Abstract
Aberrations in one-carbon metabolism were reported to increase breast cancer risk by influencing the DNA synthesis and methylation of DNA and catecholamines. However, the results of these association studies remain inconclusive. We have explored the contribution of eight genetic polymorphisms in modulating the susceptibility to breast cancer by performing a meta-analysis of worldwide studies. In total, 62 case-control studies representing 17 different populations involving 18,117 breast cancer cases and 23,573 healthy controls were included in this meta-analysis. Out of the eight polymorphisms analyzed, methylenetetrahydrofolate reductase (MTHFR) C677T exhibited positive association with the breast cancer risk in both fixed effects (OR 1.14, 95 % CI 1.10–1.17) and random effects (OR 1.10, 95 % CI 1.02–1.18) models. Solute carrier family 19 (folate transporter), member 1 (SLC19A1) G80A exhibited positive association (OR 1.16, 95 % CI 1.03–1.31) while MTR A2756G exhibited an inverse association (OR 0.78, 95 % CI 0.75–0.82) with the breast in fixed effect model alone. Significant heterogeneity was observed in the association of MTHFR C677T with breast cancer even between studies from the same geographical area, specifically among Chinese, Indians, and Turks. Subgroup analysis revealed MTHFR C677T-mediated breast cancer risk in post-menopausal women and women with low dietary intake of folate. Geographical area wise segregation of data revealed MTHFR-mediated increased breast cancer risk in populations who consume methionine-rich diet. Altitude-level variations were observed in the association of SHMT1 C1420T with breast cancer. India and Brazil of same altitude showed an inverse association with this polymorphism, while USA and China that share similar altitude showed a null association. MTHFR C677T and SLC19A1 G80A are the two polymorphisms of one-carbon metabolic pathway that increase breast cancer in the worldwide population. Dietary patterns and altitudinal variations are the likely risk modulators that are contributing toward ethnic- and population-level variations in genetic associations.
Keywords: Breast cancer, One-carbon metabolism, Polymorphisms, Altitude
Introduction
The etiology of breast cancer is complex, involving interactions between genetic and environmental factors; and epigenetic modifications. The high-penetrant genetic mutations account for less than 10 % breast cancer cases (Hoskins et al. 1995). Several genetic polymorphisms have been explored across different pathways for possible association with breast cancer (Fachal and Dunning 2015). Among these pathways, the most widely investigated pathway is the one-carbon metabolic pathway, which was so named because of the several metabolic reactions involving the transfer of one-carbon moiety from one substrate to another to form several crucial metabolic precursors.
The dietary folate in the form of folyl polyglutamates is hydrolyzed by folate hydrolase (prostate-specific membrane antigen) 1 (FOLH1) to folyl monoglutamates, which are absorbed easily by the intestinal cells (Halsted 1989). Folate reductase catalyzes the reduction of folate to dihydrofolate (DHF) and tetrahydrofolate (THF). The THF of the blood stream is transported into RBC by the solute carrier family 19 (folate transporter), membrane 1 (SLC19A1) (Brzezińska et al. 2000). The methylene moiety from the serine is transferred to THF to form 5,10-methylene THF in the presence of serine hydroxymethyltransferase 1 (soluble) (SHMT1) (Stover and Schirch 1992). The 5,10-methylene THF is the common substrate for two rate-limiting enzymes, i.e., thymidylate synthase (TYMS) and methylenetetrahydrofolate reductase (NAD(P)H) (MTHFR), which catalyze the conversion of dUMP to dTMP and FAD-dependent reduction of 5,10-methylene THF to 5-methyl THF, respectively (Trinh et al. 2002). The 5-methyl THF is the cosubstrate for the remethylation of homocysteine to methionine in the presence of 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR). The other cosubstrate, i.e., methyl cobalamin is formed due to reductive methylation of cobalamin by the 5-methyltetrahydrofolate-homocysteine methyltransferase reductase (MTRR). The MTR-MTRR holoenzyme complex, thus contributes toward the remethylation of homocysteine to methionine (Brot and Weissbach 1966). Methionine is the precursor for the synthesis of S-adenosylmethionine (SAM), which is a universal methyl donor that donates a methyl group to DNA, histones, and catecholamines. After donating a methyl group, SAM is converted to S-adenosyl homocysteine (SAH).
Since aberrant methylation of tumor suppressors and catechol estrogens, defective DNA synthesis and repair are the well-documented risk factors for breast cancer; several polymorphisms of one-carbon metabolic pathway were explored for the possible association with breast cancer. Limited studies are there on FOLH1 C1561T polymorphism in association with breast cancer (Mohammad et al. 2011; Naushad et al. 2011). Several other genetic variants of FOLH1 are also explored for their association with breast cancer (Divyya et al. 2013). SLC19A1 G80A was shown to exert breast cancer risk in Indian and Brazilian populations (Carvalho Barbosa Rde et al. 2011; Mohammad et al. 2011; Naushad et al. 2011), while no association was reported in the US population (Xu et al. 2007). SHMT1 C1420T polymorphism was shown to confer protection against breast cancer in Indian (Mohammad et al. 2011; Naushad et al. 2011), Chinese (Wu et al. 2014), and Brazilian (Carvalho Barbosa Rde et al. 2011) populations while null association was observed in the US and (Xu et al. 2007) Taiwanese (Yu et al. 2007) populations. TYMS 5′-UTR 28 bp tandem repeat polymorphism showed null association with breast cancer risk in six studies (Xu et al. 2007; Suzuki et al. 2008; Carvalho Barbosa Rde et al. 2011; Naushad et al. 2011). TYMS 3′-UTR ins6/del6 showed positive association with breast cancer in the Japanese population (Zhai et al. 2006), while it showed a null association in Indians (Naushad et al. 2011), Chinese (Zhai et al. 2006) and Germans (Justenhoven et al. 2005). MTHFR C677T is the most widely studied polymorphism of this pathway; the segregation of data based on ethnic group or populations revealed a strong association of this polymorphism in Turkey(Deligezer et al. 2005; Ozen et al. 2013), China (Cheng et al. 2008; Gao et al. 2009; Wu et al. 2012; Jiang-Hua et al. 2014), Syria (Lajin et al. 2012), Morocco (Diakite et al. 2012), and North America (Maruti et al. 2009; Bentley et al. 2010; Ramos-Silva et al. 2015). Two studies, i.e., one on multi-ethnic group (Le Marchand et al. 2004) and another on the Iranian population (Hosseini et al. 2011), showed a protective role of this polymorphism. In rest of the population (N = 10), the association of this polymorphism was either borderline or null (Shrubsole et al. 2004; Lee et al. 2004; Inoue et al. 2008; Ma et al. 2009; Hosseini et al. 2011; Prasad and Wilkhoo 2011; Akram et al. 2012; Awwad et al. 2015). MTR A2756G was investigated in ten different populations out of which it was identified as a risk factor in Iranian (Hosseini 2013) and Australian (Beetstra et al. 2008) populations. In two populations, i.e., Greece (Kakkoura et al. 2015) and China (He et al. 2014), this polymorphism showed an inverse association with breast cancer risk while in other population, it showed a null association (Platek et al. 2009; Weiwei et al. 2014; He et al. 2014). MTRR A66G was identified as a risk for breast cancer in a Russian population (Tao et al. 2009), while it was shown to have an inverse association with Thai (Sangrajrang et al. 2009; Sangrajrang et al. 2010) and Australian (Beetstra et al. 2008) populations. In another eight populations, MTRR A66G showed a null association (Kotsopoulos et al. 2008; Burcoş et al. 2010; Weiner et al. 2012).
The variations in genetic association across different populations might be attributed to gene-gene and gene-nutrient interactions which act as potential risk modulators. In the current study, we have aimed to provide a comprehensive overview of all the genetic association relevant to one-carbon metabolism as possible risk modulators for breast cancer using meta-analysis approach. This will help in identifying the genetic risk factors that are common across the different populations and also to emphasize the role of dietary and lifestyle patterns in dictating the breast cancer risk along with a given set of polymorphisms.
Materials and methods
Search strategy and selection criteria
We searched four electronic databases (PubMed, Google Scholar, Scopus, and Medline) to identify eligible studies that were published before December 2015. Articles were retrieved by using the following keywords: “GCPII/FOLH1,” “RFC1/SLC19A1,” “SHMT1,” “TYMS,” “MTHFR,” “MTR,” “MTRR,” “polymorphism,” and “breast cancer.” The reference list of the retrieved publications was also reviewed to identify additional relevant articles.
Inclusion and exclusion criteria
The inclusion criteria were the following: (a) case-control study involving unrelated individuals, (b) information on the raw data of genotypes, (c) information on ethnicity, and (d) the genotype distribution in accordance with Hardy-Weinberg equilibrium (HWE). The exclusion criteria were the following: (a) case only study, (b) meta-analysis, (c) only minor allele frequencies provided, and (d) duplication of data.
Data extraction
A standardized form was used by the investigators for independent extraction of data and for credibility of results. The following information was extracted from each published article: first author, year of publication, ethnicity (country), number of cases, number of controls, source of controls, and genotype distribution.
Segregation of data into ethnic groups and populations
The studies from the same ethnic group or population were pooled together to assess ethnicity or population-based risk. This analysis is useful in addressing the risk modulation based on specific dietary or lifestyle patterns. The number of ethnic groups, number of subjects in each group, and their association with each polymorphism were tabulated.
In silico analysis
In order to elucidate the structural and functional effects of the amino acid substitution, Polymorphism Phenotyping v2 (PolyPhen-2) (http://genetics.bwh.harvard.edu/pph2/) was used, which was based on the physical and comparative considerations. The score classifies the proteins into benign, partially damaging and highly damaging.
Statistical analysis
Using statpages.org, chi-square goodness of fit for each cases and control studies was calculated. p Value with 95 % confidence interval, odds ratio, and phi coefficient were included in the data. p Value with 0.05 or less was considered as significant, and breast cancer risk was assessed through OR. A computational tool (statdirect) was used to conduct meta-analysis of all the studies related to polymorphisms of one-carbon metabolism. The data were computed in the form of number of variant alleles and number of total alleles in cases and controls. The fixed effect model was generated using the Mantel-Haenszel and the Robins-Breslow-Greenland algorithm. These were based on conditional maximum likelihood. The random effects model was based on the DerSimonian-Laird algorithm. Non-combinability of studies was assessed based on the Cochran’s Q test. The effect of heterogeneity was quantified with the I 2 test. Publication bias was assessed based on the Horbold-Egger test.
Results
Characteristics of the included studies
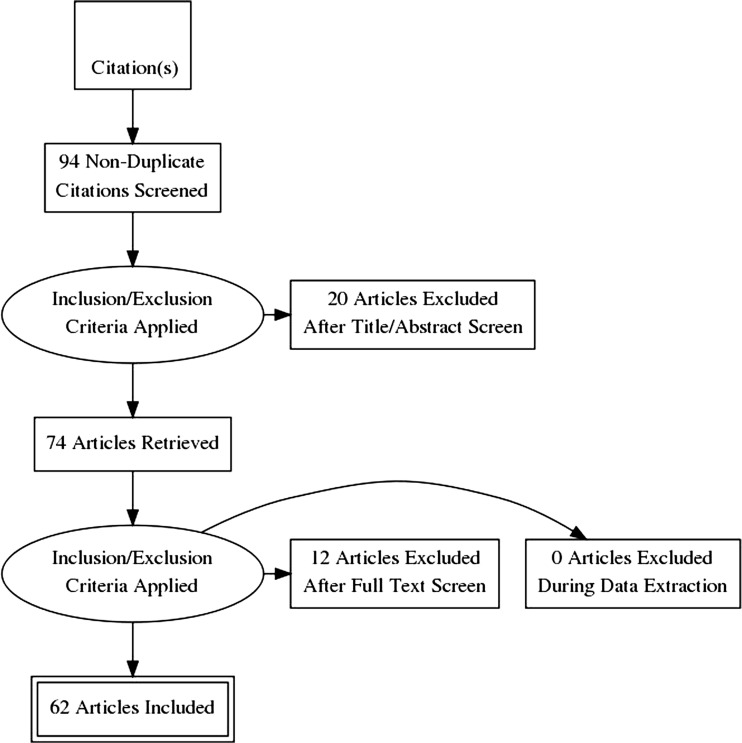
According to search, 94 potentially relevant articles were identified. After applying the inclusion and exclusion criteria, we have chosen 62 published studies representing 17 populations involving 18,117 cases of breast cancer and 23,573 healthy controls (Flowchart 1).
Flowchart 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. This illustrates the selection process of the studies
Meta-analysis databases
There are three studies from India on FOLH1 C1561T polymorphism where one study was taken as representative which shows no association of this polymorphism with breast cancer (OR 0.74, 95 % CI 0.46–1.19) (Table 1). No other population-specific data were available on FOLH1 C1561T with relevance to breast cancer.
Table 1.
Association of polymorphisms of one-carbon metabolic pathway with breast cancer risk
| Polymorphism | Number of populations (subjects) | Pooled odds ratio | Test for homogeneity | Bias | |
|---|---|---|---|---|---|
| Fixed effects | Random effects | ||||
| FOLH1 C1561T (rs61886492) T vs. C-allele |
1 (486) | 0.74 (0.46–1.19) | ND | ND | ND |
| SLC19A1 G80A (rs1051266) A vs. G-allele |
3 (3177) | 1.16 (1.03–1.30)a | 1.28 (0.98–1.67) | 7.91a | 4.78 |
| cSHMT C1420T (rs1979277) T vs. C-allele |
4 (5742) | 0.93 (0.86–1.01) | 0.86 (0.71–1.03) | 7.94a | −2.15 |
| TYMS 5′-UTR 2R/3R (rs45445694) 2R vs. 3R |
5 (5461) | 0.96 (0.88–1.04) | 0.96 (0.88–1.04) | 2.76 | 2.24a |
| TYMS 3′-UTR ins6/del6 Del6 vs. ins6-allele |
4 (2676) | 1.02 (0.91–1.14) | 1.16 (0.84–1.59) | 19.06a | 5.19 |
| MTHFR C677T (rs1801133) T vs. C-allele |
17 (51,690) | 1.14 (1.10–1.17)a | 1.10 (1.02–1.18)a | 57.88a | −0.73 |
| MTR A2756G (rs1805087) G vs. A-allele |
10 (22,584) | 0.78 (0.75–0.82)a | 0.99 (0.76–1.29) | 236.06a | 6.28a |
| MTRR A66G (rs1801394) G vs. A-allele |
11 (15,018) | 1.02 (0.97–1.07) | 1.02 (0.95–1.09) | 15.78 | −0.97 |
This table illustrates association of each folate pathway genetic polymorphism with breast cancer risk in the worldwide population. The data from the literature was grouped based on the populations. The number of populations and the number of subjects included for the analysis were depicted
ND not determined
aStatistically significant
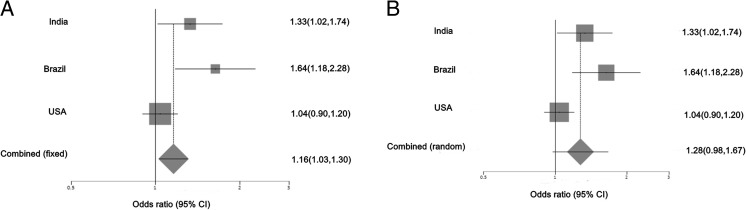
The data on SLC19A1 G80A was complied into three distinct populations, out of which Indian (OR 1.33, 95 % CI 1.02–1.74) and Brazilian (OR 1.63, 95 % CI 1.18–2.28) populations exhibited an increased risk for breast cancer. Null association was observed in US population (OR 1.04, 95 % CI 0.90–1.20). The pooled OR was significant in the fixed effect model (OR 1.16, 95 % CI 1.03–1.30). However, the random effects model showed no statistical significance (p = 0.08). Cochran’s Q tests showed evidence of heterogeneity in association (p = 0.02). There is no evidence of publication bias (p = 0.07) (Fig. 1).
Fig. 1.
Association of SLC19A1 G80A with breast cancer in three populations. This illustrates population based risk association in a fixed effects model and b random effects model
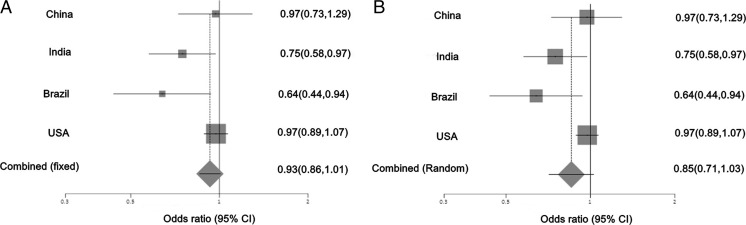
Studies on SHMT1 C1420T were segregated into four populations; out of these Indian (OR 0.75, 95 % CI 0.58, 0.97) and Brazilian (OR 0.64, 95 % CI 0.44–0.94) populations showed statistically significant protective role against breast cancer. Null association was observed in Chinese (OR 0.97, 95 % CI 0.73–1.29) and US (OR 0.97, 95 % CI 0.89–1.07) populations. Pooled OR was not statistically significant in both fixed effects and random effects models. The Cochran’s Q test of heterogeneity was positive (p = 0.047). However, there is no evidence of publication bias (p = 0.20) (Fig. 2). Altitudinal variation was observed in the association of SHMT1 C1420T with breast cancer (Fig. 3).
Fig. 2.
Association of SHMT1 C1420T with breast cancer in four populations. This illustrates population based risk association in a fixed effects model and b random effects model
Fig. 3.
Influence of altitude on SHMT1 C1420T association. The two population, i.e., Indians and Brazilians who showed protective role (light gray) and two other population, Chinese and US, who showed null association (dark gray) shared similar altitude
Studies on TYMS 5′UTR 28 bp repeat polymorphism were segregated into five populations, i.e., Chinese, Brazilian, US, Indian, and Japanese. Null association was observed in all the populations. Both fixed effects and random effects models showed null association with pooled data (p = 0.28). No evidence of heterogeneity was shown in Cochran’s Q test (p = 0.60). There was evidence for publication bias based on Horbold-Egger test (p = 0.02).
In four populations, the association of TYMS 3′UTR with breast cancer was investigated. Japanese population alone showed statistically significant risk with this polymorphism (OR 3.54, 95 % CI 1.86–6.76). However, the sample size of the study was very less. In other populations, i.e., Indian, Chinese, and Germans, null associations were observed with this polymorphism. Both fixed effects (p = 0.77) and random effects (p = 0.36) models showed null association of the pooled data. The Cochran’s Q test (p = 0.0003) showed evidence of heterogeneity in association. No publication bias was observed for this polymorphism (p = 0.20).
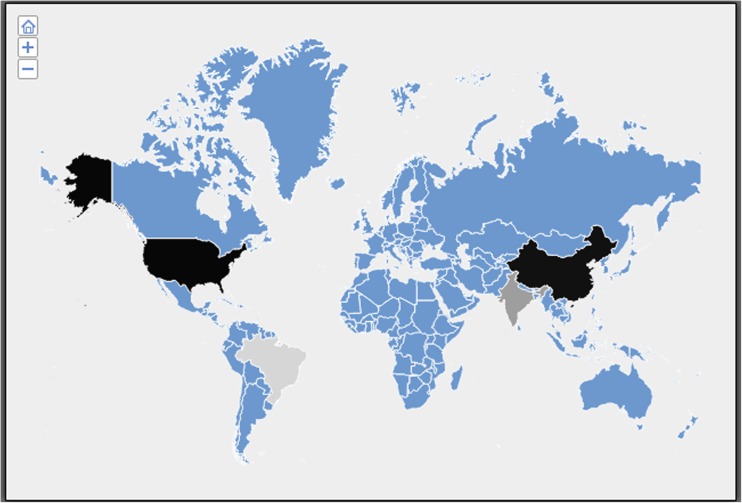
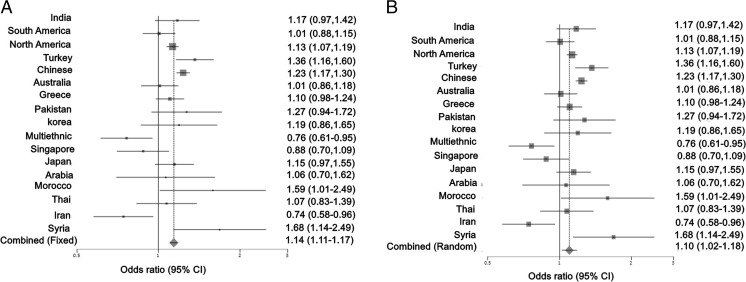
As shown in Table 2, the association of MTHFR C677T with breast cancer exhibits lot of heterogeneity across different studies. The Cochran’s Q test (Q 239.09, p < 0.0001) confirms this heterogeneity. Furthermore, the data was grouped based on populations. It was identified as a risk factor in North America, Turkey, China, Morocco, and Syria. In other populations, the association was not statistically significant. Both fixed effects (p < 0.0001) and random effects (p = 0.01) models showed significant risk for this polymorphism in the pooled analysis. The Cochran’s Q test (p < 0.0001) indicated heterogeneity in the association across different populations. No evidence of publication bias was observed (p = 0.34) (Fig. 4). Except for North America and China, all other countries showing MTHFR-mediated risk for breast cancer belong to Mediterranean origin (Fig. 5). As shown in Table 3, even within a geographical area, heterogeneity was observed with regard to association of MTHFR C677T with breast cancer, specifically among Chinese, Indians, and Turks. Subgroup analysis revealed MTHFR C677T-mediated breast cancer risk in post-menopausal women and in women with low dietary intake of folate (Tables 4 and 5).
Table 2.
Association of MTHFR C677T with breast cancer risk across different studies
| Author | MTHFR 677 T-allele frequency | OR | 95 % CI | ||
|---|---|---|---|---|---|
| Cases | Controls | Limit | Limit | ||
| Kalyan | 0.5698 | 0.4536 | 1.5892 | 0.9785 | 2.581 |
| Mir | 0.2727 | 0.5614 | 0.3084 | 0.1158 | 0.8214 |
| Naushad | 0.6139 | 0.4869 | 1.6676 | 1.0959 | 2.5376 |
| Prasad | 0.4118 | 0.5132 | 0.6777 | 0.2612 | 1.7584 |
| Barbosa | 0.4906 | 0.52 | 0.8894 | 0.6482 | 1.2205 |
| Ma | 0.4938 | 0.5028 | 0.9648 | 0.7912 | 1.1765 |
| Xu | 0.5105 | 0.478 | 1.1386 | 1.0075 | 1.2868 |
| Bentley | 0.439 | 0.43 | 1.0371 | 0.918 | 1.1717 |
| Platek | 0.3568 | 0.3548 | 1.0088 | 0.899 | 1.1321 |
| Tao | 0.2191 | 0.2218 | 0.9847 | 0.8519 | 1.1381 |
| Chen | 0.5105 | 0.478 | 1.1386 | 1.0075 | 1.2868 |
| Maruti | 0.3615 | 0.3137 | 1.2389 | 1.0148 | 1.5125 |
| Jin | 0.2989 | 0.2872 | 1.0639 | 0.6136 | 1.8447 |
| Yu | 0.2199 | 0.201 | 1.1238 | 0.8071 | 1.5649 |
| Liu | 0.5082 | 0.4973 | 1.0443 | 0.8396 | 1.2989 |
| Cheng | 0.3916 | 0.3992 | 0.9691 | 0.7839 | 1.1981 |
| Gao | 0.5305 | 0.4791 | 1.2286 | 1.0471 | 1.4416 |
| Wu | 0.4348 | 0.4696 | 0.8777 | 0.3622 | 2.1267 |
| Weiwei | 0.5539 | 0.469 | 1.4044 | 1.0904 | 1.809 |
| Jiang-Hua | 0.5063 | 0.4127 | 1.4595 | 1.2301 | 1.7315 |
| He | 0.5 | 0.427 | 1.3418 | 1.0646 | 1.691 |
| Wu | 0.6039 | 0.4036 | 2.2411 | 1.4345 | 3.5013 |
| Li | 0.3978 | 0.2879 | 1.6362 | 1.0148 | 2.6381 |
| Yuan | 0.6039 | 0.4036 | 2.2411 | 1.4345 | 3.5013 |
| Qi | 0.5359 | 0.4574 | 1.3689 | 1.0484 | 1.7874 |
| Shrubsole | 0.4873 | 0.491 | 0.9855 | 0.8758 | 1.1089 |
| Kan | 0.6083 | 0.5268 | 1.3905 | 0.9109 | 2.1224 |
| Hua | 0.4432 | 0.5355 | 0.6926 | 0.429 | 1.1182 |
| Lin | 0.2122 | 0.2016 | 1.0711 | 0.7451 | 1.5397 |
| Chou | 0.3187 | 0.3391 | 0.9134 | 0.6723 | 1.241 |
| Yu | 0.2466 | 0.2112 | 1.2253 | 0.8936 | 1.6802 |
| Justenhoven | 0.4599 | 0.4908 | 0.8837 | 0.7482 | 1.0438 |
| Reljic | 0.5962 | 0.5849 | 1.0454 | 0.6498 | 1.6818 |
| kalemi | 0.4545 | 0.45 | 1.0196 | 0.5598 | 1.857 |
| Deligezer | 0.5158 | 0.4378 | 1.3671 | 1.0043 | 1.861 |
| Ozen | 0.4828 | 0.2891 | 2.289 | 1.285 | 4.0775 |
| Ergul | 0.4032 | 0.3693 | 1.1551 | 0.8133 | 1.6405 |
| Hekim | 0.3704 | 0.3704 | 1.0067 | 0.535 | 1.8944 |
| Cam | 0.5814 | 0.516 | 1.2996 | 0.8544 | 1.9768 |
| Akram | 0.5 | 0.5 | 1 | 0.6648 | 1.5042 |
| Grieu | 0.352 | 0.389 | 0.8539 | 0.693 | 1.0522 |
| Campbell | 0.623 | 0.574 | 1.2248 | 0.9493 | 1.5802 |
| Beetstra | 0.56 | 0.4211 | 1.724 | 0.6782 | 4.3826 |
| Awward | 0.544 | 0.4902 | 1.2389 | 0.8737 | 1.7569 |
| Lee | 0.5839 | 0.5408 | 1.1907 | 0.8722 | 1.6253 |
| Le Marchand | 0.3965 | 0.4628 | 0.7632 | 0.6165 | 0.9447 |
| Inoue | 0.3418 | 0.3714 | 0.8797 | 0.7096 | 1.0905 |
| Suzuki | 0.3511 | 0.3208 | 1.1459 | 0.9749 | 1.3468 |
| Alshatwi | 0.5093 | 0.4937 | 1.064 | 0.7142 | 1.5852 |
| Sangrajrang | 0.551 | 0.5338 | 1.0713 | 0.8365 | 1.3719 |
| Hosseini | 0.4444 | 0.5185 | 0.7434 | 0.5818 | 0.9497 |
| Lajin | 0.5698 | 0.4403 | 1.6797 | 1.1563 | 2.4401 |
| Liu | 0.5364 | 0.4755 | 1.276 | 1.0532 | 1.5459 |
| Diakite | 0.5339 | 0.4188 | 1.5859 | 1.0366 | 2.4262 |
| Batschauer | 0.4272 | 0.48 | 0.8084 | 0.5014 | 1.3035 |
| Cortes | 0.4256 | 0.3324 | 1.4868 | 1.0663 | 2.0732 |
| Silva | 0.6531 | 0.5627 | 1.4613 | 1.1872 | 1.7988 |
| Maria | 0.4947 | 0.4698 | 1.1049 | 0.98 | 1.2456 |
| Ericson | 0.3571 | 0.3232 | 1.1642 | 0.9422 | 1.4384 |
| [Combined] | |||||
| Fixed | 1.1031 | 1.0707 | 1.1364 | ||
| Random | 1.1333 | 1.0734 | 1.1966 | ||
Fig. 4.
Association of MTHFR C677T with breast cancer across 17 populations. This illustrates population based risk association in a fixed effects model and b random effects model
Fig. 5.
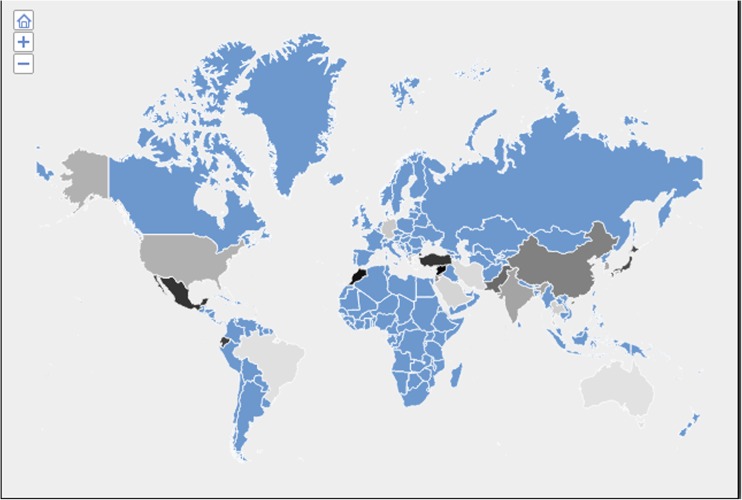
Influence of altitude on MTHFR C677T association. Iran, Singapore, Germany, Brazil, and Australia showed odds ratio ≤1.01. Saudi Arabia, Thailand, Greece, and North America showed odds ratios between 1.06 and 1.13. India, Korea, Chinese, Jordan, and Pakistan showed odds ratios between 1.17 and 1.27. Japan, Turkey, Morocco, and Syria showed odds ratios between 1.35 and 1.68. The change in odds ratio was depicted in gray to black gradation in the world map
Table 3.
Test for homogeneity between the studies on MTHFR C677T segregated based on geographical area
| Country | Total number of studies | Cochran’s Q | p value |
|---|---|---|---|
| China | 15 | 84.21 | <0.0001* |
| USA | 6 | 7.79 | 0.17 |
| Turkey | 6 | 12.54 | 0.03* |
| India | 4 | 9.07 | 0.03* |
| Taiwan | 3 | 1.93 | 0.38 |
| Australia | 3 | 1.03 | 0.60 |
| Brazil | 3 | 0.43 | 0.81 |
*Denotes statistical significance and indicative of heterogeneity in association
Table 4.
Effect of folate in modulating MTHFR C677T-mediated breast cancer risk
| Author | MTHFR T-allele freq | OR | 95 % CI | ||
|---|---|---|---|---|---|
| Cases | Control | Limit | Limit | ||
| High folate | |||||
| Shrubsole | 0.4071 | 0.4274 | 0.9202 | 0.7708 | 1.0985 |
| Lee | 0.3487 | 0.3333 | 1.0708 | 0.7376 | 1.5545 |
| Maruti | 0.3447 | 0.3212 | 1.113 | 0.8183 | 1.514 |
| Ma | 0.2724 | 0.3344 | 0.7457 | 0.5846 | 0.9511 |
| Suzuki | 0.4314 | 0.3909 | 1.1824 | 0.9644 | 1.4496 |
| Naushad | 0.1409 | 0.0461 | 3.3184 | 1.7081 | 6.4468 |
| Kakkoura | 0.3981 | 0.4035 | 0.9779 | 0.8241 | 1.1605 |
| He | 0.295 | 0.2896 | 1.0276 | 0.6859 | 1.5395 |
| Weiwei | 0.2717 | 0.2382 | 1.1929 | 0.8229 | 1.7294 |
| Chou | 0.1818 | 0.2411 | 0.7071 | 0.4221 | 1.1845 |
| Chen | 0.3911 | 0.3821 | 1.0383 | 0.8755 | 1.2314 |
| Le Marchand | 0.3243 | 0.3295 | 0.977 | 0.7912 | 1.2064 |
| [Combined] | |||||
| Fixed | 1.0046 | 0.9362 | 1.078 | ||
| Random | 1.0098 | 0.9372 | 1.088 | ||
| Low folate | |||||
| Shrubsole | 0.4312 | 0.4051 | 1.113 | 0.9375 | 1.3214 |
| Lee | 0.3358 | 0.3333 | 0.9992 | 0.4835 | 2.0652 |
| Maruti | 0.3854 | 0.3066 | 1.4177 | 1.0631 | 1.8906 |
| Ma | 0.2841 | 0.2451 | 1.2204 | 0.862 | 1.7279 |
| Suzuki | 0.4235 | 0.404 | 1.0843 | 0.8283 | 1.4193 |
| Naushad | 0.125 | 0.0865 | 1.488 | 0.827 | 2.6775 |
| Kakkoura | 0.435 | 0.3845 | 1.2324 | 1.0397 | 1.4607 |
| He | 0.3303 | 0.2787 | 1.2739 | 0.7285 | 2.2275 |
| Weiwei | 0.3412 | 0.25 | 1.5492 | 1.0879 | 2.2061 |
| Chou | 0.1053 | 0.2028 | 0.4722 | 0.2628 | 0.8484 |
| Chen | 0.4167 | 0.3581 | 1.2799 | 1.0708 | 1.5298 |
| Le Marchand | 0.3177 | 0.3025 | 1.0743 | 0.8625 | 1.338 |
| [Combined] | |||||
| Fixed | 1.1907 | 1.1037 | 1.2845 | ||
| Random | 1.1877 | 1.0722 | 1.3158 | ||
Table 5.
Effect of menopausal status on MTHFR C677T-mediated breast cancer risk
| Author | MTHFR T-allele freq | OR | 95 % CI | ||
|---|---|---|---|---|---|
| Cases | Control | Limit | Limit | ||
| Pre-menopausal | |||||
| Diakite | 0.3387 | 0.2705 | 1.3763 | 0.7999 | 2.3682 |
| Deligezer | 0.2611 | 0.227 | 1.2053 | 0.7824 | 1.857 |
| Le Marchand | 0.3129 | 0.3038 | 1.046 | 0.777 | 1.408 |
| Naushad | 0.1348 | 0.0714 | 1.9703 | 0.9701 | 4.0016 |
| Suzuki | 0.3958 | 0.412 | 0.9355 | 0.7392 | 1.1838 |
| Platek | 0.3431 | 0.3522 | 0.9613 | 0.7742 | 1.1937 |
| Ericson | 0.2982 | 0.3202 | 0.9035 | 0.708 | 1.153 |
| Ma | 0.2908 | 0.2899 | 1.0037 | 0.7255 | 1.3887 |
| [Combined] | |||||
| Fixed | 1.0026 | 0.9011 | 1.1155 | ||
| Random | 1.0026 | 0.9011 | 1.1155 | ||
| Post-menopausal | |||||
| Diakite | 0.3088 | 0.1964 | 1.8206 | 0.9154 | 3.6208 |
| Maruti | 0.3632 | 0.3153 | 1.2389 | 1.0148 | 1.5125 |
| Stevens | 0.1921 | 0.1582 | 1.2641 | 1.0014 | 1.5958 |
| Deligezer | 0.3384 | 0.2683 | 1.39 | 0.8845 | 2.1843 |
| Ziva Cerne | 0.3467 | 0.368 | 0.9111 | 0.7338 | 1.1313 |
| Le Marchand | 0.3183 | 0.3116 | 1.0316 | 0.9122 | 1.1665 |
| Naushad | 0.1013 | 0.0597 | 1.7551 | 1.0317 | 2.9857 |
| Suzuki | 0.4559 | 0.3784 | 1.3761 | 1.1014 | 1.7192 |
| Platek | 0.3444 | 0.3284 | 1.0746 | 0.9371 | 1.2324 |
| Ericson | 0.2518 | 0.2158 | 1.2238 | 0.9666 | 1.5495 |
| Ma | 0.312 | 0.3235 | 0.9486 | 0.7382 | 1.2189 |
| [Combined] | |||||
| Fixed | 1.1177 | 1.0491 | 1.1909 | ||
| Random | 1.1494 | 1.0443 | 1.265 | ||
Out of the ten populations investigated for possible association of MTR A2756G with breast cancer, the Iranian and Australian populations showed positive association; the Chinese and Greece populations showed an inverse association; and the Russian, German, Japanese, Brazilian, Indian, and US populations showed a null association. The pooled data showed the protective role of this polymorphism in fixed effects models alone (p < 0.0001). However, the random effects model showed a null association (p = 0.96). The Cochran’s Q test (p < 0.0001) showed evidence of heterogeneity. There is evidence of publication bias (p = 0.04).
Eleven studies explored the association of MTRR A66G with breast cancer. Out of these, only Russian population showed increased risk for breast cancer. Australian population showed an inverse association with breast cancer. Other nine populations, namely Chinese, Romanian, Canadian, Syrian, Polish, Thai, Japanese, and Indian showed a null association. Both fixed effects (p = 0.41) and random effects models (p = 0.59) showed a null association. The Cochran’s Q test (p < 0.11) showed no evidence of heterogeneity in association. No publication bias was observed (p = 0.26).
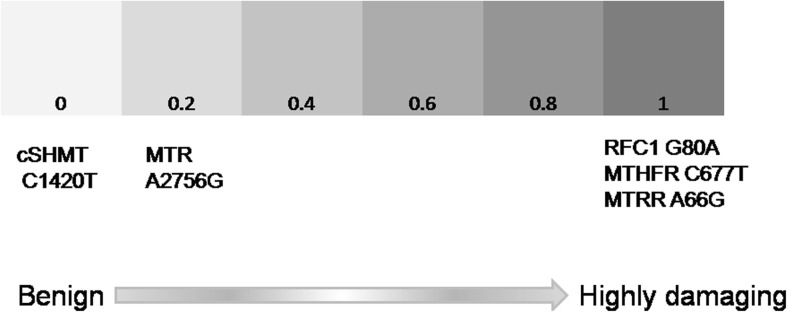
As shown in Fig. 6, in silico studies revealed the potential damaging effects of SLC19A1 G80A, MTHFR C677T, and MTRR A66G, while no damage was observed with SHMT1 C1420T and MTR A2756G polymorphisms (Fig. 6).
Fig. 6.
In silico analysis of functional relevance of genetic polymorphisms. PolyPhen-based computational analysis was carried out to elucidate effect of SNPs on the respective proteins. The damage is represented in the form of color gradient from light gray to black suggesting no damage to severe damage
Discussion
The current study attempts to investigate the role of putative genetic polymorphisms in one-carbon metabolic pathway with the etiology of breast cancer. Here, we have pooled the data from different ethnic groups and populations. Two polymorphisms, i.e., SLC19A1 G80A and MTHFR C677T, were identified as potential risk factors for breast cancer in the pooled analysis. SHMT1 C1420T and MTR A2756G showed borderline protective role against breast cancer.
The functional analysis revealed the strong association of SLC19A1 G80A and MTHFR C677T polymorphisms with structural instability and damage of the respective proteins. MTHFR C677T was shown to induce thermolability in MTHFR protein, resulting in its dissociation into inactive monomers with loss of FAD-binding capacity (Yamada et al. 2001). The SHMT1 C1420T polymorphism has no deleterious effect on SHMT1 protein. The MTR A2756G was shown to induce benign damage to the protein. The MTRR A66G showed a deleterious effect on MTRR protein. Since MTR and MTRR act together in 1:1 stoichiometric ratio to form holoenzyme complex (Yamada et al. 2006), it is likely that MTR and MTRR variant alleles act in synergy in modulating breast cancer risk.
The SLC19A1 G80A was found to be a risk factor in Indians and Brazilians, but not in US population. This lack of association with US population can be attributed to folate fortification program in the USA. SLC19A1 expression was reported to be downregulated in conditions of folate deprivation (Ifergan et al. 2008) suggesting that availability of folate might act as an effect modifier. Decreased transcription and altered function might have deleterious impact, thus influencing intracellular folate levels.
The MTHFR C677T polymorphism was found to be a risk factor in Mediterranean populations. The risk is probably attributed to change in the dietary patterns from the conventional Mediterranean diet to the processed food resulting in the deficiency of folate and other vitamins (Castro-Quezada et al. 2014). This hypothesis was substantiated by the subgroup analysis showing MTHFR C677T-mediated breast cancer risk among women with low folate intake. Our results corroborate with a previous study, which demonstrated increased risk for post-menopausal breast cancer in carriers of MTHFR 677 T-allele despite having high plasma folate levels (Ericson et al. 2009). Methionine after the synthesis of SAM and its utilization as a methyl group donor forms SAH and thus contributes toward higher homocysteine levels. The remethylation of homocysteine depends on the bioavailability 5-methyl THF and the activity of MTR and MTRR complex. The MTR-MTRR complex requires methyl cobalamine as cofactor. Thus, the synthesis of 5-methyl THF is hampered in subjects harboring the variants. The other possible contributors for the population level variation in the association are complex interactions among MTR, MTHFR, and MTRR.
The induction of futile folate cycle by SHMT1C1420T might be contributing toward the maintenance of one-carbon homeostasis through formation of 5-formyl THF from 5,10-methylene THF (Stover and Schirch 1992) thus conferring protection against breast cancer. In a previous study, we have observed higher circulating folate levels in a subject with SHMT1 TT genotype when compared to those with SHMT1 CT and CC genotypes (Naushad et al. 2011). The in silico study showing active enzyme in the presence of this polymorphism supports this hypothesis further.
The results of our meta-analysis corroborated with other meta-analyses (Yu and Chen 2012; Liang et al. 2013; Castro-Quezada et al. 2014); (Li et al. 2014a; Rai 2014; Pooja et al. 2015; Kumar et al. 2015) in showing MTHFR C677T as a risk factor for breast cancer. The null association of MTR A2756G in random effects model and an inverse association with breast cancer in Caucasians were consistent with the meta-analysis of Zhong et al. (2013). The null association of MTRR A66G with breast cancer is in agreement with the meta-analysis of Hu et al. (2010). To date, there is no other meta-analysis that explored the association of SLC19A1 G80A with breast cancer risk.
Our study showed agreement with another study (Li et al. 2014b) in demonstrating the protective role of SHMT1 C1420T against breast cancer in Asians, while null association in Caucasians. In contrast with Wang et al. (2010), we have performed a meta-analysis of TYMS polymorphisms based on alleles. And hence, null association was observed. Only Japanese population exhibited positive association of TYMS 3′UTR ins6/del6 with breast cancer.
The limitations of the current study were the following: (i) inclusion of only published studies in the current meta-analysis and (ii) this meta-analysis was based on unadjusted odds ratios as it is difficult to retrieve information on the confounding factors from each study.
To summarize, MTHFR C677T and SLC19A1 G80A are considered to be a significant risk factors for breast cancer globally. The SHMT1 C1420T seems to confer borderline protective role. The ethnic and population level variations in genetic association could be due to gene-gene interaction, gene-nutrient interaction, and genome-epigenome interactions.
Acknowledgments
This study is funded by Prof.T.Rajagopalan Research Fund conferred upon MJR.
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Akram M, Malik FA, Kayani MA. Mutational analysis of the MTHFR gene in breast cancer patients of Pakistani population. Asian Pac J Cancer Prev. 2012;13:1599–1603. doi: 10.7314/APJCP.2012.13.4.1599. [DOI] [PubMed] [Google Scholar]
- Awwad N, Yousef A-M, Abuhaliema A, et al. Relationship between genetic polymorphisms in MTHFR (C677T, A1298C and their Haplotypes) and the incidence of breast cancer among Jordanian females—case-control study. Asian Pac J Cancer Prev. 2015;16:5007–5011. doi: 10.7314/APJCP.2015.16.12.5007. [DOI] [PubMed] [Google Scholar]
- Beetstra S, Suthers G, Dhillon V, et al. Methionine-dependence phenotype in the de novo pathway in BRCA1 and BRCA2 mutation carriers with and without breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2565–2571. doi: 10.1158/1055-9965.EPI-08-0140. [DOI] [PubMed] [Google Scholar]
- Bentley AR, Raiszadeh F, Stover PJ, et al. No association between cSHMT genotypes and the risk of breast cancer in the Nurses’ Health Study. Eur J Clin Nutr. 2010;64:108–110. doi: 10.1038/ejcn.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N, Weissbach H. The role of cobamides in methionine synthesis. Enzymatic formation of holoenzyme. J Biol Chem. 1966;241(9):2024–8. [PubMed] [Google Scholar]
- Brzezińska A, Wińska P, Balińska M. Cellular aspects of folate and antifolate membrane transport. Acta Biochim Pol. 2000;47(3):735–749. [PubMed] [Google Scholar]
- Burcoş T, Toma M, Stavarachi M, et al. MTRR polymorphism and the risk for colorectal and breast cancer in Romanian patients—a preliminary study. Chirurgia. 2010;105:379–382. [PubMed] [Google Scholar]
- Carvalho Barbosa Rde C, De Cássia Carvalho Barbosa R, Menezes DC, et al. Associations of polymorphisms of folate cycle enzymes and risk of breast cancer in a Brazilian population are age dependent. Mol Biol Rep. 2011;39:4899–4907. doi: 10.1007/s11033-011-1285-1. [DOI] [PubMed] [Google Scholar]
- Castro-Quezada I, Itandehui C-Q, Blanca R-V, Lluís S-M. The Mediterranean diet and nutritional adequacy: a review. Nutrients. 2014;6:231–248. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-W, Yu J-C, Huang C-S, et al. Polymorphism of cytosolic serine hydroxymethyltransferase, estrogen and breast cancer risk among Chinese women in Taiwan. Breast Cancer Res Treat. 2008;111:145–155. doi: 10.1007/s10549-007-9754-x. [DOI] [PubMed] [Google Scholar]
- Deligezer U, Akisik EE, Dalay N. Homozygosity at the C677T of the MTHFR gene is associated with increased breast cancer risk in the Turkish population. In Vivo. 2005;19:889–893. [PubMed] [Google Scholar]
- Diakite B, Tazzite A, Hamzi K, et al. Methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Moroccan women. Afr Health Sci. 2012;12:204–209. doi: 10.4314/ahs.v12i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divyya S, Shree D, Naushad SM, et al. Association of glutamate carboxypeptidase II (GCPII) haplotypes with breast and prostate cancer risk. Gene. 2013;516:76–81. doi: 10.1016/j.gene.2012.11.047. [DOI] [PubMed] [Google Scholar]
- Ericson UC, Ivarsson MI, Sonestedt E, et al. Increased breast cancer risk at high plasma folate concentrations among women with the MTHFR 677T allele. Am J Clin Nutr. 2009;90(5):1380–1389. doi: 10.3945/ajcn.2009.28064. [DOI] [PubMed] [Google Scholar]
- Fachal L, Dunning AM. From candidate gene studies to GWAS and post-GWAS analyses in breast cancer. Curr Opin Genet Dev. 2015;30:32–41. doi: 10.1016/j.gde.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Gao C-M, Tang J-H, Cao H-X, et al. MTHFR polymorphisms, dietary folate intake and breast cancer risk in Chinese women. J Hum Genet. 2009;54:414–418. doi: 10.1038/jhg.2009.57. [DOI] [PubMed] [Google Scholar]
- Halsted CH. The intestinal absorption of dietary folates in health and disease. J Am Coll Nutr. 1989;8(6):650–658. doi: 10.1080/07315724.1989.10720340. [DOI] [PubMed] [Google Scholar]
- He JM, Pu YD, Wu YJ, et al. Association between dietary intake of folate and MTHFR and MTR genotype with risk of breast cancer. Genet Mol Res. 2014;13:8925–8931. doi: 10.4238/2014.October.31.7. [DOI] [PubMed] [Google Scholar]
- Hoskins KF, Stopfer JE, Calzone KA, et al. Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA. 1995;273(7):577–585. doi: 10.1001/jama.1995.03520310075033. [DOI] [PubMed] [Google Scholar]
- Hosseini M. Role of polymorphism of methyltetrahydrofolate-homocysteine methyltransferase (MTR) A2756G and breast cancer risk. Pol J Pathol. 2013;64:191–195. doi: 10.5114/pjp.2013.38138. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Mojgan H, Massoud H, Ahmad E. MTHFR polymorphisms and breast cancer risk. Arch Med Sci. 2011;1:134–137. doi: 10.5114/aoms.2011.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Jia H, Guo-Wu Z, et al. MTRR A66G polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;124:779–784. doi: 10.1007/s10549-010-0892-1. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Jansen G, Assaraf YG. The reduced folate carrier (RFC) is cytotoxic to cells under conditions of severe folate deprivation. RFC as a double edged sword in folate homeostasis. J Biol Chem. 2008;283:20687–20695. doi: 10.1074/jbc.M802812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Robien K, Wang R, et al. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2008;29:1967–1972. doi: 10.1093/carcin/bgn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang-Hua Q, De-Chuang J, Zhen-Duo L, et al. Association of methylenetetrahydrofolate reductase and methionine synthase polymorphisms with breast cancer risk and interaction with folate, vitamin B6, and vitamin B 12 intakes. Tumour Biol. 2014;35:11895–11901. doi: 10.1007/s13277-014-2456-1. [DOI] [PubMed] [Google Scholar]
- Justenhoven C, Hamann U, Pierl CB, et al. One-carbon metabolism and breast cancer risk: no association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol Biomarkers Prev. 2005;14:3015–3018. doi: 10.1158/1055-9965.EPI-05-0592. [DOI] [PubMed] [Google Scholar]
- Kakkoura MG, Demetriou CA, Loizidou MA, et al. Single-nucleotide polymorphisms in one-carbon metabolism genes, Mediterranean diet and breast cancer risk: a case-control study in the Greek-Cypriot female population. Genes Nutr. 2015;10:453. doi: 10.1007/s12263-015-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsopoulos J, Joanne K, Zhang WW, et al. Polymorphisms in folate metabolizing enzymes and transport proteins and the risk of breast cancer. Breast Cancer Res Treat. 2008;112:585–593. doi: 10.1007/s10549-008-9895-6. [DOI] [PubMed] [Google Scholar]
- Kumar P, Yadav U, Rai V. Methylenetetrahydrofolate reductase gene C677T polymorphism and breast cancer risk: evidence for genetic susceptibility. Meta Gene. 2015;6:72–84. doi: 10.1016/j.mgene.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajin B, Alhaj Sakur A, Ghabreau L, Alachkar A. Association of polymorphisms in one-carbon metabolizing genes with breast cancer risk in Syrian women. Tumour Biol. 2012;33:1133–1139. doi: 10.1007/s13277-012-0354-y. [DOI] [PubMed] [Google Scholar]
- Lee S-A, Kang D, Nishio H, et al. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36:116–121. doi: 10.1038/emm.2004.17. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Haiman CA, Wilkens LR, et al. MTHFR polymorphisms, diet, HRT, and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2004;13:2071–2077. [PubMed] [Google Scholar]
- Liang H, Hongjie L, Yulan Y, et al. Methylenetetrahydrofolate reductase polymorphisms and breast cancer risk in Chinese population: a meta-analysis of 22 case–control studies. Tumor Biol. 2013;35:1695–1701. doi: 10.1007/s13277-013-1234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Kai L, Wusheng L, Xi D. Association of 677 C > T (rs1801133) and 1298 A > C (rs1801131) Polymorphisms in the MTHFR gene and breast cancer susceptibility: a meta-analysis based on 57 individual studies. PLoS One. 2014;9:e71290. doi: 10.1371/journal.pone.0071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ma Y, Jin C, et al. Polymorphism of cytosolic serine hydroxymethyltransferase and breast cancer risk: evidence from a meta-analysis. Tumour Biol. 2014;35:7361–7367. doi: 10.1007/s13277-014-1964-3. [DOI] [PubMed] [Google Scholar]
- Ma E, Iwasaki M, Junko I, et al. Dietary intake of folate, vitamin B6, and vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case-control study in Brazilian women. BMC Cancer. 2009;9:122. doi: 10.1186/1471-2407-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruti SS, Ulrich CM, Jupe ER, White E. MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case-control study. Breast Cancer Res. 2009;11:R91. doi: 10.1186/bcr2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad NS, Yedluri R, Addepalli P, et al. Aberrations in one-carbon metabolism induce oxidative DNA damage in sporadic breast cancer. Mol Cell Biochem. 2011;349:159–167. doi: 10.1007/s11010-010-0670-8. [DOI] [PubMed] [Google Scholar]
- Naushad SM, Pavani A, Digumarti RR, et al. Epistatic interactions between loci of one-carbon metabolism modulate susceptibility to breast cancer. Mol Biol Rep. 2011;38:4893–4901. doi: 10.1007/s11033-010-0631-z. [DOI] [PubMed] [Google Scholar]
- Ozen F, Erdis E, Sik E, et al. Germ-line MTHFR C677T, FV H1299R and PAI-1 5G/4G variations in breast carcinoma. Asian Pac J Cancer Prev. 2013;14:2903–2908. doi: 10.7314/APJCP.2013.14.5.2903. [DOI] [PubMed] [Google Scholar]
- Platek ME, Shields PG, Marian C, et al. Alcohol consumption and genetic variation in methylenetetrahydrofolate reductase and 5-methyltetrahydrofolate-homocysteine methyltransferase in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:2453–2459. doi: 10.1158/1055-9965.EPI-09-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooja S, Carlus J, Sekhar D, et al. MTHFR 677C > T polymorphism and the risk of breast cancer: evidence from an original study and pooled data for 28031 cases and 31880 controls. PLoS One. 2015;10:e0120654. doi: 10.1371/journal.pone.0120654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad VVTS, Wilkhoo H. Association of the functional polymorphism C677T in the methylenetetrahydrofolate reductase gene with colorectal, thyroid, breast, ovarian, and cervical cancers. Onkologie. 2011;34:422–426. doi: 10.1159/000331131. [DOI] [PubMed] [Google Scholar]
- Rai V. The methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in Asian populations. Asian Pac J Cancer Prev. 2014;15:5853–5860. doi: 10.7314/APJCP.2014.15.14.5853. [DOI] [PubMed] [Google Scholar]
- Ramos-Silva A, Figuera LE, Soto-Quintana OM, et al. Association of the C677T polymorphism in the methylenetetrahydrofolate reductase gene with breast cancer in a Mexican population. Genet Mol Res. 2015;14:4015–4026. doi: 10.4238/2015.April.27.16. [DOI] [PubMed] [Google Scholar]
- Sangrajrang S, Sato Y, Sakamoto H, et al. Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: a case-control study in Thai women. Breast Cancer Res Treat. 2010;123:885–893. doi: 10.1007/s10549-010-0804-4. [DOI] [PubMed] [Google Scholar]
- Sangrajrang S, Suleeporn S, Yasunori S, et al. Genetic polymorphisms of estrogen metabolizing enzyme and breast cancer risk in Thai women. Int J Cancer. 2009;125:837–843. doi: 10.1002/ijc.24434. [DOI] [PubMed] [Google Scholar]
- Shrubsole MJ, Gao Y-T, Cai Q, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–196. doi: 10.1158/1055-9965.EPI-03-0273. [DOI] [PubMed] [Google Scholar]
- Stover P, Schirch V. Enzymatic mechanism for the hydrolysis of 5,10-methenyltetrahydropteroylglutamate to 5-formyltetrahydropteroylglutamate by serine hydroxymethyltransferase. Biochemistry. 1992;31(7):2155–2164. doi: 10.1021/bi00122a037. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Matsuo K, Hirose K, et al. One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis. 2008;29:356–362. doi: 10.1093/carcin/bgm295. [DOI] [PubMed] [Google Scholar]
- Tao MH, Shields PG, Nie J, et al. DNA promoter methylation in breast tumors: no association with genetic polymorphisms in MTHFR and MTR. Cancer Epidemiol Biomarkers Prev. 2009;18:998–1002. doi: 10.1158/1055-9965.EPI-08-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh BN, Ong CN, Coetzee GA, et al. Thymidylate synthase: a novel genetic determinant of plasma homocysteine and folate levels. Hum Genet. 2002;111(3):299–302. doi: 10.1007/s00439-002-0779-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Jun W, Baocheng W, et al. The association between two polymorphisms in the TYMS gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;128:203–209. doi: 10.1007/s10549-010-1314-0. [DOI] [PubMed] [Google Scholar]
- Weiner AS, Boyarskikh UA, Voronina EN, et al. Polymorphisms in the folate-metabolizing genes MTR, MTRR, and CBS and breast cancer risk. Cancer Epidemiol. 2012;36:e95–e100. doi: 10.1016/j.canep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Weiwei Z, Liping C, Dequan L. Association between dietary intake of folate, vitamin B6, B12 & MTHFR, MTR Genotype and breast cancer risk. Pak J Med Sci. 2014;30:106–110. doi: 10.12669/pjms.301.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-Y, Ni J, Xu W-J, et al. Interactions between MTHFR C677T-A1298C variants and folic acid deficiency affect breast cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2012;13:2199–2206. doi: 10.7314/APJCP.2012.13.5.2199. [DOI] [PubMed] [Google Scholar]
- Wu X, Zou T, Cao N, et al. Plasma homocysteine levels and genetic polymorphisms in folate metabolism are associated with breast cancer risk in Chinese women. Hered Cancer Clin Pract. 2014;12:2. doi: 10.1186/1897-4287-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gammon MD, Zhang H, et al. Polymorphisms of one-carbon-metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis. 2007;28:1504–1509. doi: 10.1093/carcin/bgm061. [DOI] [PubMed] [Google Scholar]
- Yamada K, Chen Z, Rozen R, Matthews RG. Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase. Proc Natl Acad Sci U S A. 2001;98:14853–14858. doi: 10.1073/pnas.261469998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Gravel RA, Toraya T, Matthews RG. Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proc Natl Acad Sci U S A. 2006;A103(25):9476–9481. doi: 10.1073/pnas.0603694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-P, Wu M-H, Chou Y-C, et al. Breast cancer risk associated with multigenotypic polymorphisms in folate-metabolizing genes: a nested case-control study in Taiwan. Anticancer Res. 2007;27:1727–1732. [PubMed] [Google Scholar]
- Yu L, Chen J. Association of MTHFR Ala222Val (rs1801133) polymorphism and breast cancer susceptibility: an update meta-analysis based on 51 research studies. Diagn Pathol. 2012;7:171. doi: 10.1186/1746-1596-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Gao J, Hu Z, et al. Polymorphisms in thymidylate synthase gene and susceptibility to breast cancer in a Chinese population: a case-control analysis. BMC Cancer. 2006;6:138. doi: 10.1186/1471-2407-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Xu J, Li W, et al. Methionine synthase A2756G polymorphism and breast cancer risk: an up-to-date meta-analysis. Gene. 2013;527:510–515. doi: 10.1016/j.gene.2013.06.054. [DOI] [PubMed] [Google Scholar]