Abstract
Hepatocellular carcinoma (HCC) is a disease usually diagnosed in its advanced-stage, and is frequently not amenable to curative surgical treatment. Also, HCC is resistant to chemotherapy and less vulnerable to radiation therapy compared to normal hepatic parenchyma. Both of these facts render the efficacy of adjuvant and palliative treatments problematic. Selective internal radiation therapy (SIRT) with 90Y-bearing microspheres is characterized by preferentially delivering substantially high doses of radiation to a liver tumor dose simultaneously limiting the damage to its non-tumorous cells, providing an opportunity for effective local tumor control and even tumor regression therapy. The current article reviews the specific characters, dosimetry, possible applications, and special considerations toward the pre-existing radiation therapy of 90Y microsphere SIRT in treating HCC.
Keywords: Hepatocellular carcinoma, 90Y microspheres, Selective internal radiation therapy, 99mTc macroaggregated albumin, Transarterial chemoembolization, External beam radiation therapy, Radiation lobectomy
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and the third leading cause of cancer-related deaths [1]. The annual incidence of HCC is more than 1 million worldwide [2]. Approximately 80% of reported HCC cases are from East Asia and sub-Sahara Africa, which are areas greatly influenced by the prevalence of hepatitis B virus (HBV) infections [3]. Resection of the tumors, including partial hepatectomy and liver transplantation, are the only curative therapies currently available to patients suffering from HCC. However, only about 10% of patients are eligible for surgery [4]. Until now, patients with HCC have been frequently given poor prognoses because of the diagnoses of most cases is at the advanced stage [5]. In addition, HCC is resistant to chemotherapy [6] and is less vulnerable to radiation therapy compared to normal hepatic parenchyma [7]. Moreover, patients with HCC usually have liver cirrhosis or portal vein thrombosis, which both contribute to poor hepatic reserve and prevent aggressive treatments of the HCC. Hence, the estimated median survival of HCC is only 8 months [8].
Approximately 80% of the blood supply to a hepatocellular carcinoma is via the hepatic artery. On the other hand, 75% of the blood supply to a normal hepatic parenchyma comes from the portal vein [9-11]. Therefore, an anticancer regimen preferentially delivered via the hepatic artery may be advantageous in treating HCC by increasing the toxicity of the tumor and decreasing the damage done to normal hepatic tissue. As a result, transarterial embolic therapy, either with bland embolization only or combined infusion with a chemotherapeutic agent, has been widely applied as a palliative, alternative, or interim treatment for patients not eligible for curative surgery.
For patients with HCC, it is estimated that there is either a 5% or a 50% risk of radiation-induced liver disease when the whole liver is irradiated by external beam radiation with mean doses of 32 and 40 Gy, respectively [7]. Compared to external beam radiation therapy (EBRT), selective internal radiation therapy (SIRT) by transarterial infusion with 90Y-bearing microspheres—the high energy beta particle (maximum energy: 2.27 MeV; mean energy: 0.94 MeV) emitted by the decay of 90Y to 90Zr and minimal penetration range (average: 2.5 mm; maximum: 11 mm) in tissue—is potentially capable of delivering much higher radiation doses to tumors while the radiation exposure to the normal hepatic parenchyma remains within tolerable limits [12].
2. Comparison of microspheres
90Y, with a physical half-life of 64.2 hours, is produced by neutron bombardment of 89Y in a nuclear reactor. It decays to stable 90Zr with emitting high-energy beta particles. One GBq of 90Y delivers an absorbed dose of dose 50 Gy per kilogram of tissue. Currently there are two types of commercially available 90Y-bearing microspheres: glass-based (TheraSphere®, MDS Nordion, Ottawa, Ontario, Canada) and resin-based (SIR-Spheres®, Sirtex Medical Limited, Sydney, Australia) microspheres [13]. The characteristics of these microspheres are listed in Table 1.
| TheraSphere® | SIR-Spheres® | |
|---|---|---|
| Material | Glass-based | Resin-based |
| Diameter | 20-30 μm | 20-60 μm |
| Activity per sphere | 2500 Bq | 50 Bq |
| Specific gravity | 3.2 g/ml | 1.6 g/ml |
| Relative embolic potential | Low | High |
| Relative pressure for infusion | High | Low |
3. Procedure and dosimetry
The therapy consists of two angiographic procedures. The first procedure delivers 99mTc macroaggregated albumin (MAA) to the liver in order to simulate the deposition of the therapeutic microspheres that will follow. Planar and single photon emission computed tomography (SPECT) images are obtained to detect any uptake of 99mTc MAA outside of the liver, particularly in the gastrointestinal tract and lungs. Gastrointestinal uptake usually can be avoided by coiling the involved artery. Hepatopulmonary shunting, if resulting in more than 30 Gy going to the lungs, is a contraindication for 90Y SIRT and should be carefully evaluated. The second procedure delivers 90Y-bearing microspheres, with the calculated radioactivity based on the prior 99mTc MAA scan and other patient-specific factors, to the same liver about 1 to 2 weeks later. Images of bremsstrahlung SPECT/CT or internal pair production positron emission tomography/computed tomography (PET/CT) of 90Y may be acquired to confirm the distribution of the microspheres [14] (Figure 1).
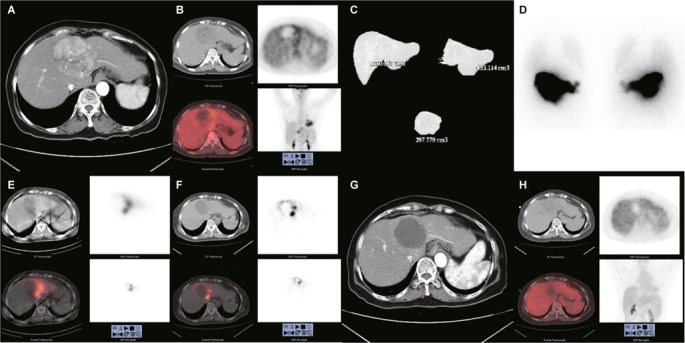
The formation of arteriovenous anastomoses or shunts may occur as part of neoplastic vasculature within tumors. These shunts may allow microspheres to bypass the terminal arterioles/ capillaries of the hepatic tumor and enter the lungs, leading to radiation pneumonitis [15]. Estimating lung damage due to liver-tolung shunting can be accomplished by calculating the percentage of lung shunting based on the planar scan of chest/abdomen with 148-185 MBq of 99mTc MAA injected to the hepatic arteries [16]. Previous studies have demonstrated that the lungs may tolerate up to 30 Gy with a single injection of 90Y microspheres and up to 50 Gy for multiple injections [15]. Hence, potential lung shunting demonstrated on 99mTc MAA scan resulting in doses greater than 30 Gy should have their treatment discontinued.
As mentioned earlier, 40-Gy whole-liver external beam radiation has been found to demonstrate a 50% incidence of radiationinduced liver disease, and the incidence steeply rises with doses above 30 Gy. However, liver doses with SIRT often exceed 50 Gy and, as found in the literature, may even exceed 100 Gy in a single infusion [17, 18]. Yet extrapolating the estimates of complication probability derived from the data of external beam radiation seems unsuitable for SIRT since the occurrence of radiation- induced liver disease is rare in the latter. The non-uniform distribution of microspheres within the liver, which leads to a very high tumor-cell-to-normal-liver-cell ratio of absorbed dose, has been proposed to explain the different effects between EBRT and SIRT [19, 20]. Currently, little is known about the maximum tolerable dose of non-tumor liver parenchyma in SIRT. Although there have been recommendations to set non-tumor dose limits as low as 70 Gy in non-cirrhotic livers and 50 Gy in cirrhotic livers [21], these limits are still arbitrarily defined and need to be confirmed in prospective studies [22].
Based on the partition model, the survival of HCC patient is better when the absorbed dose of SIRT in the tumor is > 120 Gy according to previous studies [23, 24].
Several methods have been used for determining HCC treatment. For resin-based microspheres, three different methods can be applied, including empirical, body surface area (BSA), and partition methods [25]. The simplest way is the first, the empirical method (Table 2), which needs only two to three parameters: the fraction of liver that is tumorous, the lung shunting fraction, and the target liver range. However, this method greatly depends on tumor load and does not consider other patient-specific factors, such as liver function reserve. It has been found to relate to an unacceptable clinical and laboratory toxicity profile and thus has been not recommended for routine use [26, 27].
| Extent of disease | |
| Fraction of liver involvement | Base activity (GBq) |
| > 50% | 3 |
| 25-50% | 2.5 |
| < 25% | 2 |
| Lung shunting | |
| Fraction of counts in the lung | Dose modifier |
| < 10% | 1.0 |
| 10-15% | 10-15% |
| 15-20% | 0.6 |
| > 20% | Do not proceed. |
| Target | |
| Part of liver | Dose modifier |
| Whole liver | 1.0 |
| Right lobe only | 0.7 |
| Left lobe only | 0.3 |
Prescribed activity = base activity × lung shunting modifier × target liver fraction modifier.
The second method is based on body-surface area, or BSA. It is a semi-empirical method and has been used safely in many clinical trials. The activity is determined by the following equation:
Activity (GBq) = [BSA (m2) – 0.2] + (tumor volume/total liver volume)
The BSA method is a relatively conservative estimation of activity compared to the empirical method. The main limitation of this method is the absence of target volume in the calculation, resulting in possible under-treatment (for example, a small patient with a large liver) or over-treatment (a large patient with a small liver) [26, 28]. In addition, the differences of individual intrahepatic distribution of radioactivity in tumor and non-tumor livers are not considered, which is also disadvantage for patients with hyper- or hypo-vascular tumors [22].
The third method is the so-called partition method. This assessment partitions the radioactivities with their individual masses in the tumor and normal liver according to the 99mTc-MAA SPECT and contrast-enhanced CT. Although this method is very intuitive and based on the aforementioned principle of calculating and balancing the need for minimum amount for the maximum tumoricidal effect with maximum tolerable normal liver doses, the actual execution is usually difficult and time-consuming, particularly when there are multiple small tumors throughout a liver. The dosimetry uses the medical internal radiation dose (MIRD) model. In short, the partition method provides an average of the activity per unit of tumor mass and per unit of normal liver mass respectively, which can be used for estimating average doses to deliver to the tumor(s) and to the normal liver. The calculation uses following equations:
Equation 1:
Lung shunting fraction = Radioactivity counts of lung / (Radioactivity counts of lung + Radioactivity counts of liver)
Equation 2:
T:N (tumor to normal liver ratio) = (Radioactivity counts of tumor / Mass of tumor) / (Radioactivity counts of normal liver / Mass of normal liver)
Equation 3:
Activity (GBq) = {Dose to normal liver (Gy) × [T:N × Mass of tumor (kg) + Mass of normal liver (kg)] } / [50 (Gy ∙ kg ∙ GBq-1) × (1 - lung shunting fraction)]
Equation 4:
Dose to tumor (Gy) = Dose to normal liver (Gy) × T:N
Equation 5:
Dose to lungs (Gy) = 50 (Gy ∙ kg ∙ GBq-1) × Activity (GBq) × Lung shunting fraction / mass of lungs (kg)
For glass-based microspheres, the recommended dose to the liver is between 80 to 150 Gy [29]. The calculation of activity is also based on the MIRD model. However, unlike the partition method used in resin-based microspheres, this only considers average dose to the entire affected liver—i.e., both the tumorous and normal liver parts.
Activity (GBq) = [Dose (Gy) × Mass of affected liver (kg)] / [50 (Gy ∙ kg ∙ GBq-1) × (1 – lung shunting fraction)]
4. Indication and contraindication
The use of resin-based microspheres (SIR-Spheres®) is approved for treatment of advanced non-operable liver cancer in the European Union (EU) as well as unresectable metastatic liver tumors from primary colorectal cancer with adjuvant intra-hepatic artery chemotherapy of Floxuridine by the Food and Drug Administration in the United States (U.S. FDA). Glass-based microspheres (TheraSphere®) have a Humanitarian Device Exception for the treatment of unresectable hepatocellular carcinoma by the U.S.’s FDA and are approved for the treatment of hepatic neoplasia in the EU and Canada [30].
Generally, patients with HCC that are considered for SIRT have unresectable conditions, a life expectancy of at least 3 months, and are ambulatory/capable of self-care (a score less than or equal to 2 according to the Eastern Cooperative Oncology Group, ECOG [27]. On the other hand, contraindications for SIRT are determined by the potentially irreversible radiationinduced organ damage. For instance, significant and uncorrectable blood flow (either by angiogram or pretreatment Tc-99m MAA scan) to the gastrointestinal tract may cause severe gastric or intestinal ulcer and even hollow-organ perforation. Excessive shunting to lungs that can potentially exceed the 30-Gy lung dose limit noted by Tc-99m MAA scan may induce radiation pneumonitis. A large tumor burden with limited hepatic reserve or biochemical evidence of reduced liver function may elicit the post-therapeutic liver failure. Prior external beam radiotherapy involving the liver may increase the possibility of radiationinduced liver disease due to accumulated radiation doses [13].
Although SIRT with 90Y-bearing microspheres has an acceptable safety profile, studies comparing 90Y-bearing microspheres to other palliative loco-regional therapies and thus consensus about the optimal use of 90Y-bearing microspheres are currently lacking [31]. However, SIRT with 90Y-bearing microspheres, especially with the glass-based microspheres, because of their low embolic potential, may be preferred to transarterial chemoembolization (TACE) in the presence of portal vein occlusion [32-34]. SIRT is also not currently listed among possible treatment options for HCC in some guidelines [35]. However, there have been suggestions to use SIRT as a bridging treatment or as a main therapy for patients with diffuse intrahepatic tumor involvement [36]. Nevertheless, the therapy may be considered for patients with an insufficient hepatic reserve, poor performance status, and the comorbidities of a resectable disease [37]. Potential indications for specific clinical conditions are listed in the following paragraphs.
4.1. Bridging therapy for subsequent liver transplantation
Patients with early-stage hepatocellular carcinoma can be candidates for liver transplantation as a curative treatment. However, organ donors, compared to those in need of organs, are few and far between, causing long waiting list for transplantation. Hence, patients in need of a liver transplant may be at a high risk for disease progression, which would subsequently render them ineligible for transplantation surgery. To decrease the probability of disease progression while waiting, these patients are usually treated with various kinds of locoregional therapies, such as TACE and percutaneous ablation, with the aim of temporarily restricting the tumor growth. It is in this context that SIRT has also been proposed for theoretically being better able to control tumor growth and disease progression as compared to the aforementioned traditional approach. However, SIRT has not been widely applied and validated due to its relatively high cost [38].
4.2. Downsizing/downstaging therapy for subsequent curative surgery
For patients with intermediate-stage hepatocellular carcinoma, shrinkage of the tumor may convert the initially unresectable tumor to a resectable tumor, or perhaps put the patient in an eligible condition for liver transplantation [39]. The SIRT method has been compared to the traditional TACE method regarding its effect on downsizing/downstaging tumors to allow for liver transplantation, and the data have shown a higher percentage of SIRT patients with tumor downstaging (58%, compared to 31% of TACE) [40].
4.3. “Radiation lobectomy” to induce hypertrophy of liver remnant for subsequent curative surgery
Portal vein embolization is a standard technique for patients with liver malignancies that are not amenable to surgical resection owing to the small liver remnant that would remain after surgery. Portal vein embolization induces contralateral hypertrophy by redirecting the portal blood flow, which would increase the volume of the post-surgery liver remnant [41, 42]. However, there has been concern raised about the progression of diseases left untreated while waiting for the hypertrophy process to have its effect [43]. Recently “radiation lobectomy”, defined as the transarterial lobar infusion of 90Y-bearing microspheres, has been found to induce similar or superior volumetric changes as portal vein embolization in hepatic lobes. It induces marked hypotrophy of the treated hepatic lobe and evident hypertrophy of the contralateral lobe. By limiting the rate of portal blood flow diversion, this therapy also effectively treats the cancer itself and reduces the risk of preinterventional tumor progression [42, 44].
4.4. Local control and palliative therapy for inoperable disease
In a retrospective analysis consisting of 122 HCC-affected patients treated with TACE and 123 HCC-affected patients treated with glass-based SIRT, the overall survival of patients in all-stages (median survival of TACE and SIRT are 17.4 and 20.5 months, respectively, P = 0.232) or in the intermediate-stage (17.2 and 17.5 months, respectively, P = 0.42) were found to be similar [45]. However, significantly more frequent fatigue, nausea, anorexia, and abdominal pain were observed in patients treated with TACE, suggesting a more favorable safety profile and a potentially reduced need for hospitalization to treat adverse effects with SIRT treatment [46-49].
5. Safety of repeated selective internal radiation therapies
Repeated selective internal radiation therapies of liver tumors theoretically create a higher risk of developing radiation-induced liver disease because of the accumulated unrecoverable injuries done to the liver. Lam et al. retrospectively analyzed 8 patient treated by repeated selective internal radiation therapies with resin-based microspheres and the BSA method [50]. Two of them died shortly after the second treatment (at 84 and 107 days), and their deaths were attributed to radiation-induced liver disease. Both patients underwent whole liver treatment twice (cumulative activities of 3.08 and 2.66 GBq), and were heavily treated with multiple systemic chemotherapy and tumor resections before the 1st SIRT and between two selective internal radiation therapies. The authors concluded that repeated SIRT of the same targeted liver volume, especially in whole liver treatments and high cumulative activities, might increase the risk for development of radiation-induced liver disease.
On the other hand, Zarva et al. performed repeated SIRTs to 21 patients with advanced liver tumors [51]. The authors also used resin-based microspheres and the BSA method for treatments. However, they treated patients only 1 lobe at a time, and there was an interval between selective internal radiation therapies of both lobes of 4 to 6 weeks if both liver lobes needed to be treated in a single SIRT cycle. An average of 1.6 whole-liver treatments in 3.0 unilobar selective internal radiation therapies (liver lobes sequentially) were given to patients with a mean total activity of 2.57 GBq. No radiation-induced liver disease was observed in any of their patients. They concluded that there was a significantly better tolerance to SIRT in patients with sequential lobar treatments than in patients receiving single-session wholeliver treatments.
6. Safety of sirt after ebrt
There is limited literature discussing the safety of performing SIRT after EBRT. In one retrospective study consisting of 31 patients, dose-volume analysis of the liver showed that the fraction of liver exposed to ≥ 30 Gy (V30) was the strongest predictor of hepatotoxicity [52]. Patients with V30 > 13% had hepatotoxicity, and fatal radiation-induced liver disease occurred in two patients with the highest mean liver doses of EBRT (20.9 Gy and 23.1 Gy) and also at the highest cumulative liver doses (91.8 Gy and 149 Gy). Hence, SIRT may be safe only for patients with limited hepatic exposure to previous EBRT, and should be used cautiously if the prior V30 of the liver exceeded about 10%.
7. Conclusions
SIRT with 90Y-bearing microspheres offers an opportunity for effective local tumor control and even tumor regression in patients with unresectable hepatocellular carcinomas. Moreover, it causes less adverse effects as compared to TACE, as well as decreasing the need for prolonged hospitalization. Radiation lobectomy seems promising as an alternative technique to portal vein embolization for patients with liver malignancies not amenable to surgical resection owing to small liver remnant after surgery. However, more evidence from prospective studies among patients with HCC at various stages are necessary to confirm the usefulness of SIRT.
Contributor Information
Yu-Chin Wu, Email: kubler0514@gmail.com.
Chia-Hung Kao, Email: d10040@mail.cmuh.org.tw.
References
- 1.Fitzmorris P, Shoreibah M, Anand BS, Singal AK. Management of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2015;141:861–76. doi: 10.1007/s00432-014-1806-0. [DOI] [PubMed] [Google Scholar]
- 2.Kalva SP, Thabet A, Wicky S. Recent advances in transarterial therapy of primary and secondary liver malignancies. Radiographics. 2008;28:101–17. doi: 10.1148/rg.281075115. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264-73 e1. [DOI] [PMC free article] [PubMed]
- 4.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–5. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 5.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::AID-CNCR2820560437>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM. Treatment of Hepatocellular Carcinoma. Curr Treat Options Gastroenterol. 2004;7:431–41. doi: 10.1007/s11938-004-0002-8. [DOI] [PubMed] [Google Scholar]
- 7.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–21. doi: 10.1016/S0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 9.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–77. [PMC free article] [PubMed] [Google Scholar]
- 10.Ackerman NB, Lien WM, Kondi ES, Silverman NA. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to “small” and “large” tumors. Surgery. 1969;66:1067–72. [PubMed] [Google Scholar]
- 11.Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg. 1989;76:545–8. doi: 10.1002/bjs.1800760607. [DOI] [PubMed] [Google Scholar]
- 12.Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff DC, Gupta S, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25(1):S41–55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med. 2010;40:105–21. doi: 10.1053/j.semnuclmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Edeline J, Gilabert M, Garin E, Boucher E, Raoul JL. Yttrium-90 microsphere radioembolization for hepatocellular carcinoma. Liver. Cancer. 2015;4:16–25. doi: 10.1159/000343878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995;33:919–24. doi: 10.1016/0360-3016(95)00039-3. [DOI] [PubMed] [Google Scholar]
- 16.Lau WY, Ho S, Leung TW, Chan M, Ho R, Johnson PJ, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int J Radiat Oncol Biol Phys. 1998;40:583–92. doi: 10.1016/S0360-3016(97)00818-3. [DOI] [PubMed] [Google Scholar]
- 17.Burton MA, Gray BN, Klemp PF, Kelleher DK, Hardy N. Selective internal radiation therapy: distribution of radiation in the liver. Eur J Cancer Clin Oncol. 1989;25:1487–91. doi: 10.1016/0277-5379(89)90109-0. [DOI] [PubMed] [Google Scholar]
- 18.Andrews JC, Walker SC, Ackermann RJ, Cotton LA, Ensminger WD, Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–44. [PubMed] [Google Scholar]
- 19.Yorke ED, Jackson A, Fox RA, Wessels BW, Gray BN. Can current models explain the lack of liver complications in Y-90 microsphere therapy? Clin Cancer Res 1999; 5: 3024s-30 s. [PubMed]
- 20.Gray BN, Burton MA, Kelleher D, Klemp P, Matz L. Tolerance of the liver to the effects of Yttrium-90 radiation. Int J Radiat Oncol Biol Phys. 1990;18:619–23. doi: 10.1016/0360-3016(90)90069-V. [DOI] [PubMed] [Google Scholar]
- 21.Lau WY, Kennedy AS, Kim YH, Lai HK, Lee RC, Leung TW, et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82:401–7. doi: 10.1016/j.ijrobp.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Braat AJ, Smits ML, Braat MN, van den Hoven AF, Prince JF, de Jong HW, et al. 90Y Hepatic Radioembolization: An Update on Current Practice and Recent Developments. J Nucl Med. 2015;56:1079–87. doi: 10.2967/jnumed.115.157446. [DOI] [PubMed] [Google Scholar]
- 23.Lau WY, Leung WT, Ho S, Leung NW, Chan M, Lin J, et al. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br J Cancer. 1994;70:994–9. doi: 10.1038/bjc.1994.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho S, Lau WY, Leung TW, Chan M, Johnson PJ, Li AK. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med. 1997;24:293–8. doi: 10.1007/BF01728766. [DOI] [PubMed] [Google Scholar]
- 25.Welsh JS, Kennedy AS, Thomadsen B. Selective Internal Radiation Therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma Int J Radiat Oncol Biol Phys. 2006;66:S62–73. doi: 10.1016/j.ijrobp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Lam MG, Louie JD, Abdelmaksoud MH, Fisher GA, Cho-Phan CD, Sze DY. Limitations of body surface area-based activity calculation for radioembolization of hepatic metastases in colorectal cancer. J Vasc Interv Radiol. 2014;25:1085–93. doi: 10.1016/j.jvir.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Kao YH, Tan EH, Ng CE, Goh SW. Clinical implications of the body surface area method versus partition model dosimetry for yttrium-90 radioembolization using resin microspheres: a technical review. Ann Nucl Med. 2011;25:455–61. doi: 10.1007/s12149-011-0499-6. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol. 2012;35:91–9. doi: 10.1097/COC.0b013e3181f47583. [DOI] [PubMed] [Google Scholar]
- 31.Vilarinho S, Taddei T. Therapeutic strategies for hepatocellular carcinoma: new advances and challenges. Curr Treat Options Gastroenterol. 2015;13:219–34. doi: 10.1007/s11938-015-0049-8. [DOI] [PubMed] [Google Scholar]
- 32.Bruix J, Sherman M, American Association for the Study of Liver D Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 34.Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15:335–45. doi: 10.1097/01.RVI.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- 35.Sacco R, Mismas V, Marceglia S, Romano A, Giacomelli L, Bertini M, et al. Transarterial radioembolization for hepatocellular carcinoma: An update and perspectives. World J Gastroenterol. 2015;21:6518–25. doi: 10.3748/wjg.v21.i21.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelic S, Sotiropoulos GC, Group EGW. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21 Suppl 5: v59-64. [DOI] [PubMed]
- 37.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–86. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 39.Inarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D’Avola D, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594–601. doi: 10.1016/j.ejso.2012.02.189. [DOI] [PubMed] [Google Scholar]
- 40.Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–8. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 41.Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104–9. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029–36. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617–22. doi: 10.1038/sj.bjc.6604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–96. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 45.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140: 497-507 e2. [DOI] [PMC free article] [PubMed]
- 46.El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechene A, Abdella H, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35:627–35. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714–23. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305–14. doi: 10.1002/cncr.24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–30. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Lam MG, Louie JD, Iagaru AH, Goris ML, Sze DY. Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36:1320–8. doi: 10.1007/s00270-013-0547-9. [DOI] [PubMed] [Google Scholar]
- 51.Zarva A, Mohnike K, Damm R, Ruf J, Seidensticker R, Ulrich G, et al. Safety of repeated radioembolizations in patients with advanced primary and secondary liver tumors and progressive disease after first selective internal radiotherapy. J Nucl Med. 2014;55:360–6. doi: 10.2967/jnumed.113.127662. [DOI] [PubMed] [Google Scholar]
- 52.Lam MG, Abdelmaksoud MH, Chang DT, Eclov NC, Chung MP, Koong AC, et al. Safety of 90Y radioembolization in patients who have undergone previous external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:323–9. doi: 10.1016/j.ijrobp.2013.05.041. [DOI] [PubMed] [Google Scholar]


