3 (KV1.3, NM-019270) mRNA was purchased from Dharmacon, Inc. (Chicago, IL). Ols were seeded at a density of 0.35 × 106/well into 6-well plates or 0.1 × 106/well into 12-well plates. According to the manufacturer instruction, KV1.3-siRNA and NT-siRNA were transfected at the final concentration of 25 nM for 72 h to gain the protein level knockdown in presence of Dharma FECT Transfection Reagent (Dharmacon, Inc). Transfected cells were then treated with 50 ng/ml Tat for 48 h.
2.5. Apoptosis Assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (Tunel) staining was used to measure apoptotic Ols. Cultures plated on coverslips were fixed in 4% paraformaldehyde for 1 h at room temperature and permeabilized with 0.1% Triton X-100 for 30 min. Tunel was performed according to manufacturer`s instructions for the in situ cell death detection kit (Fluorescein, Roche Applied Science, Indianapolis, IN). Cells were incubated for 90 min at 37 °C in Tunel reaction mixture contained terminal deoxynucleotidyl transferase and fluorescein-labeled nucleotides (1:50). After washing, coverslips were mounted in vectashield mounting medium with Dapi stain (Vector Laboratories, Burlingame, CA) and Ols were visualized by a fluorescent microscope. Apoptosis was assessed in each of 3 experimental preparations by examining 10 visual fields per experimental group.
2.6. Brain slices cultures
Coronal brain slices were prepared from 21 day old Sprague-Dawley rats. The slice culture was performed as described by Stoppini et al (Stoppini et al., 1991). Briefly, after anesthesia with isoflurane, the rats were quickly decapitated by small animal decapitator (Stoelting, 51330). The whole brain was dissected and fixed onto the stage of vibrotome (Campden instruments, MA752 Motorised advance vibroslice). Slices of 400 µm were cut and separated in oxygenated artificial cerebrospinal fluid contained (in mM): NaCl 124.0, KCl 3.0, CaCl2 2.0, MgCl2 2.0, NaH2PO4 1.25, NaHCO3 26.0, and glucose 10.0, pH 7.2. The slices were then transferred on to culture inserts plated in 6-well plate with 1 ml/well pre-warmed (37 °C) culture medium. It is important to keep the slices placed on the interface between air and medium. The culture medium consisted of 50% MEM with HEPES (Gibco), 25% horse serum (Atlanta biological, Flowery Branch, GA), 25% Hank`s solution (Invitrogen), and 6.5 mg/ml glucose. The 6-well plates were then kept in incubator at 37 °C with 5% CO2, and the medium was refreshed every three days.
2.7. Cryostat sections
The cultured slices were washed three times with PBS after treatments, then fixed with 4% paraformaldehyde for 24 h. After removal of 4% paraformaldehyde, the graded (10%, 20%, and 30%) sucrose/PBS solutions were sequentially added, each for 24 h, to slices for cryoprotection. The slices were then orientated and embedded within OCT and quickly frozen in −80°C. The slice tissue blocks were cut into 10 µm sections by Leica cryostat (LEICA CM1850 UV).
2.8. Immunofluorescence detection of myelin sheaths
The prepared brain sections were blocked and permeablized in 10% goat serum/0.1 % Triton/PBS solution for 30 min at room temperature, and incubated with anti-rat MBP antibody (1:200, MAB386, Chemicon/Millipore, Billerica, MA) in a humidified chamber at 4 °C overnight. The sections were washed three times with PBS and then incubated with goat anti-rat fluorescent secondary antibody (1:1000, A11006, Invitrogen) for 2 h at room temperature. After washing, sections were mounted with coverslips in vectashield mounting medium with Dapi (Vector Laboratories, Inc.), and myelin sheaths were visualized under fluorescent microscope.
2.9. Western blot
Following experimental treatments, cells or brain tissues were washed twice with ice-cold PBS. The whole cell lysates were prepared in RIPA buffer (BioRad, Hercules, CA) while brain tissues were lysated in tissue extraction reagent (Invitrogen), followed by a freeze-thaw cycle for complete lysis. After quantification of protein concentrations with BCA protein assay kit (ThermoFisher Scientific, Waltham, MA), 15 µg of total protein was loaded onto 10% SDS-polyacrylamide gels, separated by electrophoresis, and transferred to a polyvinylidene difuoride (PVDF) membrane. The PVDF membrane was then blocked in 5% dry milk in Tris-buffered saline, followed by overnight incubation of primary antibodies diluted in blocking buffer at 4 °C. Primary antibodies were rabbit polyclonal anti-KV1.3 (1:100, Alomone Lab, Israel), rat monoclonal anti-MBP (1:500, Chemicon/Millipore) and mouse monoclonal anti-β-actin (1:5000, Sigma). Afterwards, membranes were washed in TBS with 0.1% Tween for 10 min × 4 times and incubated for 1.5 h at room temperature with HRP-conjugated anti-rabbit, anti-rat or anti-mouse secondary antibodies (1:10,000, all from Jackson ImmunoResearch Laboratories, West Grove, PA). After washing, membranes were finally incubated with Pierce ECL Western blotting substrate (Thermo Scientific, Rockford, IL) and, imaged by the FluorChem M system (ProteinSimple, Santa Clara, CA). Band densities were measured by Alphaview software (ProteinSimple).
2.10. Over-expression of KV1.3 in HEK293 cells
HEK293 cells were seeded in 6-well plates at a density of 0.6 × 106/well and, grew overnight to reach 70–90% confluent. Lipofectamine 2000 reagent was diluted in Opi-MEM (Invitrogen) at a ratio 5 µl: 95 µl. The pJK or pJK/KV1.3 plasmid at amount of 2.5 µg was diluted in Opi-MEM to make the final volume at 100 µl. Diluted plasmids were added to each tube of diluted lipofectamine 2000 and, incubated at room temperature for 15 min. DNA-lipofectamine complex were applied to cells. Cells were collected and lysed after 48 h. The pJK and pJK/KV1.3 plasmids were gifts provided by Professor Erich Gulbins from Department of Molecular Biology, University of Duisburg-Essen, Essen, Germany (Szabo et al., 2005).
2.11. Protein pull-down assay
The HEK293 whole cell lysate was prepared same as described above. Rat brain tissue sample was homogenized in the isotonic sucrose homogenization buffer (2 ml) contained 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease inhibitor cocktail (Thermo Scientific, Rochester, NY) in a glass-grinding vessel with a motor driven Teflon pestle at 700 r.p.m.. The homogenate was then centrifuged at 800 g for 10 min at 4 °C. The supernatant was aspirated into a new tube and centrifuged at 12,000 g for 15 min at 4 °C. Pellets were dissolved in a buffer contained 10 mM HEPEs, 1% Triton X-100 (pH 7.4) and proteinase inhibitor cocktail. Then the tube was centrifuged at 12,000 g for 15 min at 4 °C. The supernatant (membrane enriched part) was collected for pull down assay. GST-HIV-1-Tat recombinant protein (1 µg for transfected-HEK293 and 10 µg for rat brain tissue protein) were added to protein lysate with rotation overnight at 4 °C. The next day, 50 µl sepharose beads (GE health) were added to the tube for another 3 h rotation. The beads were collected by centrifugation at 5,000 g for 2 min and followed with three times wash by PBS. Then the beads were added with 30 µl 2 × loading buffer and boiled at 95 °C for 10 min to elute the binding protein complex. The eluted samples were subjected to Western Blot for analysis of KV1.3 presence with specific KV1.3 antibody (1:1000, NeuroMab).
2.12. Statistics
All data are expressed as mean ± S.D. unless otherwise indicated. Statistical analyses were performed by student`s t test or one-way ANOVA followed by a Fisher`s least-significant difference test for multiple comparisons. The difference between groups was considered significant at p < .05.
3. Results
3.1. HIV-1 protein Tat enhancement of KV1.3 current in Ol lineage cells
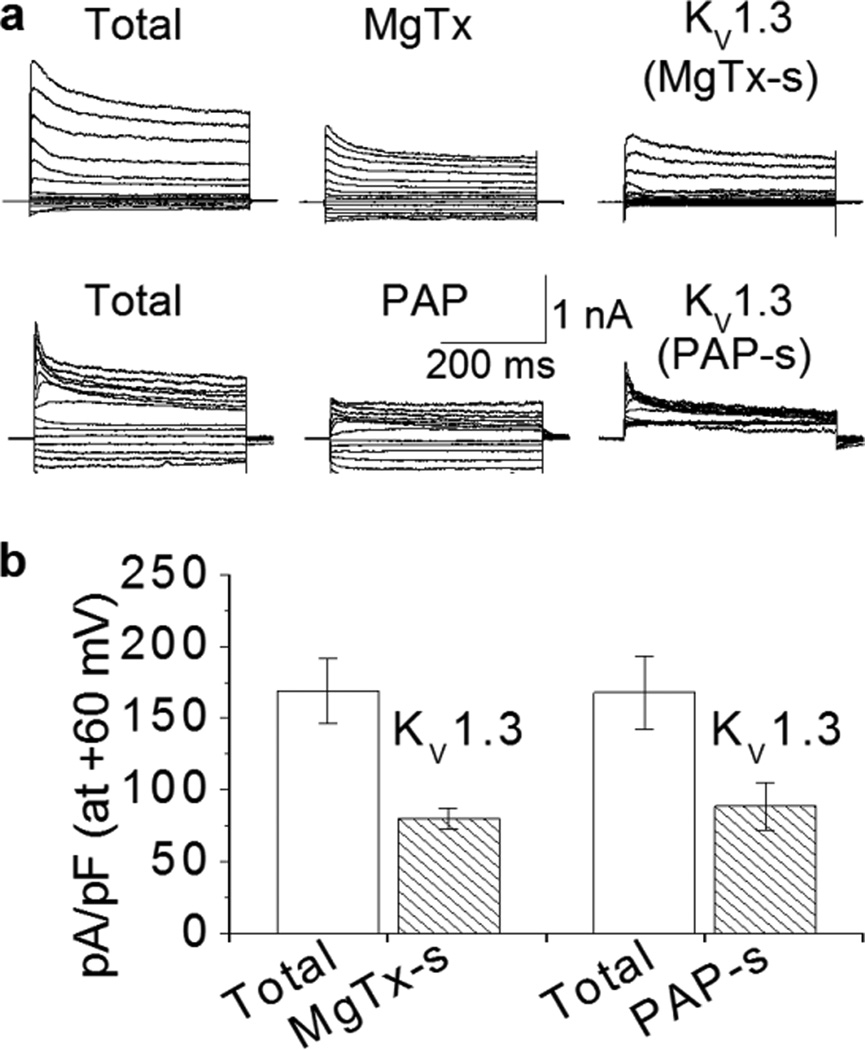
To determine if HIV-1 protein Tat induces Ol/myelin injury via activation of KV1.3 channels, we first examined the expression of KV1.3 current in Ol cells. Two specific KV1.3 channel antagonists, MgTx and PAP, were utilized to isolate KV1.3-conducted K+ currents. Whole-cell outward K+ currents, induced by voltage-steps, were recorded in Ols at the age of 14 DIV in the absence (total) and presence of specific KV1.3 antagonist 5 nM MgTx (IC50 ~1 nM) or 10 nM PAP (IC50 = 2 nM (Schmitz et al., 2005)) in the perfusate. KV1.3 currents were calculated by subtraction of the outward K+ currents recorded 20 minutes after the entrance of MgTx (MgTx) or PAP (PAP) into the bath chamber from the outward K+ currents recorded in the absence of MgTx or PAP in the perfusate (Total). These MgTx-sensitive (MgTx-s) and PAP-sensitive (PAP-s) currents are KV1.3 currents as illustrated at the right column (Fig. 1a). These results demonstrated that KV1.3 contributes approximately 50% of the total currents in Ol lineage cells (Fig. 1b).
Figure 1. Expression of KV1.3 current in Ols.
Representative traces are whole-cell outward K+ currents recorded in Ols before (total) and after super-fusion of Ols with PAP (10 nM) or MgTx (5 nM). KV1.3-excluded currents showed in the middle column were recorded 20 min after super-fusion of Ols with each specific KV1.3 antagonist as indicated. The KV1.3 antagonist-sensitive currents (MgTx-s and PAP-s), which represent the KV1.3 current, are calculated by subtraction of individual KV1.3-excluded current from corresponding total current and shown in the right column. Panel b, whole-cell current density and the subtracted-KV1.3 current density at +60 mV (n = 6 of each antagonist super-fusion). KV1.3 current contributes to approximately 50% of the total outward K+ currents in Ols.
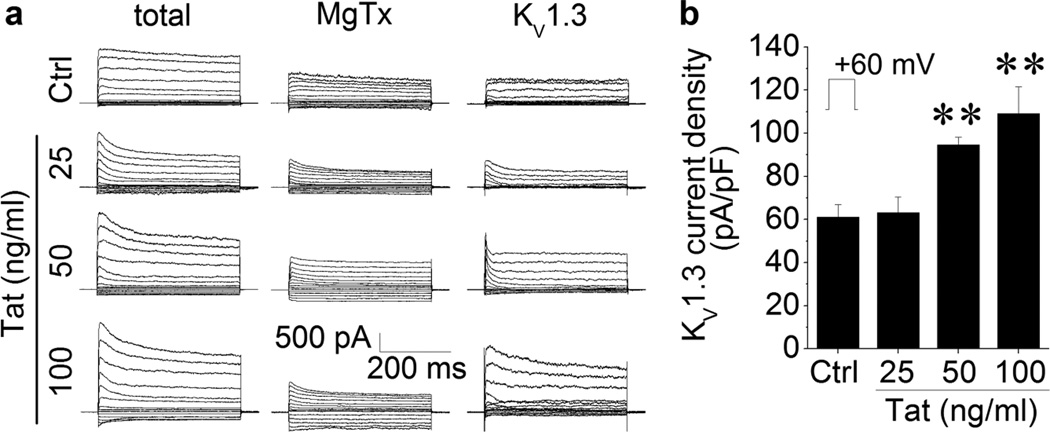
After demonstration of KV1.3 expression in the Ols, we next tested effects of Tat on KV1.3 current recorded in the Ols. Experiments were carried out on four groups of Ols, one was untreated controls (Ctrl, n = 6) and the others were incubated with Tat at three doses (Tat, 25, 50, and 100 ng/ml, n = 6 for each dose) for 2 h before conducting electrophysiology recording. Whole-cell outward K+ currents were recorded in the absence (Total) and presence of MgTx (MgTx). The KV1.3 (KV1.3) currents were calculated as described above. Incubation of the Ols with Tat at doses of 50 and 100 ng/ml produced an increase of KV1.3 currents (Fig. 2a). The average peak current densities, when measured at +60 mV, were 61.17 ± 5.66 pA/pF in control Ols, 94.60 ± 3.59 pA/pF in cells treated with 50 ng/ml Tat, and 109.03 ± 12.59 pA/pF in Ols treated with 100 ng/ml Tat (Fig. 2b). The difference is statistically significant, indicating Tat enhancement of KV1.3 current in Ols.
Figure 2. Tat increased KV1.3 current on Ols.
Data were obtained from Ols with (Tat) or without (Ctrl) incubation of Tat for 2 h at doses as indicated. Panel a shows representative whole cell outward K+ currents recorded before (total, left column) and 15 min after addition of 5 nM MgTx (MgTx, middle column) to the bath. The KV1.3 currents were then isolated by subtraction of outward K+ currents recorded in the presence of MgTx from the total currents (KV1.3, right column). Panel b is a summary bar graph illustrating average KV1.3 current density (pA/pF) obtained from Ols without (Ctrl) and with Tat treatment (25, 50, 100 ng/ml). Note that incubation of 50 ng/ml and 100 ng/ml Tat significantly increased KV1.3 current density measured at +60 mV. ** p < .01 vs. control Ctrl. n = 6 in each group.
3.2. Involvement of KV1.3 in Tat-induced Ol injury
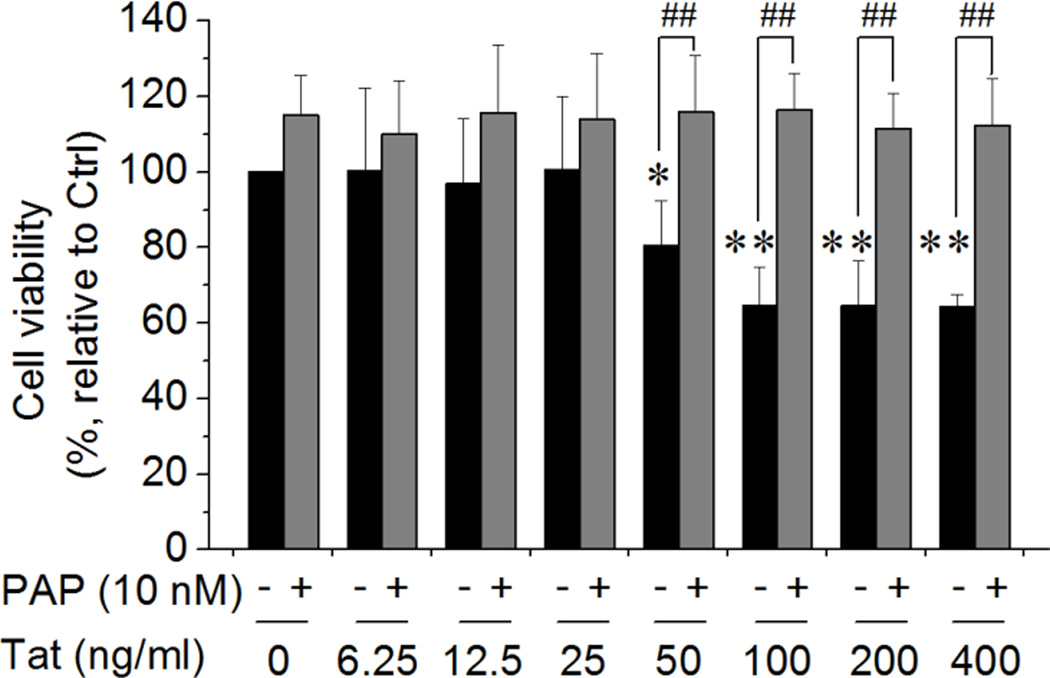
It has been reported that enhancement of outward K+ current causes neural cell apoptosis (Yu, 2003; Yu et al., 1997). To explore whether the Tat enhancement of KV1.3 current is associated with Tat-mediated neurotoxic activity, we examined involvement of KV1.3 channel in Tat-induced Ol injury by MTT assay in the absence and presence of pre-added (1 h earlier) PAP (10 nM), a specific KV1.3 antagonist. Our results showed that addition of Tat to the culture media for 48 h significantly decreased Ol viability in a concentration-dependent manner. At the concentrations of 50 ng/ml and 100 ng/ml, Tat reduced Ol viability to 80.50% ± 6.81% of control level (p < .05) and 64.76% ± 5.83% of control level (p < .01), respectively, indicating that Tat induces Ol injury in vitro (Fig. 3). Increase of Tat concentrations to 200 ng/ml and 400 ng/ml did not produce further decrease of Ol viability (Fig. 3). The Tat-induced decrease of Ol cell viability was blocked by pre-treatment of the Ols with PAP, demonstrating an involvement of KV1.3 in Tat-induced Ol injury (Fig. 3). It has been shown that Tat concentration can reach as high as ~40 ng/ml in the serum of HIV-1-positive patients, with potential higher concentrations at local tissue sites because Tat in vivo might be sequestered by endogenous anti-Tat and/or by glycosaminoglycans (Westendorp et al., 1995; Xiao et al., 2000). Thus, Tat at a concentration of 50 ng/ml was considered as being physiological relevant and adopted for the subsequent studies reported hereinafter.
Figure 3. Blockage of KV1.3 prevented Tat-induced Ol injury.
Ol cultures were exposed to Tat for 48 h at different concentrations as indicated with or without pre-addition of 10 nM PAP for 1 h. Tat produced a significant reduction of Ol cell viability at concentrations ≥ 50 ng/ml. Tat-mediated reduction of Ol cell viability was prevented by addition of a specific KV1.3 antagonist PAP, suggesting the Tat-mediated reduction of Ol cell viability was mediated via KV1.3. Data presented were from 6 independent experiments. *p < .05 and **p < .01 vs. control; ##p < .01 as indicated.
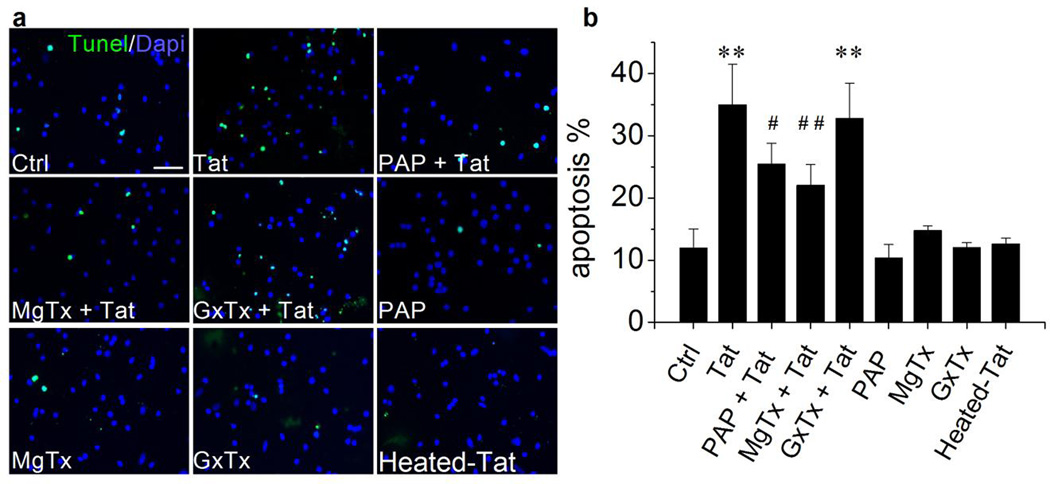
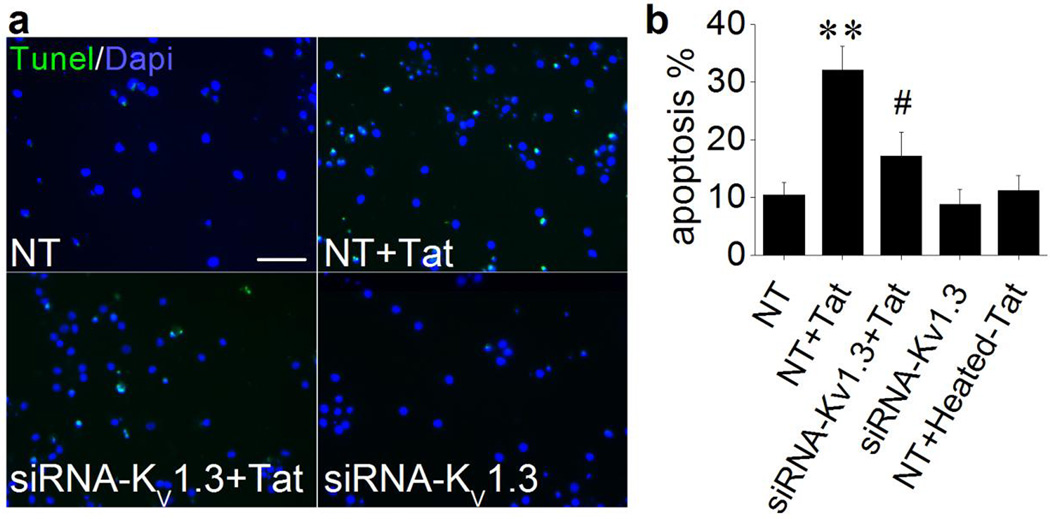
Increasing evidence indicates that activation of KV channels is an essential pathway in programmed cell death (Burg et al., 2006; Remillard and Yuan, 2004) and activation of apoptotic pathway has been observed in both HAND patients and Tat transgenic mice (Hauser et al., 2009; Jayadev et al., 2007). Whether Tat enhancement of KV1.3 current contributes to Tat-induced Ol apoptosis was further investigated using pharmacological and molecular genetic approaches. As anticipated, addition of Tat to the Ol culture for 48 h resulted in Ol apoptosis as detected by Tunel assay. Heated Tat (boiled for 15 minutes) had no significant effect on inducing Ol apoptosis (Fig. 4). The Tat-induced apoptosis was significantly attenuated by pre-addition (1 h earlier) of specific KV1.3 antagonists PAP (10 nM) or MgTx (5 nM) to the culture media. In contrast, pre-addition of Guangxi Toxin (GxTx), a specific antagonist for K+ channel KV 2.1 which is also expressed in Ols (Liu et al., 2015), failed to block Tat-induced Ol apoptosis. Addition of PAP, MgTx or GxTx each alone had no apparent effects on Ol apoptosis. These results showed a clear involvement of KV1.3 in Tat-induced Ol apoptosis (Fig. 4). The Tat-induced Ol apoptosis was also blocked through genetic knockdown of KV1.3 gene. As shown in Fig. 5, transfection of Ols with KV1.3-siRNA for 72 h prevented Tat-induced Ol apoptosis. In contrast, Ols transfected with Non-targeting (NT) siRNA (controls) did not block Tat-induced Ol apoptosis. KV1.3-siRNA per se had no effect on Ol cell apoptosis. In parallel with the experimental data obtained with pharmacological approaches, these results further demonstrated an involvement of KV1.3 in Tat-induced Ol injury.
Figure 4. Involvement of KV1.3 in Tat-induced Ol apoptosis.
Ols were exposed to Tat (50 ng/ml) for 48 h with or without pre-addition (1 h earlier) of PAP (10 mM), MgTx (5 nM), Panel a exhibits Tunel staining (green in color) revealed that Tat induced Ol apoptosis (Tat) and the Tat-induced Ol apoptosis was attenuated by pre-addition of PAP (PAT+Tat), MgTx (MgTx+Tat) or GxTx (GxTx+Tat) to the cultures. Intact cell nuclei were visualized with Dapi (blue in color). In a subset of cultures, PAP (PAP), MgTx (MgTx), GxTx (GxTx) and heat-inactivated Tat (50 ng/ml, heated-Tat) were tested individually. Panel b shows average percentage of apoptotic cells from five independent experiments. There were nine different experimental treatments in each experiments and 10 randomly selected visual fields were counted for each experimental treatment. As showed in Panel b, Tat-induced Ol apoptosis was attenuated by either specific KV1.3 antagonist PAP or MgTx, but not by GxTx, a specific antagonist for KV2.1, demonstrating an involvement of KV1.3 in Tat-induced Ol apoptosis. ** p < .01 vs. control; #p < .05 vs. Tat; ## p < .01 vs. Tat. Scale bar equals 20 µm.
Figure 5. Knockdown of KV1.3 gene prevented Tat-induced Ol apoptosis.
Cultured Ols were transfected with KV1.3-siRNA for 72 h to knockdown KV1.3 gene or with non-targeting siRNA as a control. Following siRNA transfection, 50 ng/ml Tat or heat-inactivated Tat was applied to the transfected cells for another 48 h. Representative Tunel staining images (Panel a) showed that knockdown of KV1.3 gene prevented Tat-induced Ol apoptosis (siRNA-KV1.3+Tat). In contrast, non-targeting siRNA (NT) failed to block Tat-induced Ol apoptosis (NT+Tat). Non-targeting siRNA (NT) and KV1.3-siRNA (siRNA-KV1.3) per se had no apparent effect on basal levels of apoptosis. Also, Ols transfected with NT and treated with Heat-inactivated Tat (NT + Heated-Tat) had no apparent effect (image not shown). This study was conducted in three independent experiments. Apoptotic cells were counted from 10 randomly selected visual fields in each experiment with different experimental treatments, and then calculated and shown in Panel b that knockdown of KV1.3 gene prevented Tat-induced Ol apoptosis. ** p < .01 vs. NT; # p < .05 vs. NT + Tat. Scale bar equals 20 µm.
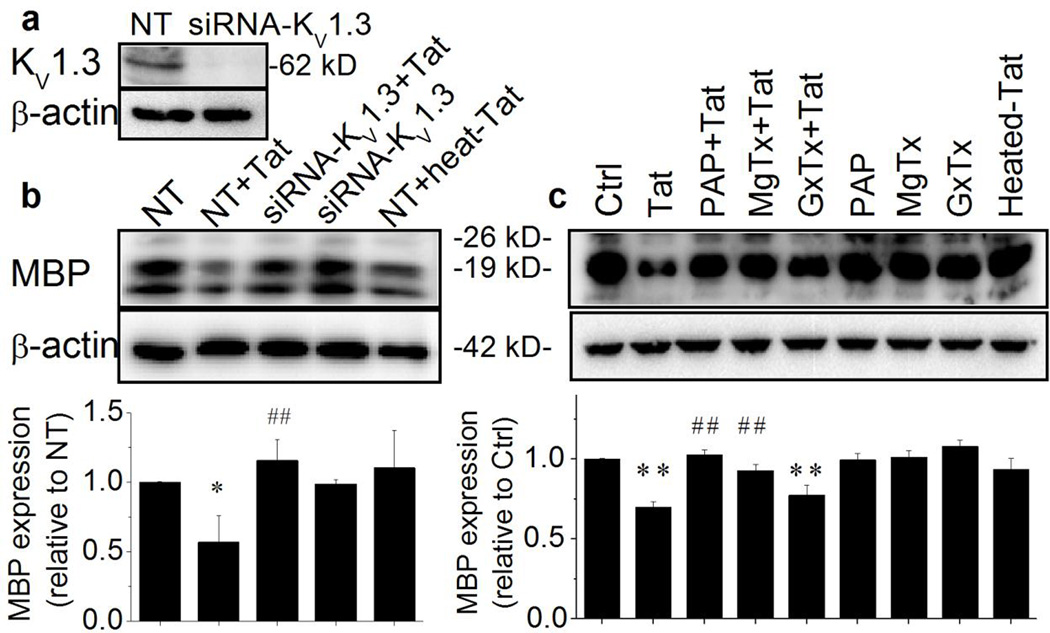
3.3. Tat reduction of MBP expression via KV1.3
MBP is an essential myelin structural protein and decreased levels of MBP expression in Ols reflect an impaired ability of Ols on axonal myelination. Classically, MBP has three isoforms that arise from different transcription start sites and the protein band at 18.5-kDa is considered as the predominant isoform essential for CNS myelin stability (Smith et al., 2012; Vassall et al., 2013). To determine Tat influence on MBP expression, we specifically examined the protein band at 18.5-kDa by Western blot analyses. The same treatments as described in assessing Ol cell apoptosis (Fig. 5) were employed in the experiments. The effectiveness of siRNA-KV1.3 on knockdown KV1.3 gene was first evaluated. As shown in Fig. 6a, Ols transfected with siRNA-KV1.3 revealed the KV1.3 gene silence as evidenced by a significant reduction on the levels of KV1.3 protein expression when compared with the KV1.3 protein expression levels in non-targeting siRNA (NT)-treated Ols. The involvement of KV1.3 on Tat-induced alteration on MBP expression was then determined through molecular genetic and pharmacological approaches. As illustrated in Fig. 6c, treatment of Ols with Tat significantly decreased the levels of MBP expression compared to the MBP levels detected in non-treated controls (p < .01). The Tat-induced decrease of MBP expression was blocked by specific KV1.3 channel antagonists PAP and MgTx, but not by a KV2.1 antagonist GxTx, demonstrating activation of KV1.3 underlies Tat-mediated decrease of Ol MBP expression. Such experimental results were duplicated by using siRNA to knockdown of KV1.3 gene (Fig. 6b). These results showed Tat decreases Ol myelination ability via KV1.3.
Figure 6. Knockdown of KV1.3 gene abolished Tat reduction of MBP expression.
Ols were treated the same as described in Figs. 4 and 5. Data presented were from three independent experiments. Panel a shows a representative gel demonstrating a successful KV1.3 protein knockdown as a consequence of KV1.3 gene silencing. Panel b exhibits representative western blot results showing Tat-induced reduction on MBP expression was blocked by knockdown of KV1.3 gene (upper). The band densitometric bar graph (lower) revealed a significant Tat reduction of MBP expression and the Tat-associated reduction of MBP expression was abolished by genetic KV1.3 silence. Panel c illustrates Tat reduction of MBP expression was pharmacologically attenuated by specific KV1.3 antagonists, PAP and MgTx, but not by GxTx, a specific KV2.1 antagonist, demonstrating that Tat decrease of Ol MBP expression via KV1.3. *p < .05 vs. ctrl or non-targeting siRNA (NT); ** p < .01 vs. control or NT; #p < .05 vs. Tat or NT + Tat; ## p < .01 vs. Tat or NT + Tat.
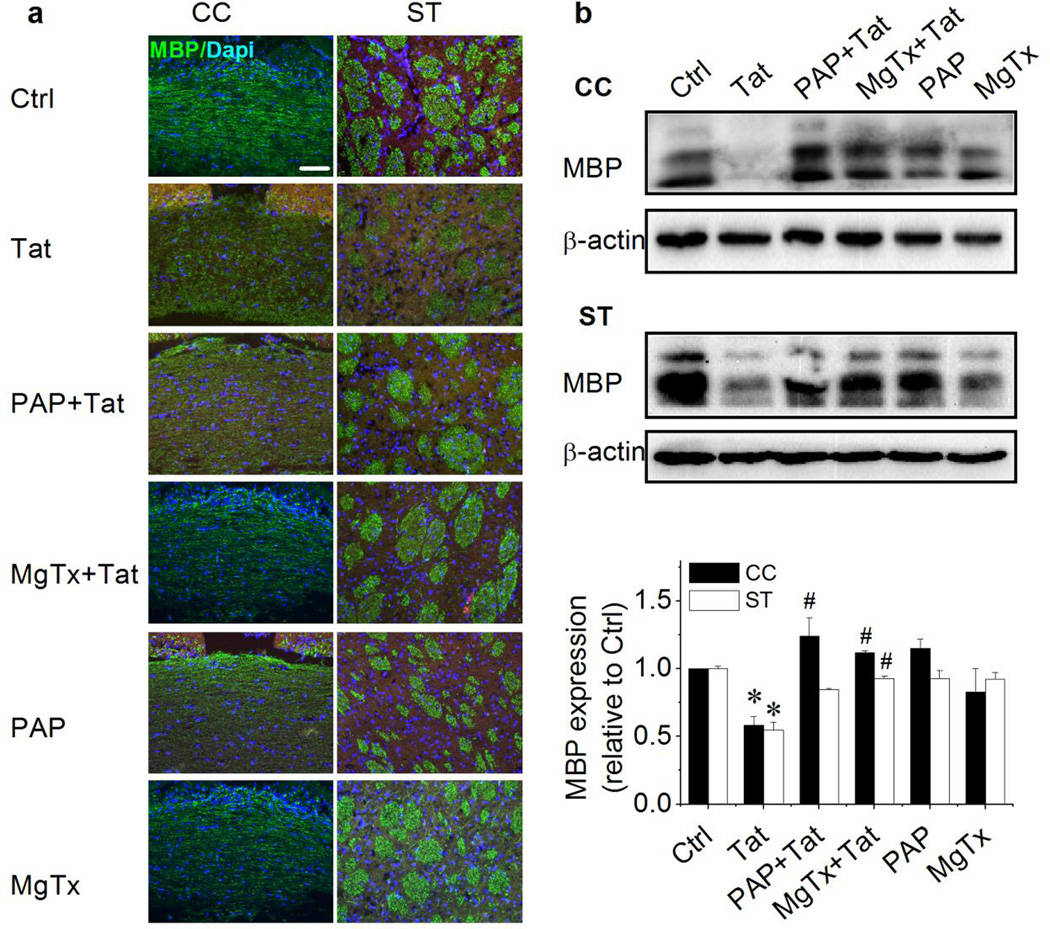
Since there were no neurons in Ol cell cultures in above experiments, it was difficult to evaluate if Tat decrease of MBP expression reflects HIV-1-associated Ol/myelin damage seen in HIV-1-infected individuals. Alternatively, cultured brain slices were utilized as an ex vivo Ol/myelin injury model to address possible pathophysiological processes in an in vivo condition. Coronal brain slice cultures, prepared from young adult rats, were utilized to examine Tat alteration of myelination. Tat was added to slice culture for 72 h and specific KV1.3 antagonists were added 1 h prior to addition of Tat. The treated and untreated slices were then subject to MBP immunostaining to examine myelin integrity and myelination was evaluated by western blot analyses of MBP expression. The brain regions of corpus callosum and striatum were selected for analyses of myelin sheath integrity. The MBP reactivity in the corpus callosum was continuously aligned and exhibited an intact appearance in control (Ctrl) brain slices, whereas the MBP reactivity in the corpus callosum was discontinuous aligned and disorganized in brain slices treated with Tat (Fig. 7a left). In comparison with the clear and intense axonal bundles seen in the striatum of control brain slices, the striatum of Tat-treated brain slices showed impaired axonal bundles (Fig. 7a right). These abnormalities of myelinated nerve fibers and bundles were not observed in the slices treated with Tat combined with KV1.3 antagonists PAP and MgTx, illustrating the blockade of Tat-induced impairments of myelin sheaths in both brain regions by KV1.3 antagonists. In parallel with the morphological changes observed in the corpus callosum and striatum, Tat decreased the levels of MBP protein expression in these two brain regions and the Tat-associated decrease of MBP protein expression was also blocked by specific KV1.3 antagonists (Fig. 7B), further support the notion that Tat induces Ol/myelin/white matter injury via KV1.3.
Figure 7. Tat-induced myelin injury in brain slices and its blockage by KV1.3 antagonists.
Cultured brain slices were exposed to 50 ng/ml Tat for 72 h with or without pre-addition (1 h earlier) of 10 nM PAP or 5 nM MgTx. Panel a, representative images of MBP immunofluorescence staining in corpus callosum (CC) and striatum (ST). Intact cell nuclei were visualized with Dapi. Tat impaired the myelin and nerve bundle integrity in the CC and ST. Panel b, western blot analyses of MBP expression in brain tissues of the CC and the ST dissected out from cultured brain slices and lysed in tissue extraction buffer. Tat decreased MBP expression both in the CC (upper) and in the ST (middle). Band densitometry data are shown in the bar graph (lower). Data are expressed as mean ± S.E.M. after normalized to β-actin shown in each gel. Note that Tat-induced reduction of MBP expression was blocked by PAP and MgTx. Experiments were done in triplicates. Scale bar equals 20 µm *P < .05 vs. ctrl; #P < .05 vs. Tat.
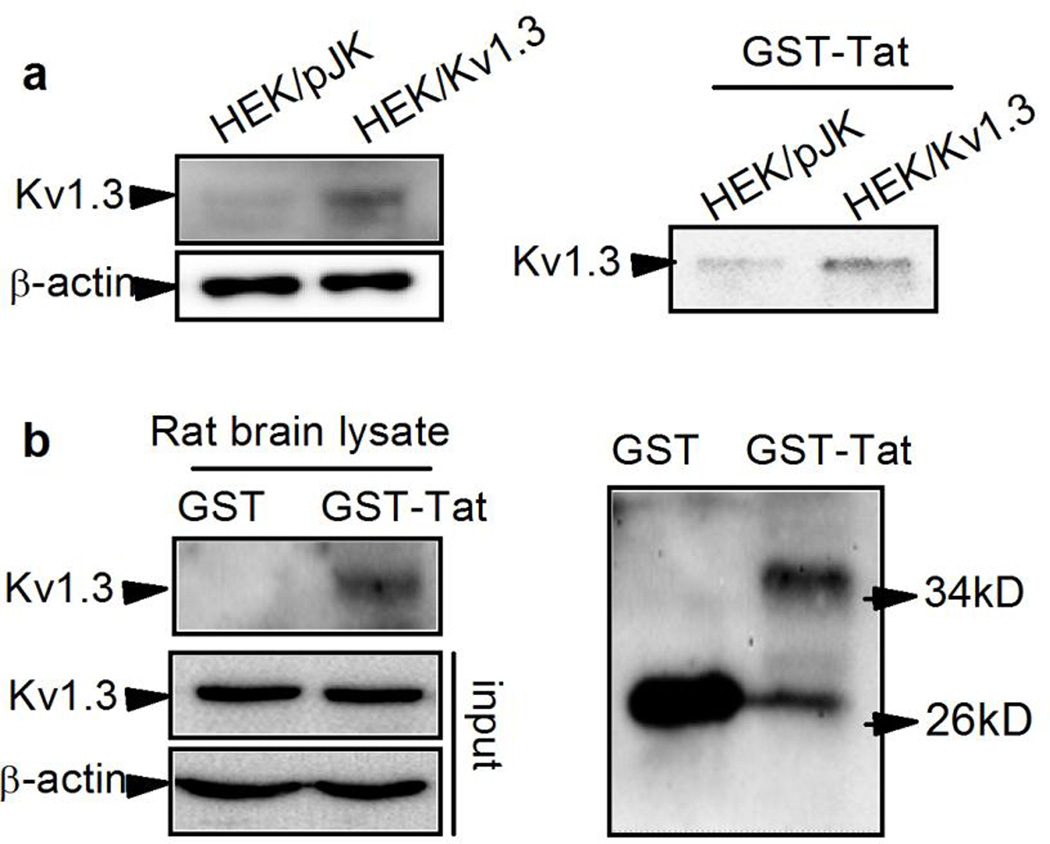
3.4. Tat interacts with KV1.3 channel
Having demonstrated the involvement of KV1.3 in Tat-induced Ol/myelin injury, we next sought to examine if Tat interacts directly with KV1.3 at protein level. For this purpose, we transfected HEK293 cells with pJK/KV1.3 plasmid, followed by pull-down of GST or GST-HIV-Tat recombinant proteins (GST-Tat) with total HEK293 cell lysates. As anticipated, KV1.3 protein was successfully over-expressed in HEK293 cells (Fig. 8a, left panel right lane) and, the recombination of GST-Tat was confirmed by western blot (Fig. 8b right panel). KV1.3 was precipitated by GST-Tat in lysates from HEK293 cells infected with pJK/KV1.3 (HEK/KV1.3), but not detected in pJK-infected cells (HEK/pJK, Fig. 8a right panel), suggesting Tat was associated with KV1.3 in HEK293 cells. The association of Tat and KV1.3 proteins was also detected in rat brain homogenate by the GST pull-down assay. As showed in Fig. 8b (upper left), KV1.3 was detectable in precipitated fraction of rat brain with addition of GST-Tat but not in fraction with GST per se, suggesting that Tat interacts with KV1.3 in rat brain tissue as well.
Figure 8. Tat interacts with KV1.3 protein.
GST-Tat recombinant protein was added to pJK-KV1.3 transfected HEK293 whole cell lysate or rat brain tissue homogenate for protein pull-down assay. Panel a, Tat interacted directly with KV1.3 expressed in HEK293 cells. Left in panel a is representative blot results showing KV1.3 protein was successfully overexpressed in HEK293 cells transfected with pJK-KV1.3 (HEK/KV1.3) plasmids. Right in panel a shows pull-down assay demonstrating that Tat can interact with KV1.3 protein overexpressed in HEK293 cells. Shown in Panel b are the pull-down results illustrating GST-Tat interacts with KV1.3 protein in rat brain tissue lysates (left). The expression of KV1.3 in rat brain is showed in the lower left. Right in panel b shows the bands of GST (lower bands) in both GST and Tat band (upper band) only in GST-Tat.
3.5. Tat enhances Ol KV1.3 current by retarding channel inactivation and downregulation of channel protein phosphorylation
The results presented above showed that Tat enhances Ol KV1.3 current, leading to Ol/myelin injury. To understand how Tat enhances Ol KV1.3 current, we investigated the impact of Tat on Ol KV channel kinetics. Primary Ols were treated with 50 ng/ml Tat for 2 h before recording, and whole cell K+ currents were recorded before (Tat) and after addition of 5 nM MgTx to the bath solution (MgTx + Tat) for 20 min. Tat did not shift the activation curve of Ol K+ currents, with V1/2 = 21.52 ± 4.19 mV in control cells and V1/2 = 20.93 ± 4.55 mV in Tat-treated cells (n = 6, p >.05). However, the inactivation curve shifted to the right after Tat treatment and, the shift was blocked by MgTx (Fig. 9a). To confirm the Tat-induced K+ channel kinetic change results in K+ current density change, the total K+ current density was analyzed in Tat-treated Ols before and after application of MgTx in the bath solution. Tat increased the total outward K+ currents in Ols and the increase was blocked by MgTx (Fig. 9b). These results suggest KV1.3 is primarily involved in the Tat-induced inactivation curve shift, implying Tat retardation of KV1.3 channel inactivation and resultant enhancement of KV1.3 current.
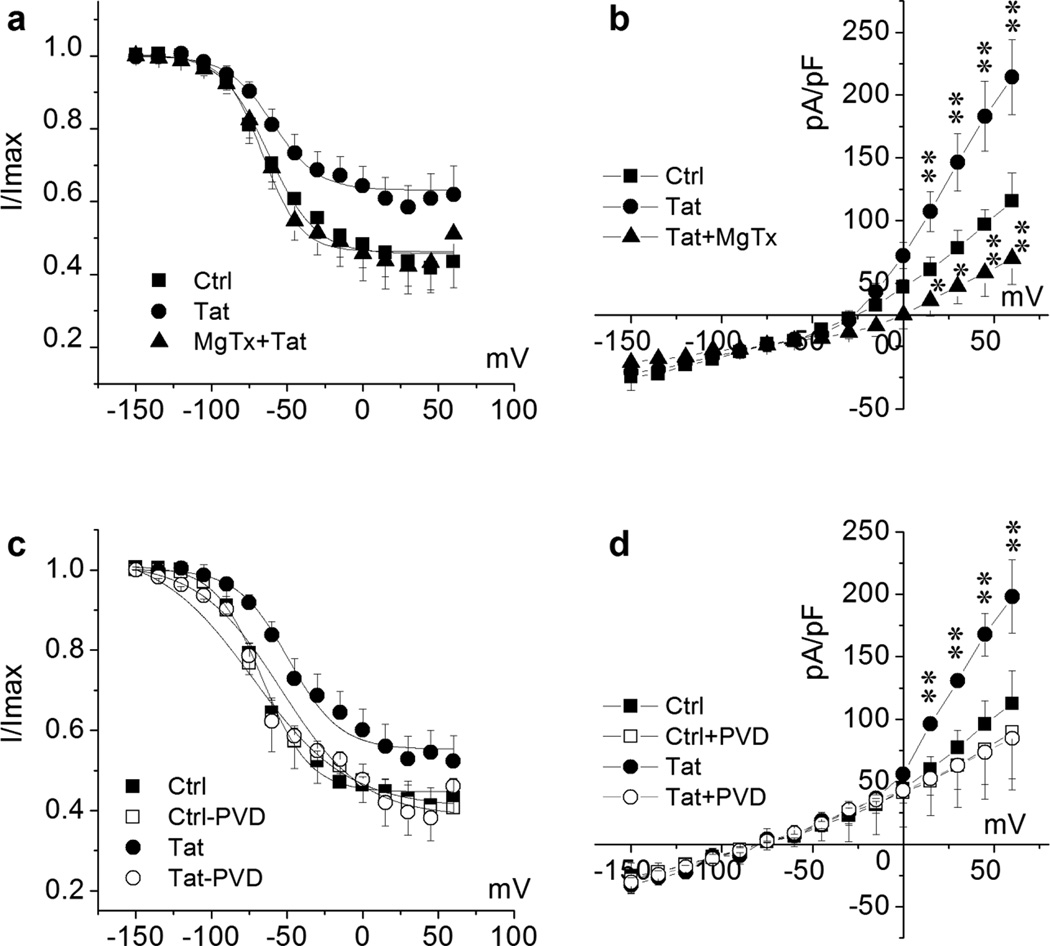
Figure 9. Tat alteration of KV1.3 channel inactivation and channel phosphorylation regulation.
For channel inactivation analysis, recoded cells were held at −80 mV during voltage-clamping. K+ channels were pre-activated by voltage steps from −150 mV to +60 mV with 15 mV increments and then tested at the depolarization pulse of +40 mV. Data are expressed as mean ± S.E.M.. Normalized data points were fitted with the Boltzmann equation: I/Imax=1/{1+exp[(V-V1/2)/k]}. Cultured Ols were treated with Tat (50 ng/ml) for 2 h prior to recording. Non-treated Ols were recorded as control (Ctrl). Panels a and b, Tat-treated cells were recorded before (Tat) and after (Tat + MgTx) application of 10 nM MgTx in the bath. Fitted inactivation curve of each group as indicated is shown in Panel a. n = 8 in each group. V1/2 of Tat was −57.99 ± 2.23 mV compared with −62.39 ± 1.79 mV in Ctrl; and V1/2 was −66.41 ± 2.03 mV in Tat + MgTx, indicating an involvement of KV1.3 in Tat-mediated right shift of the inactivation curve. MgTx blocked Tat-enhanced total outward K+ current density (Panel b). Panels c and d illustrate that bath application of PVD (200 µM), a tyrosine phosphatase inhibitor, blocked Tat-induced right-shift of inactivation curve (Panel b) and Tat-mediated increase of mean peak current density as plotted in the I-V curves in Panel c (n = 6), demonstrating that Tat may enhance Ol KV1.3 current by downregulation of phosphorylation of KV1.3 tyrosine residues. Fitted inactivation curve of each group in Panel b as indicated (n = 6). V1/2 of Tat-treated group was −49.06 ± 2.62 mV in comparison with −66.32 ± 1.46 mV of Ctrl. V1/2 of Tat-PVD group was −65.01 ± 4.53 mV compared with −66.60 ± 3.78 mV of Ctrl-PVD. * p < .05 vs. Ctrl, ** p < .01 vs. Ctrl.
It is known that KV1.3 channel contains several regulatory tyrosine residues at N- and C-termini, as well as p-loop (Colley et al., 2009; Fadool, 1998). Tyrosine kinases phosphorylate KV1.3 at these residues and cause channel inactivation, which is an important mechanism for KV1.3 activity regulation. As Tat retards KV1.3 inactivation, we next examined the possibility that Tat alters KV1.3 channel kinetics by disturbing the tyrosine kinase-mediated KV1.3 phosphorylation regulation. Pervanadate (PVD), a universal tyrosine phosphatase inhibitor, was introduced to evaluate whether Tat intervenes with channel phosphorylation. PVD was prepared freshly prior to experimental use by mixing sodium orthovanadate with H2O2 as described previously (Holmes et al., 1996). Outward K+ currents were recorded on Tat-treated Ols (50 ng/ml for 2 h) in the absence and presence of PVD (200 µM for 10 min) in the bath solution. As shown in Fig. 9c, Tat induced a right-shift of inactivation curve and PVD prevented the Tat-induced right-shift, suggesting that Tat hinders channel inactivation by disturbing tyrosine kinase-mediated channel phosphorylation. To confirm the phosphorylation-associated inactivation curve shift is contributory to the current enhancement, the K+ current density was also determined in the absence and presence of PVD in the bath solution. Tat increased the total outward K+ currents in Ols as expected and the increase was counteracted by PVD (Fig. 9d), demonstrating Tat enhancement of Ol KV1.3 current via alteration of channel protein tyrosine phosphorylation.
Discussion
It is widely believed that HIV-1 persists in the brain despite of cART. The virus persistent in a latent or restricted manner in monocyte-macrophages, microglia and astrocytes continues to play a significant disease-inciting role. Indeed, HAND remains a subcortical dementia with notable evidence of affected white matter tracts within the corpus callosum, internal capsule and superior longitudinal fasciculus (Hoare et al., 2011; Sarma et al., 2014; Wohlschlaeger et al., 2009). How HIV-1 brain infection causes white matter damage is not fully understood. In the present study, we investigated the effects of HIV-1 protein Tat on Ols, the myelin-forming cells in the CNS. Our results demonstrated that Tat induces Ol injury via activation of voltage-gated K+ channel KV1.3. Such an effect can be blocked by knockdown of KV1.3 gene or by specific KV1.3 antagonists MgTx and PAP, but not by GxTx, a specific KV 2.1 antagonist, suggesting an involvement of KV1.3 in Tat-induced Ol injury. Further studies revealed that Tat interacts with KV1.3 at protein level, resulting in a prolonged channel activation via a decrease of tyrosine phosphorylation of KV1.3 channel. These results, to our knowledge, are the first demonstrations that Tat induces Ol injury via KV1.3.
The consensus view regarding HAND pathogenesis has been related to cerebral white matter damage (Gongvatana et al., 2009; Leite et al., 2013). Many cellular and viral factors are involved in HIV-1-associated brain white matter damage. Amongst them is viral protein Tat. It has been shown that Tat continues to be detectable in the CSF of HIV-infected patients with well-controlled viremia in the cART era (Johnson et al., 2013). With a broad range of actions on promoting inflammation and producing cytotoxic stress to various types of cells in the CNS (Bagashev and Sawaya, 2013), Tat might continue playing an important role for HIV-1-associated white matter damage in the cART era. Indeed, Tat has recently been shown to act on Ols with a detrimental consequence (Hauser et al., 2009; Zou et al., 2015). In an agreement with the aforementioned findings, our in vitro studies revealed that Tat had a direct toxic effect on the Ols in an Ol primary culture system (purity > 85%) (Figs. 3, 4, 5). The detrimental effect of Tat on Ols was validated on ex vivo studies that treatment of rat brain slices in culture with Tat produced myelin damage and a reduction of MBP expression in corpus callosum and striatum (Fig. 6). Combined with the results of increased apoptosis (Figs. 4 and 5), the reduction of MBP expression may reflect a decrease of Ol cell numbers. These results may explain, at least in part, why anti-inflammatory therapies did not achieve a great benefit in HAND patients (Tan and McArthur, 2012). However, Tat was found to increase Ol cell numbers in the hilus of the dentate gyrus in a study on rats with intrahippocampal injection of Tat (Fitting et al., 2010). The discrepancy may be caused by potentially inclusion of the population of immature Ols in that study (Fitting et al., 2010), since proliferation of Ol lineage cells could increase the probability to repair damaged myelin designedly as the response to Tat injection in vivo. This notion is supported by an earlier report that mild myelin damage was associated with an increase in Ol numbers, but the Ol numbers decreased as damage became more severe in HIV-1-infected brains (Esiri and Morris, 1996; Esiri et al., 1991). In conjunction with a previous gross anatomical study on HIV-1-infected brains with demyelination which detected a reduction of corpus callosum volume (Wohlschlaeger et al., 2009), we posit that Tat induces mature Ol injury, resulting in and loss of cerebral cortical white matter. Such a pathological change is concordant with the pattern of white matter damage observed in HAND patients.
Studies have shown that excessive cellular K+ efflux and/or intracellular K+ depletion are common characteristics in early apoptosis (Bortner and Cidlowski, 2007; Remillard and Yuan, 2004). KV1.3 is positively associated with apoptosis in different types of cells, including retinal ganglion cell (Jimenez-Perez et al., 2016), lymphocytes (Bock et al., 2002; Valencia-Cruz et al., 2009), and various cancer cells (Abdul and Hoosein, 2006; Brevet et al., 2008; Brevet et al., 2009). In agreement with these studies, our results showed Tat-induced apoptosis in cultured Ols was attenuated either by knockdown KV1.3 gene or by specific KV1.3 antagonists, but not by a KV2.1 antagonist (Fig. 4), suggesting that Tat triggers Ol apoptosis by increasing K+ efflux particularly through KV1.3. Moreover, blockade of KV1.3 rescued Tat-induced MBP reduction (Figs. 6, 7), indicating that KV1.3 is involved in HIV-1-related myelin damage. However, KV1.3 inhibition only partially blocked Tat-induced apoptosis (Fig. 4), implying that Tat may cause Ol injury through other mechanisms, such as targeting at glutamate receptors (Zou et al., 2015). Myelin impairments may cause further axonal injury related to neurodegeneration in HAND, because myelinated axons exhibit a myelin-dependency during development (Alizadeh et al., 2015). Additionally, the increased K+ efflux from Ols in an in vivo condition may affect neuronal function through elevating the levels of extracellular K+, instead of clearing K+ released from neurons during axonal firings (Gipson and Bordey, 2002; Menichella et al., 2006). An earlier study from our laboratory also showed that blockade of KV1.3 or knockdown KV1.3 expression in microglial cells attenuated Tat-induced microglia neurotoxic activity (Liu et al., 2013). Taking together, these findings indicate that activation of KV1.3 plays an important role in Tat-associated neurotoxicity.
It has been shown that the activities of several different cloned KV channels are suppressed after phosphorylation of the channels by both receptor and non-receptor tyrosine kinases (Fadool, 1998). Indeed, there are several tyrosine residues on KV1.3 channel protein and different combinations of phosphorylated tyrosine residues are required for current suppression as consequences of different triggering signals (Bowlby et al., 1997; Colley et al., 2004; Fadool et al., 2000). Our GST-pull down assay results showed KV1.3 presence in GST-Tat-precipitated protein complex, indicating a direct interaction between Tat and KV1.3 protein (Fig. 8). Such an interaction may cause an interruption of phosphorylation regulation of KV1.3 channel. This notion is supported by experimental results that tyrosine phosphatase inhibitor PVD attenuated Tat enhancement of KV1.3 currents and prevented Tat-induced right-shift of KV1.3 channel inactivation curve (Figs. 9b and 9c). Although we did not examine direct phosphorylation of KV1.3 channels due to the paucity of efficient antibody, our available data (Figs. 2 and 9a) indicate that Tat may increase KV1.3 currents by decrease of channel protein tyrosine phosphorylation and prolonged channel in activation state as revealed by a right-shift of inactivation curve (Fig. 9a). However, the tyrosine kinase-associated KV1.3 channel modulation can be influenced by adaptor proteins through direct protein-protein association involving Src homology (SH) domains (Colley et al., 2009; Marks and Fadool, 2007). As Tat binds to SH3 domains of Grb2 (Rom et al., 2011), it is also possible that Tat interacts indirectly with KV1.3 via adaptor proteins, of which Grb2 might be a potential candidate. In addition to protein-protein interaction and tyrosine phosphorylation regulation of KV1.3 channel activity, mobilization of cytoplasm KV1.3 channels to plasma membrane could also be possible for underlying mechanism for Tat-induced increase of the KV1.3 current in Ols. We separated the Ol proteins into cytosol and membrane fractions by membrane protein extraction kit (Biovision Incorporation), then the levels of KV1.3 in each fraction were evaluated. Tat increased the levels of KV1.3 in the cytosol fraction but no significant increase in membrane fraction (data not shown), suggesting the translocation of KV1.3 from cytosol to the plasma membrane was not involved in observed Tat-associated increase of KV1.3 current in Ols.
It is worth pointing out that the evaluations on Ol cell viability/apoptosis and myelin morphology in brain slices were conducted at different time points, 48 h and 72 h, respectively. This is because, at the same concentrations, a longer time was needed to generate significant morphological changes in brain slices than apoptotic changes seen in primary cell cultures. It is also worth pointing out that, in addition to its toxic effect to mature Ols and myelin sheaths, Tat may also induce disturbance of myelin maintenance via KV1.3 channels, such as Ol progenitor cells proliferation and differentiation, leading to myelin/white matter abnormalities as seen in the brains of HIV-1-infected patients. As basal activity of KV channels controls Ol proliferation and differentiation (Liu et al., 2015; Tegla et al., 2011; Vautier et al., 2004), the alteration of KV1.3 channel activity by Tat may possibly disturb Ol lineage cell proliferation and differentiation, and ultimately myelin maintenance processes. In addition, KV1.3 was shown to play a crucial role in controlling cell cycle activation (Tegla et al., 2011). This may explain why PAP-treated Ols in our studies exhibited higher cell viability (Fig. 3) than the control Ols.
In summary, our present studies demonstrated that HIV-1 protein Tat induces Ol/myelin injury by enhancing outward K+ current conducted by a voltage-gated K+ channel KV1.3 by using specific KV1.3 antagonists and siRNA knockdown of KV1.3 gene. This is the first demonstration that Tat targets at Ol KV1.3 channels by direct protein-protein interaction and interruption of channel phosphorylation/de phosphorylation processes. These findings may serve not only to elucidate the mechanisms underlying HIV-1-associated brain white matter damage seen in HIV-1-infected patients in the era of cART, but also to provide a potential target for the development of therapeutic strategies for amelioration of HIV/Tat-induced brain myelin/white matter injury.
Highlights.
HIV-1 brain infection causes cerebral white matter damage and the underlying mechanisms are not fully understood.
Virus trans-activator of transcription (Tat) protein, which is toxic to neural cells, can be released to the extracellular space during disease.
Tat increased whole cell Kv1.3 current recorded in rat oligodendrocytes via downregulation of channel protein phosphorylation, leading to prolonged channel activation and consequent cell apoptosis.
Tat-induced cultured oligodendrocyte apoptosis and myelin sheath damage in brain slices were blocked by specific Kv1.3 antagonists or by knockdown of Kv1.3 gene, suggesting an involvement of Kv1.3 in Tat-induced oligodendrocyte/myelin sheath injury.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01 NS 063878; R01 NS077873. The pJK-KV1.3 plasmid was kindly provided by Dr. Erich Gulbins, Department of Molecular Biology, University of Duisburg-Essen, Essen, Germany.
Abbreviations
- cART
combination antiretroviral therapy
- GxTx
Guangxi toxin
- HIV-1
human immunodeficiency virus type 1
- HAND
HIV-1-associated neurocognitive disorders
- MgTx
Margatoxin
- Ol
oligodendrocyte
- PAP
5-(4-phenoxybutoxy) psoralen
- PVD
Pervanadate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare that they have no conflict of interests.
References
- Abdul M, Hoosein N. Reduced Kv1.3 potassium channel expression in human prostate cancer. J Membr Biol. 2006;214:99–102. doi: 10.1007/s00232-006-0065-7. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, et al. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. doi: 10.3389/fnmol.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B, et al. Characterization of delayed rectifier Kv channels in oligodendrocytes and progenitor cells. J Neurosci. 1997;17:8234–8245. doi: 10.1523/JNEUROSCI.17-21-08234.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagashev A, Sawaya BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. doi: 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A, et al. HIV-gp120 affects the functional activity of oligodendrocytes and their susceptibility to complement. J Neurosci Res. 1997;50:946–957. doi: 10.1002/(SICI)1097-4547(19971215)50:6<946::AID-JNR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bock J, et al. Actinomycin D-induced apoptosis involves the potassium channel Kv1.3. Biochem Biophys Res Commun. 2002;295:526–531. doi: 10.1016/s0006-291x(02)00695-2. [DOI] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby MR, et al. Modulation of the Kv1.3 potassium channel by receptor tyrosine kinases. J Gen Physiol. 1997;110:601–610. doi: 10.1085/jgp.110.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet M, et al. Expression of K+ channels in normal and cancerous human breast. Histol Histopathol. 2008;23:965–972. doi: 10.14670/HH-23.965. [DOI] [PubMed] [Google Scholar]
- Brevet M, et al. Deregulation of 2 potassium channels in pancreas adenocarcinomas: implication of KV1.3 gene promoter methylation. Pancreas. 2009;38:649–654. doi: 10.1097/MPA.0b013e3181a56ebf. [DOI] [PubMed] [Google Scholar]
- Burg ED, et al. K+ channels in apoptosis. J Membr Biol. 2006;209:3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- Chang L, et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009a;47:1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Long-range linkage on chromosome 6p of VEGF, FKBP5, HLA and TNF alleles associated with transplant rejection. Mol Immunol. 2009b;47:96–100. doi: 10.1016/j.molimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, et al. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci U S A. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley B, et al. Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Receptors Channels. 2004;10:25–36. [PMC free article] [PubMed] [Google Scholar]
- Colley BS, et al. Brain-derived neurotrophic factor modulation of Kv1.3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neurosci. 2009;10:8. doi: 10.1186/1471-2202-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Morris CS. Cellular basis of HIV infection of the CNS and the AIDS dementia complex: oligodendrocyte. J NeuroAIDS. 1996;1:133–160. doi: 10.1300/j128v01n01_07. [DOI] [PubMed] [Google Scholar]
- Esiri MM, et al. Fate of oligodendrocytes in HIV-1 infection. AIDS. 1991;5:1081–1088. doi: 10.1097/00002030-199109000-00003. [DOI] [PubMed] [Google Scholar]
- Fadool DA. Tyrosine phosphorylation downregulates a potassium current in rat olfactory bulb neurons and a cloned Kv1.3 channel. Ann N Y Acad Sci. 1998;855:529–532. doi: 10.1111/j.1749-6632.1998.tb10621.x. [DOI] [PubMed] [Google Scholar]
- Fadool DA, et al. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol. 2000;83:2332–2348. doi: 10.1152/jn.2000.83.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, et al. Dose-dependent long-term effects of Tat in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2010;20:469–480. doi: 10.1002/hipo.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson K, Bordey A. Analysis of the K+ current profile of mature rat oligodendrocytes in situ. J Membr Biol. 2002;189:201–212. doi: 10.1007/s00232-002-1014-8. [DOI] [PubMed] [Google Scholar]
- Glass JD, et al. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosztonyi G, et al. Human immunodeficiency virus (HIV) distribution in HIV encephalitis: study of 19 cases with combined use of in situ hybridization and immunocytochemistry. J Neuropathol Exp Neurol. 1994;53:521–534. doi: 10.1097/00005072-199409000-00012. [DOI] [PubMed] [Google Scholar]
- Hauser KF, et al. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, et al. White-Matter damage in Clade C HIV-positive subjects: a diffusion tensor imaging study. J Neuropsychiatry Clin Neurosci. 2011;23:308–315. doi: 10.1176/jnp.23.3.jnp308. [DOI] [PubMed] [Google Scholar]
- Holmes TC, et al. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S, et al. The glial response to CNS HIV infection includes p53 activation and increased expression of p53 target genes. J Neuroimmune Pharmacol. 2007;2:359–370. doi: 10.1007/s11481-007-9095-x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Perez L, et al. Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation. J Biol Chem. 2016;291:3569–3580. doi: 10.1074/jbc.M115.678995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Nath A. New insights into immune reconstitution inflammatory syndrome of the central nervous system. Curr Opin HIV AIDS. 2014;9:572–578. doi: 10.1097/COH.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Kuroda J, et al. Inhibition of myelin formation by HIV-1 gp120 in rat cerebral cortex culture. Arch Virol. 1994;137:81–99. doi: 10.1007/BF01311175. [DOI] [PubMed] [Google Scholar]
- Leite SC, et al. Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J Magn Reson Imaging. 2013;38:1488–1493. doi: 10.1002/jmri.24129. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J Neurosci. 2015;35:352–365. doi: 10.1523/JNEUROSCI.2606-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. HIV-1 Tat protein increases microglial outward K(+) current and resultant neurotoxic activity. PLoS One. 2013;8:e64904. doi: 10.1371/journal.pone.0064904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Fadool DA. Post-synaptic density perturbs insulin-induced Kv1.3 channel modulation via a clustering mechanism involving the SH3 domain. J Neurochem. 2007;103:1608–1627. doi: 10.1111/j.1471-4159.2007.04870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, et al. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci. 2006;26:10984–10991. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia BA, et al. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Peavy G, et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. The HNRC Group. J Clin Exp Neuropsychol. 1994;16:508–523. doi: 10.1080/01688639408402662. [DOI] [PubMed] [Google Scholar]
- Pomara N, et al. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Power C, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34:339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Price RW, et al. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rom S, et al. HIV-1 Tat binds to SH3 domains: cellular and viral outcome of Tat/Grb2 interaction. Biochim Biophys Acta. 2011;1813:1836–1844. doi: 10.1016/j.bbamcr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma MK, et al. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, et al. Heterogeneous expression of voltage-gated potassium channels of the shaker family (Kv1) in oligodendrocyte progenitors. Brain Res. 1999;843:145–160. doi: 10.1016/s0006-8993(99)01938-1. [DOI] [PubMed] [Google Scholar]
- Schmitz A, et al. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- Smith GS, et al. Classic 18.5- and 21.5-kDa myelin basic protein isoforms associate with cytoskeletal and SH3-domain proteins in the immortalized N19-oligodendroglial cell line stimulated by phorbol ester and IGF-1. Neurochem Res. 2012;37:1277–1295. doi: 10.1007/s11064-011-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, et al. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Szabo I, et al. A novel potassium channel in lymphocyte mitochondria. J Biol Chem. 2005;280:12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- Tan IL, McArthur JC. HIV-associated neurological disorders: a guide to pharmacotherapy. CNS Drugs. 2012;26:123–134. doi: 10.2165/11597770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tegla CA, et al. C5b-9-activated, K(v)1.3 channels mediate oligodendrocyte cell cycle activation and dedifferentiation. Exp Mol Pathol. 2011;91:335–345. doi: 10.1016/j.yexmp.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Cruz G, et al. K(bg) and Kv1.3 channels mediate potassium efflux in the early phase of apoptosis in Jurkat T lymphocytes. Am J Physiol Cell Physiol. 2009;297:C1544–C1553. doi: 10.1152/ajpcell.00064.2009. [DOI] [PubMed] [Google Scholar]
- Vassall KA, et al. The effects of threonine phosphorylation on the stability and dynamics of the central molecular switch region of 18.5-kDa myelin basic protein. PLoS One. 2013;8:e68175. doi: 10.1371/journal.pone.0068175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautier F, et al. Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia. 2004;48:337–345. doi: 10.1002/glia.20088. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wohlschlaeger J, et al. White matter changes in HIV-1 infected brains: a combined gross anatomical and ultrastructural morphometric investigation of the corpus callosum. Clin Neurol Neurosurg. 2009;111:422–429. doi: 10.1016/j.clineuro.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- Xiao H, et al. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Yu SP, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- Zou S, et al. Oligodendrocytes Are Targets of HIV-1 Tat: NMDA and AMPA Receptor-Mediated Effects on Survival and Development. J Neurosci. 2015;35:11384–11398. doi: 10.1523/JNEUROSCI.4740-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]