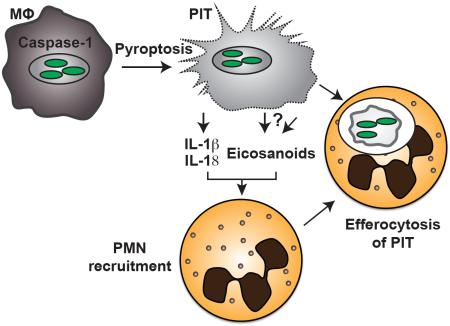
Abstract
Inflammasomes activate caspase-1, initiating a lytic form of programmed cell death termed pyroptosis, which is an important innate immune defense mechanism against intracellular infections. We recently demonstrated in a mouse infection model of pyroptosis that instead of releasing bacteria into the extracellular space, bacteria remain trapped within the pyroptotic cell corpse, termed the pore-induced intracellular trap (PIT). This trapping mediates efferocytosis of the PIT, with its associated bacteria, by neutrophils; bacteria are subsequently killed via neutrophil ROS. Using this pyroptosis model, we now show that the pro-inflammatory cytokines IL-1β and IL-18 and inflammatory lipid mediators termed eicosanoids are required for effective clearance of bacteria downstream of pyroptosis. We further show that IL-1β, IL-18, and eicosanoids affect this in part by mediating neutrophil recruitment to the PIT. This is in addition to our prior findings that complement is also important to attract neutrophils. Thus, the PIT initiates a robust and coordinated innate immune response involving multiple mediators that attract neutrophils to efferocytose the PIT and its entrapped bacteria.
Keywords: Pore-induced intracellular trap (PIT), Pyroptosis, Inflammasome, Caspase-1, neutrophil recruitment
Graphical abstract
Introduction
Inflammasomes oligomerize into multi-protein complexes, which serve as caspase-1 activating platforms. Caspase-1 mediates the secretion of the pro-inflammatory cytokines IL-1β and IL-18 and initiates a lytic and inflammatory form of programmed cell death [1], termed pyroptosis. Additionally, caspase-1 activation has recently been linked to the production of eicosanoid mediators in vivo. In a model of systemic NLRC4 inflammasome activation using an engineered flagellin toxin, Von Moltke et al found that caspase-1 activation triggered a rapid calcium flux that stimulated cyclooxygenase to produce a lethal quantity of eicosanoid mediators [2]. Whether caspase-1 driven eicosanoids serve a beneficial function during infection remains to be elucidated.
Pyroptosis is an innate immune defense mechanism against intracellular infection [3]. Following caspase-1 activation, GSDMD pore-formation in the plasma membrane causes water influx, cell swelling, and eventually lysis. Pyroptosis is also induced by caspase-11, and is the likely mechanism by which caspase-11 defends against cytosolic Gram-negative bacteria [4],[5]. Removing the intracellular replicative niche is obviously beneficial to the host, yet the experimental difficulties in studying pyroptosis in vivo have limited our understanding of its role in host defense [6].
We recently described how pyroptosis leads to the formation of pore-induced intracellular traps [7]. Instead of simply expelling pathogens from the cell corpse following pyroptosis, live bacteria are trapped within the cell corpse, as are insoluble proteins and large organelles. This trapping prevents the pathogen from disseminating to neighboring cells and slightly damages the bacteria without killing them.
Pyroptosis, as the first step in clearance, therefore enables the host to remove the protective, intracellular replicative niche of the pathogen. A second step is therefore required to finally eliminate the pathogen. This is accomplished by phagocytosis of the PIT and associated bacteria by neutrophils [7]. The engulfment of apoptotic bodies and necrotic debris by professional phagocytes – termed efferocytosis – has been well studied and is essential in promoting tissue homoeostasis [8], and we have extended the use of this term to include efferocytosis of PITs. We previously demonstrated how the concerted action of complement and scavenger receptors are required for efferocytosis of PITs by neutrophils in vivo [7].
We know very little, however, about how neutrophils are recruited to the site of pyroptosis, and how the other caspase-1-driven mediators may participate in this process. What are the chemoattractants, DAMPs or cytokines that are responsible for bringing neutrophils and PITs together? The inflammatory environment following lytic cell death is complex, which makes it likely that multiple recruitment signals play a role. Here, we show that the pro-inflammatory cytokines IL-1β and IL-18 and the lipid inflammatory mediators eicosanoids and leukotrienes, are required for clearance of Salmonella enterica serovar Typhimurium (S. Typhimurium) following pyroptosis. Genetic deletion or pharmacological inhibition of these components results in the lack of neutrophil recruitment to the site of pyroptosis in vivo.
Results
IL-1β and IL-18 are partially required for clearance of FliCind S. Typhimurium
Given the complexity of the inflammatory environment induced by lytic cell death, we hypothesized that there were additional mediators of neutrophil recruitment to the site of pyroptosis, and that these mediators are required for clearance downstream of pyroptosis. To study pyroptosis in vivo, we utilize an engineered strained of S. Typhimurium that expresses flagellin under a doxycycline inducible promoter [7]. Briefly, we co-infect mice with 103 CFUs of two S. Typhimurium strains: FliCind (ampR), which expresses flagellin under a doxycycline inducible promoter, and WT (kanR). Both strains are in a flgB∷Tn10 background that lacks chromosomal flagellin expression. Once the infection has been established, mice are injected with doxycycline and harvested at the indicated time point. A competitive index (CI) is expressed as the log of FliCind/WT CFUs, such that this 10-fold difference is expressed as a CI of −1.0. Consistent with previous results [3],[7], Casp1−/−Casp11−/− mice fail to clear FliCind (Figure 1A).
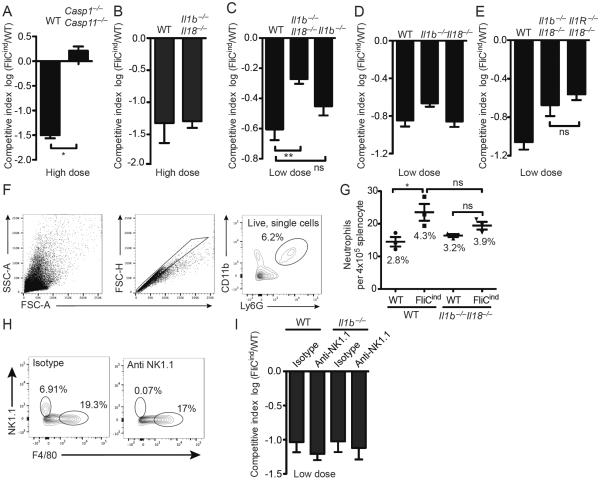
Figure 1. IL-1β, IL-18, and pyroptosis cooperatively clear FliCind S. Typhimurium.
(A–E, H, I) Mice (n=5) were infected with (A, B) high dose (105 CFUs) or (C–I) low dose (103 CFUs) (of 1:1 ratio of FliCind AmpR and WT KanR S. Typhimurium IP for 17 hours prior to doxycycline injection; organs were harvested 7 hours later. CIs are expressed as the log FliCind/WT (Amp/Kan CFUs). (F–G) Mice were infected with WT or FliCind S. Typhimurium and treated with doxycycline at 24 hours pi for 5 hours. Spleens were prepared for single cell suspension, stained and analyzed by flow cytometry. Neutrophils were defined as the CD11b+ Ly6G+. (F) The gating strategy represents a WT mouse infected with FliCind S. Typhimurium. (G) The mean percentage of neutrophils of the total splenocytes population is stated in the figure for each condition. (H–I) For NK-cell depletion, mice were injected with 75 ng anti-NK1.1 PK136 or isotype control C1.18.4 (BioXCell) at −3 and −1 dpi. Depletion of NK1.1-positive cells was confirmed by flow cytometry (left). NK cells were defined as NK1.1+ F4/80−, and the percentage of each cell type is indicated. All data are shown as mean + SE of n=3 (F, G) and n=5 (A–E, H, I) mice and are from single experiments representative of two (A, H, I) or three (B–G) experiments. * p < 0.05; two-tailed student t-test.
Caspase-1 cleaves pro-IL-1β and IL-18, which results in their secretion from macrophages. We previously used a relatively high dose infection, which is the standard dosing used in S. Typhimurium pathogenesis research [9]. This 105 CFU dose (equally divided between FliCind and control) showed that pyroptosis is sufficient to mediate clearance of a high dose infection with 105 CFU FliCON S. Typhimurium (constitutive flagellin expression) even in Il1b−/−Il18−/− mice (Figure 1B) [3]. This was an important advance, as it was the first strong evidence that pyroptosis acts in vivo and that the function of pyroptosis could be separated from IL-1β and IL-18. However, during an actual infection, a more parsimonious model is that pyroptosis would work hand-in-hand with IL-1β and IL-18 to drive bacterial clearance. With this in mind, we hypothesized that this high inoculum triggers a strong inflammatory response in the tissue that could bypass the need for IL-1β and IL-18, but that a low dose infection would have greater sensitivity to detect a role for IL-1β and IL-18.
We therefore re-examined the role of IL-1β and IL-18 in a low dose (103 CFU) infection. Here, similar to Casp1−/−Casp11−/− mice (Figure 1A), Il1b−/−Il18−/− deficient mice were partially defective in their ability to clear FliCind S. Typhimurium (Figure 1C). Mice deficient in either Il1b−/− or Il18−/− cleared FliCind Salmonella similar to WT mice (Figure 1D), indicating that either IL-1β or IL-18 are act redundantly to promote clearance of bacteria in PITs. Even though IL-1α also plays a prominent role in neutrophil migration [10], this cytokine was not required for clearance of FliCind S. Typhimurium, as Il1b−/−Il18−/− and Il1R−/−Il18−/− mice cleared this strain equally well (Figure 1E). IL-1β promotes neutrophil recruitment and inflammation [10]. We used a flow cytometry assay to determine the effect of pyroptosis upon neutrophil influx, and found that induction of FliCind expression triggered a significant neutrophil influx in spleen of wild type mice, but not Il1b−/−Il18−/− mice (Figure 1F–G). There was a trending (not statistically significant) effect in Il1b−/−Il18−/− mice that may become significant under other experimental conditions, which could be due to IL-1α or other cytosolic DAMP release by pyroptosis. This suggests that IL-1β and IL-18 may contribute to the clearance of FliCind S. Typhimurium by recruiting neutrophils to the PIT, but that these functions can be overcome when higher bacterial burdens are present in tissues. It remains to be determined if neutrophil recruitment is primarily driven by IL-1β or IL-18, or both, but based on prevailing understanding of these cytokines, IL-1β is more likely to be important.
IL-18 has significant priming effects upon NK and T cells during bacterial infection [11]. With regard to NK cells, anti-NK1.1 depletion in Il1b−/− mice did not replicate the defect of Il1b−/−Il18−/− mice (Figure 1H). Therefore, the mechanism by which IL-18 functions redundantly with IL-1β in this model remains to be further elucidated.
Prostaglandins and leukotrienes are partially required for clearance of FliCind S. Typhimurium
Following extravasation of immune cells from the vasculature, the secondary phagocyte must chemotax towards the site of the PIT. Eicosanoids are lipid mediators that enhance neutrophil and inflammatory monocyte chemotaxis [12],[13]. Von Moltke et al. showed that systemic NLRC4 inflammasome activation triggers an eicosanoid-storm, which rapidly leads to vascular permeability and death [2]. However, whether eicosanoids function downstream of inflammasomes during defense against infection was not addressed. Eicosanoids are produced from arachidonic acid released from phospholipid membranes by cPLA2. Arachidonic acid can then be metabolized by cyclooxygenases to produce prostaglandins, or lipoxygenases to produce leukotrienes (Figure 2A) [12]. We therefore hypothesized that prostaglandins and/or leukotrienes may play a role in neutrophil recruitment to the PIT.
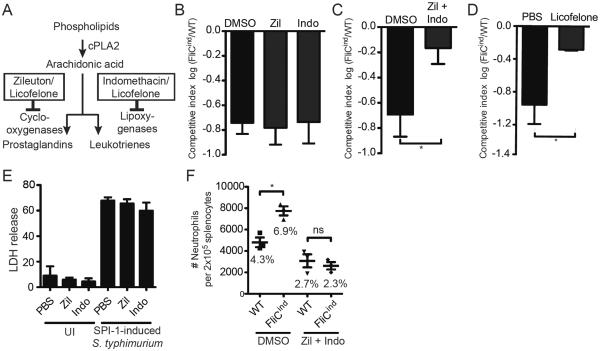
Figure 2. Prostaglandins and leukotrienes are required to clear FliCind S. Typhimurium.
(A) Schematic overview of eicosanoid synthesis and inhibitor action. (B–D) Mice (n=5) were infected with 103 CFUs of 1:1 ratio of FliCind AmpR and WT KanR S. Typhimurium IP for 17 hours, then treated with doxycycline in the presence of (B, C) DMSO vehicle, (B) indomethacin, zileuton, (C) a combination of indomethacin and zileution, or (D) licofelone and harvested 7 hours later. CIs are expressed as the log FliCind/WT (Amp/Kan CFUs). (E) WT BMMs in vitro were pre-treated with PBS, zileuton, or indomethacin for 4 hours, and infected with S. Typhimurium MOI 50 SPI-1 in the presence of inhibitors. Cytotoxicity was measured by % LDH release at 2 hours post infection. (F) Mice (n=3) were infected with WT or FliCind S. Typhimurium and both groups of mice were treated with doxycycline and inhibitors as in (A), and splenocytes analyzed by flow cytometry. Neutrophils were defined as CD11b+ Ly6G+. The mean percentage of neutrophils of the total splenocytes population is stated in the figure for each condition. All data are shown as mean + SE of n=5 (B–D), and n=3 (F) mice. All panels (A–F) are representative of three independent experiments. * p < 0.05; two-tailed student t-test.
While inhibition of leukotriene or prostaglandin production individually with zileuton (a LOX inhibitor) or indomethacin (a COX inhibitor) (Figure 2A), respectively, had no effect on FliCind clearance (Figure 2B), simultaneous inhibition of both pathways prevented FliCind clearance (Figure 2C). Blockade of both pathways by licofelone, which inhibits both COX and LOX (Figure 2A) [14],[15] similarly prevented FliCind clearance (Figure 2D). Thus, leukotrienes and prostaglandins appear to act in parallel. These inhibitors did not affect the process of pyroptosis itself in vitro (Figure 2E), although there is a theoretical possibility that Zileuton and Indomethacin can inhibit inflammasome activation in vivo.
To assess the role of eicosanoids downstream of pyroptosis, we used our flow cytometry based assay to analyze the recruitment of neutrophils to the spleen. When treating WT mice with zileuton and indomethacin, there is no increase in the number of neutrophils in the spleen following the induction of pyroptosis (Figure 2F), indicating that prostaglandins and leukotrienes are required for the recruitment of PMNs to the PIT. This is consistent with their known roles in chemotaxis. However, inhibiting eicosanoids generally prevents neutrophil migration even in the absence of pyroptosis, as there is a decrease in the number of splenic neutrophils in WT mice infected with WT S. Typhimurium and treated with Indomethacin and Zileuton compared to WT mice infected with WT S. Typhimurium and treated with DMSO Figure 1F).
Discussion
Many pathogens replicate within the macrophage intracellular niche. In response, the innate immune sensors attempt to detect cellular perturbations or microbial ligands in the cytosol via inflammasomes, activating caspase-1 or -11, and triggering pyroptosis. After membrane rupture, the organelles and cytoskeleton do not simply disperse, instead remaining associated in cell corpse, which we have named the pore-induced intracellular trap (PIT) [7]. The still viable bacterium remains entrapped within the PIT, and is only killed after phagocytosis of the PIT and entrapped bacterium by a secondary phagocyte, typically a neutrophil. The process of phagocytosing apoptotic or necrotic cells has been termed efferocytosis, from latin “effere”, meaning “to bury” a corpse, referring to the process by which dead cells are removed by phagocytes. In vitro evidence suggests that necroptosis and necrosis also results in PITs, but whether they are indeed efferocytosed in vivo by a similar mechanism remains to be investigated.
But how does the neutrophil know where to find the PIT within tissue? Direct recruitment of neutrophils to the site of pyroptosis should be essential to eliminating the pathogen. We previously demonstrated that scavenger receptors are important in mediating efferocytosis of PITs, and that complement also mediates neutrophil recruitment to the site of pyroptosis and/or contributes in opsonization of PITs. However, given the complex inflammatory environment during lytic cell death, we reasoned that additional signals likely contribute to neutrophil recruitment. Indeed, besides inducing pyroptosis, caspase-1 also induces secretion of IL-1β and IL-18. Il1b−/−Il18−/− mice had significantly reduced neutrophil recruitment after the induction of pyroptosis in the tissue. There is a strong mechanistic rationale for IL-1β in this regard, as it is well established to induce upregulation of adhesion molecules on endothelial cells, promoting neutrophil diapedesis into the tissues [16]. The most likely effect of IL-18 is to stimulate IFN-γ production by NK cells or T cells, but one would expect this do act downstream of IL-1β, rather than redundantly. Therefore, how IL-18 acts in parallel to IL-1β in this model remains uncertain, and will be investigated in future work. The effect of IL-1β and IL-18 in our model is incomplete, only ablating about half of the clearance effect. We speculate that their role may be more pronounced in other tissues – the spleen naturally has high blood flow and diapedesis may not be required for neutrophils to migrate towards the PIT as the splenic red pulp has an open blood system without an endothelial lining [17].
After diapedesis, neutrophils must chemotax to the site of the pyroptotic corpse. Because eicosanoids are released during systemic NAIP/NLRC4 inflammasome activation in vivo [2], and are know to have chemotactic properties, we investigated their role in clearing bacteria entrapped within PITs. Prostaglandin and leukotriene production together were required for recruitment of neutrophils and inflammatory monocytes to the tissue after pyroptosis, and clearance of the PIT-trapped bacteria. These eicosanoids may act via a multitude of mechanisms. For example, PGI2 promotes vasodilation, which may permit increased neutrophil extravasation from the capillaries [12]. LTB4 broadly acts as a chemoattractant for neutrophils [18], and LTB4 as well as the eicosanoid HXA3 have known roles in neutrophil migration across epithelial layers during Salmonella infection [19],[20]. These eicosanoids may be produced prior to pyroptosis intrinsically by the cell in which the inflammasome is activated. Prostaglandins could be synthesized from cyclooxygenase 1 (COX1) as suggested by von Moltke et al. [2], or may originate from COX2, as priming events have occurred prior to induction of pyroptosis in our experiments. It is also possible that the prostaglandins and leukotrienes originate from discreet cells, for example, prostaglandins may arise from the pyroptosing cell [2], while leukotrienes may arise from neutrophils in the vicinity [18]. Our data is the first example of the beneficial link between eicosanoids downstream of inflammasome activity. However, more work will be needed to determine if the cell undergoing pyroptosis is indeed the source of these eicosanoid mediators.
Concluding remarks
Given the complex inflammatory environment that follows lytic cell death, it is not inconceivable that yet additional chemokines, cytokines or DAMPs participate in recruitment of neutrophils to the site of pyroptosis. We have shown that different tissue burdens of bacteria may highlight different inflammatory mediators. Further investigation should also address if secondary phagocytes that efferocytose PITs formed by necroptosis and necrosis require the action of a different set of recruitment signals and efferocytosis mediators, since IL-1β and IL-18 may not be elaborated from necroptotic cells as they become PITs.
Materials and Methods
Mice, mouse infections and treatments
Wild type C57BL/6 (Jackson Labs # 000664), Casp1−/−Casp11−/−[21], Il18−/− (Jackson Labs #004130), and Il1b−/− [22], Il18−/−(Jackson Labs #004130) mice were housed in pathogen-specific free facility. All animal protocols were approved by the Institutional Animal Care and Use Committee at UNC-CH and met guidelines of the US NIH for the humane care of animals. For competitive index experiments, 8–10 week old mice were infected with 1000 colony forming units (CFUs) of a 1:1 ratio of stationary phase flgB∷Tn10 pWSK129 (WT, empty vector, kanamycin resistant) and flgB∷Tn10 pEM87 (FliCind plasmid, FliCind, ampicillin resistant) S. Typhimurium in PBS by intraperitoneal (IP) injection. The flgB∷Tn10 mutation prevents expression of the chromosomal flagellin, fliC and fljB, and also provides tetracycline/doxycycline resistance. At 17 hours post infection (hpi), mice were treated with 0.8mg of doxycycline (Fischer) to induce expression of FliC under the control of a tetracycline-driven promoter. When indicated, the following inhibitors were delivered IP at the same time as doxycycline: 10mg/kg indomethacin (Sigma), 10mg/kg zileuton (Sigma), or 50mg/kg licofelone. Spleens were harvested at 24hpi (which is 7h after doxycycline injection), homogenized and dilutions plated onto LB + antibiotics. Competitive index are log flgB∷Tn10 FliCind CFU/ flgB∷Tn10 CFU. For NK cell depletion, mice were injected with 75ng anti-NK1.1 PK136 or isotype control C1.18.4 (BioXCell) at −3 and −1 dpi. Depletion of NK1.1-positive cells was confirmed by flow cytometry.
Macrophage culture and infection
Bone marrow derived macrophages (BMM) were isolated as described[3]. BMMs were infected with S. Typhimurium MOI 40 by centrifugation at 250×g 5min. At 30min post infection, cells were washed with PBS and replaced with media containing 15ug/ml gentamicin (Sigma). For eicosanoid and leukotriene inhibition, WT BMMs were pre-treated for 8 hours with 10μM indomethacin or 10μM zileuton, respectively, prior to infection and for the duration of the experiment. For SPI-1 induction, a 37°C overnight (o/n) culture was back-diluted 1:40 and incubated at 37°C for 3 hours prior to infection. Supernatants were collected at the 1 hour post infection. Cell lysis was determined by measuring LDH release [23].
Bacterial strains
S. Typhimurium strains were derived from ATCC 14028s. Strains and plasmids are listed in Supporting Information Table 1. All strains used in CI were in a flgB∷Tn10 background to eliminate chromosomal flagellin expression and provide doxycycline resistance.
Cell isolation and flow cytometry
Mice were infected with 1×103 CFUs flgB∷Tn10 pWSK129 (empty vector, kanamycin resistant) (WT) or flgB∷Tn10 pEM87 (FliCind plasmid, ampicillin resistant) (FliCind) S. Typhimurium in PBS by intraperitoneal (IP) injection, and treated with doxycycline as described above. Single cells were isolated from spleen, treated with Collagenase D, RBC lysis buffer, and flushed through a cell strainer (Falcon). Cells were Fc-blocked with anti-CD16/CD32 and stained with CD11b-APC (M1/70), Ly6G-APC Cy7 (1A8), NK-1.1-PE (PK136) (BD), and F4-80-PacBlue (BM8) (eBioscience), fixed and analyzed on a Becton Dickinson LRSIII (HTS) (UNC Flow Cytometry Core Facility).
Statistical analysis
All analysis and graphical presentations were made using Prism. All error bars represent SE. P-values were determined by a two-tailed student t-test.
Supplementary Material
Acknowledgements
We thank Dat Mao for technical assistance, Stefan Laufer (U. Tübingen, Germany) for licofelone reagent. This work was funded by NIH grants AI097518 (EAM), AI057141 (IJ), AI119073 (EAM). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Abbreviations
- (PIT)
pore-induced intracellular trap
Footnotes
Conflict of interest Disclosure The authors declare no financial or commercial conflict of interest.
Author contributions IJ and JL performed the experiments. IJ and EAM conceived, designed and analyzed the experiments, and wrote the paper. SL provided essential reagents.
References
- 1.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. DOI: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 2.Moltke Von J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, Van Rooijen N, Brown CR, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. DOI: 10.1038/nature11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. DOI: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. DOI: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JP-Y, Coers J, Aderem A, et al. Canonical Inflammasomes Drive IFN-γ to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host Microbe. 2015;18:320–332. doi: 10.1016/j.chom.2015.07.016. DOI: 10.1016/j.chom.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–326. doi: 10.1016/j.mib.2013.04.004. DOI: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. Journal of Experimental Medicine. 2016;213:2113–2128. doi: 10.1084/jem.20151613. DOI: 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poon IKH, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010;17:381–397. doi: 10.1038/cdd.2009.195. DOI: 10.1038/cdd.2009.195. [DOI] [PubMed] [Google Scholar]
- 9.Beuzón CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–1352. doi: 10.1016/s1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
- 10.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 11.Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, Whitmire JK, et al. Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium. Immunity. 2015;43:987–997. doi: 10.1016/j.immuni.2015.10.010. DOI: 10.1016/j.immuni.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. DOI: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Szabady RL, McCormick BA. Control of neutrophil inflammation at mucosal surfaces by secreted epithelial products. Front Immunol. 2013;4:220. doi: 10.3389/fimmu.2013.00220. DOI: 10.3389/fimmu.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni S, Pal Singh V. Licofelone-A Novel Analgesic and Anti-Inflammatory Agent. Current Topics in Medicinal Chemistry. 2007;7:251–263. doi: 10.2174/156802607779941305. DOI: 10.2174/156802607779941305. [DOI] [PubMed] [Google Scholar]
- 15.Meirer K, Steinhilber D, Proschak E. Inhibitors of the arachidonic acid cascade: interfering with multiple pathways. Basic Clin. Pharmacol. Toxicol. 2014;114:83–91. doi: 10.1111/bcpt.12134. DOI: 10.1111/bcpt.12134. [DOI] [PubMed] [Google Scholar]
- 16.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. DOI: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. DOI: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 18.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. DOI: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyrkalska SD, Candel S, Angosto D, Gómez-Abellán V, Martín-Sánchez F, García-Moreno D, Zapata-Pérez R, et al. Neutrophils mediate Salmonella Typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat Commun. 2016;7:12077. doi: 10.1038/ncomms12077. DOI: 10.1038/ncomms12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. DOI: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 22.Shornick LP, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Ferguson TA, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayamajhi M, Zhang Y, Miao EA. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol. Biol. 2013;1040:85–90. doi: 10.1007/978-1-62703-523-1_7. DOI: 10.1007/978-1-62703-523-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.