Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin are one of the most frequently used classes of drug worldwide and inhibit prostaglandin (PG) production by inhibiting cyclooxygenase activity. Although NSAIDs are broadly used against inflammatory diseases, they have side effects including alimentary canal disorders, kidney damage, infection and cardiovascular disorders. Thus, it is necessary to elucidate the pathophysiological role of each PG in various diseases to develop better therapies with fewer and milder side effects. PGD2 is a PG that was identified in 1973 by Hamberg and is produced by the activities of cyclooxygenase and either hematopoietic or lipocalin-type PGD synthase. PGD2 exerts its physiological effects by stimulating two distinct G protein-coupled receptors, namely D prostanoid receptor (DP) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). The physiological role of PGD2 remains controversial. Some studies have reported that PGD2 has bronchoconstrictory and pro-inflammatory effects inducing immune cell accumulation. In contrast, other groups have reported that PGD2 has anti-inflammatory effects by inhibiting the recruitment of dendritic cells and neutrophils. We have investigated the pathophysiological role of PGD2 using various disease models and reported on its anti-inflammatory actions. Here, we review the anti-inflammatory roles of PGD2 and the underlying mechanisms.
Keywords: inflammation, PGD2, tumor, vascular permeability
PROSTAGLANDINS
In 1935, Goldblatt and Ulf reported that human testis and ovine vesicular gland contain physiologically active substances that induce the contraction of smooth muscle [3]. Since these substances were recognized as products of the prostate gland at that time, they are termed prostaglandins (PGs). Nowadays, it is known that several tissues and organs can produce PGs.
PGs are produced when arachidonic acid is released from the plasma membrane by phospholipase A2. Prostaglandin H2 (PGH2) is synthesized from arachidonic acid by cyclooxygenase (COX). Then, PGs are synthesized from PGH2 by PG synthases. PGs with a prostanoic acid skeleton have various physiological activities and play a substantial role in the inflammatory response. There are four types of PGs: PGE2, PGI2, PGD2 and PGF2α. PGE2 is one of the most physiologically abundant PGs, and it induces peripheral blood relaxation, resulting in increased blood flow, as well as fever and pain. PGI2 causes vasodilatation and inhibits platelet aggregation, and PGF2α contracts the uterine smooth muscles, which is necessary for parturition.
The inflammatory response is an important biological defense to exclude foreign substances and repair damaged tissue. There are four features of inflammation, namely dolor (pain), tumor (swelling), calor (heat) and rubor (redness). Increased blood flow and vascular permeability cause rubor and calor. Edema with vascular permeability and the production of pain signaling factors cause tumor and dolor, respectively. Disruption of the regulatory balance of the inflammatory response, either for congenital or acquired reasons, can result in excessive or inappropriately sustained inflammation, which sometimes leads to carcinogenesis. On the other hand, the loss of a proper inflammatory response delays wound healing.
Nonsteroidal anti-inflammatory drugs (NSAIDs), as prototypically represented by aspirin, are broadly used against inflammatory diseases. NSAIDs have potent anti-inflammatory effects by inhibiting the activity of COX and the production of PGs. However, these drugs are not universally safe, since there have been several reports showing that the administration of NSAIDs raises the risks of alimentary canal disorders, kidney damage, infection and cardiovascular conditions [1, 19]. To develop a “better aspirin” with fewer side effects, we should re-examine the physiological function of each PG.
PGD2 was identified by Hamberg in 1973 [4]. There are two PGD synthases, hematopoietic PGD synthase (H-PGDS) and lipocalin-type PGD synthase (L-PGDS), both of which metabolize PGH2 to PGD2. PGD2 exerts biological functions via two different receptors: D prostanoid receptor (DP) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) [11]. L-PGDS is mainly expressed in central nerves and is well-known to induce sleep [18]. On the other hand, H-PGDS is the main source of PGD2 in peripheral tissue, and there have been some reports suggesting that it can induce allergic inflammation. Hematopoietic lineage cells, mainly mast cells, express H-PGDS. PGD2 promotes T cell migration via CRTH2 [6] and aggravates asthma [7]. In contrast, there have been some studies suggesting that PGD2 exerts anti-inflammatory effects via DP, such as inhibiting the migration and activation of neutrophils, basophils, dendritic cells and T cells [11]. Thus, to date, there has been no consensus opinion about whether the major physiological actions of PGD2 are pro- or anti-inflammatory. In the following chapters, we summarize the anti-inflammatory aspects of PGD2 that we have discovered using murine models of inflammatory diseases.
PGD2 AND TUMOR ANGIOGENESIS
Numerous immune cells infiltrate into rapidly growing tumors. This response causes sustained inflammation that facilitates tumor growth. As mentioned above, inflammatory responses protect the body against foreign invaders; however, tumors can escape such responses and utilize them to help their own replication. Macrophages and neutrophils accumulate in the tumor microenvironment and produce cytokines and growth factors that boost angiogenesis and thereby support tumor growth by increasing the supply of oxygen and nutrients. The inhibition of aberrant inflammation is a therapeutic strategy against tumors.
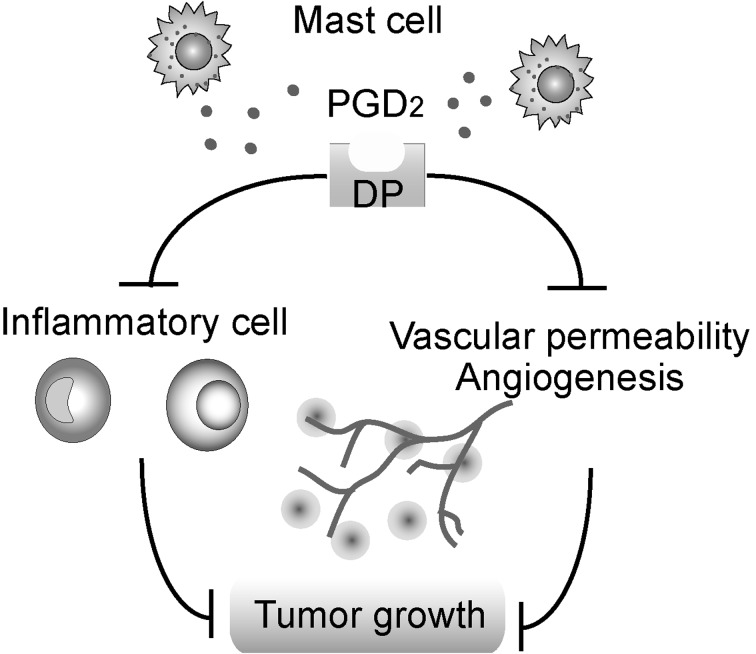
We reported the first investigation of the role of PGD2 in tumor growth [13]. H-PGDS gene deficient (Hpgds−/−) mice were utilized for the following experiments. Host deficiency of H-PGDS significantly decreased the production of PGD2 and strongly accelerated the growth of implanted Lewis lung carcinoma (LLC). Inside of growing tumors, necrotic areas are often observed due to ischemia. In Hpgds−/− mice, the necrotic area was much smaller than that of wild-type (WT) mice. This observation suggested that the depletion of H-PGDS-derived PGD2 creates a favorable environment for the survival of tumor cells. In the tumors grown on Hpgds−/− mice, the number of infiltrating monocytes, macrophages and neutrophils was increased in comparison to those in tumors grown on WT mice. Accompanying these phenomena, the production of inflammatory cytokines and chemokines, such as tumor necrosis factor α, interleukin 6, monocyte chemoattractant protein-1 and vascular endothelial growth factor, was upregulated in the tumors grown on Hpgds−/− mice. Morphological studies also showed that host H-PGDS deficiency accelerated vascular leakage and angiogenesis in the tumor mass. These results suggest that H-PGDS-derived PGD2 suppressed implanted LLC-induced inflammation and angiogenesis. This was the first report showing that PGD2 suppresses inflammation in vivo.
We next examined which type of cell was responsible for the production of PGD2 in the implanted LLC tumor mass and found that c-Kit- or FcεR1-positive mast cells markedly expressed H-PGDS. To clearly show whether the mast cell-derived PGD2 attenuates tumor expansion, we generated mast cell-specific Hpgds−/− mice by transplanting bone marrow-derived mast cells into mast cell-deficient (Kitwsh/wsh) mice. Similar to systemic gene deficiency of H-PGDS, mast cell-specific H-PGDS deficiency also accelerated tumor growth, production of cytokines, vascular permeability and angiogenesis. Of interest, infiltration of mast cells was often observed in peripheral areas of the tumor mass, especially those nearby the neovasculature. These observations evidenced that mast cell-derived PGD2 suppresses inflammation and angiogenesis in the tumor microenvironment. Mast cells are well-known to initiate allergy by producing histamine and leukotriene. Recent studies revealed that mast cells also promote tumor growth by secreting pro-inflammatory/pro-angiogenic cytokines, such as tumor necrosis factor α, vascular endothelial growth factor and matrix metalloproteinase [9]. On the other hand, we identified PGD2 as a mast cell-derived anti-inflammatory/anti-angiogenic factor in the implanted LLC tumor mass [13]. Based on our observations and those of other groups, we concluded that mast cell deficiency significantly increases the growth rate of tumors and mast cells possess anti-tumorigenic activities. However, it is interesting that mast cells secrete both pro- and anti-inflammatory substances simultaneously. Further investigations are required to reveal the pathophysiological implications of mast cell-derived PGD2 for tumor growth.
In our subsequent studies, we examined the contribution of PGD2 receptor signaling in tumor growth using DP- or CRTH2-deficient mice [14]. Genetic deficiency of DP but not CRTH2 promoted tumor growth by promoting vascular permeability, angiogenesis and inflammatory cell infiltration. As expected, the administration of a DP agonist significantly inhibited inflammation, angiogenesis and tumor growth in WT mice.
These results revealed new tumor-suppressing factors and demonstrated that a small number of mast cells can have large anti-inflammatory effects in the tumor environment (Fig. 1). Based on these findings, PGD2 signaling may be a new therapeutic target in cancer.
Fig. 1.
The role of mast cell-derived PGD2 in tumor growth.
PGD2, COLITIS AND COLITIS-ASSOCIATED COLON CANCER
Colorectal cancer is one of the most common cancers in Japan. Chronic and severe inflammation of the gut due to genetics, lifestyle factors, microbial infections and ulcerative colitis are major risk factors of colorectal cancer. Infiltrating immune cells in colorectal tissue produce physiologically active substances, such as reactive oxygen species, cytokines and growth factors. These substances induce aberrant cell proliferation and ultimately the development of cancer [2]. In this chapter, we discuss the role of PGD2 in chronic gut inflammation and the subsequent development of cancer [8].
The colitis-associated colon cancer model was generated by the administration of the large intestine-specific carcinogen azoxymethane (AOM) and dextran sulfate sodium salt (DSS). In AOM/DSS treated WT mice, body weight loss with sustained diarrhea was observed, and tumors were detected in the colonic epithelium. In the AOM/DSS-treated Hpgds−/− mice, the severity of colitis was worsened, and the number of tumors was increased compared with those in WT mice. Furthermore, we detected the aberrant activation of Wnt/β-catenin signaling, which results in carcinogenesis in Hpgds−/− colon tissue.
Of interest, as seen in the implanted LLC tumor model, infiltrating c-Kit/FcεR1-positive mast cells strongly expressed H-PGDS in inflamed colon tissues. Indeed, mast cell-specific H-PGDS deficiency exacerbated the colitis and subsequent carcinogenesis. Mast cell-derived PGD2 was also found to attenuate chronic colon inflammation and carcinogenesis. We further showed that the administration of DP agonist was beneficial against colitis and reduced the rate of carcinogenesis in this murine model.
In the LLC-transplanted model as described above, mast cells were observed in the marginal part of the tumor tissue, where the proliferative activity of cancer cells and the vascular density are high. On the other hand, in the colitis-associated colon cancer model, mast cells accumulated around the developing tumors. Interestingly, both systemic and mast cell-specific H-PGDS deficiency increased the number of infiltrating mast cells in both the LLC-transplanted and colitis-associated colon cancer models. Mast cell-derived PGD2 may modulate the contribution of mast cells to inflammatory responses by inhibiting their infiltration into the site of a developing tumor.
PGD2 AND ACUTE LUNG INJURY
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) occur due to sepsis, lung inflammation, aspiration and certain medical practices, such as artificial respiration and blood transfusion. ALI/ARDS are characterized by tissue edema and neutrophil infiltration, which are both attributable to an increased epithelial and endothelial permeability. ALI and ARDS have poor prognoses, and there is no effective treatment against them.
Here, we describe the contribution of PGD2 to the progression of ALI/ARDS, with a focus on its producer-effector interactions in vivo. The administration of endotoxin lipopolysaccharides caused tissue edema and induced neutrophil infiltration in the WT mouse lung. Genetic deficiency of H-PGDS or DP aggravated all of the symptoms. Experiments involving bone marrow transplantation between WT and Hpgds−/− mice showed that PGD2 derived from alveolar non-hematopoietic lineage cells, such as endothelial and epithelial cells, promotes vascular barrier function during the early phase (day 1) of ALI. On the other hand, neutrophil-derived PGD2 attenuates infiltration and cytokine expression by neutrophils during the later phase (day 3). Treatment with a DP agonist or a degradation product of PGD2, 15-deoxy-Δ (12,14)-PGJ2, exerted a therapeutic action against ALI. Data obtained from experiments involving bone marrow transplantation between WT and Ptgdr1−/− mice suggested that DP signaling in alveolar endothelial cells is crucial for the anti-inflammatory reactions of PGD2. In vitro, DP agonism directly enhanced endothelial barrier formation, and 15-deoxy-Δ (12,14)-PGJ2 attenuated migration and cytokine expression by neutrophils.
These observations indicate that PGD2 signaling between alveolar endothelial/epithelial cells and infiltrating neutrophils produces anti-inflammatory effects in ALI and suggest that enhancing this signaling pathway could have therapeutic potential for this disease.
PGD2 AND DERMATITIS
In the above chapters, we described that H-PGDS-derived PGD2 inhibits vascular permeability, angiogenesis and inflammation via activating DP in LLC, Colitis, Colitis-associated Colon Cancer and ALI. However, there have been controversial reports showing its pro-inflammatory or anti-inflammatory role in some inflammatory disease models. Indeed, it has been reported that PGD2 had a pro-inflammatory effect in a mouse asthma model [12]. Mast cells accumulate in focal sites of dermatitis and asthmatic trachea [5]. We hypothesized that the accumulated mast cells may produce a relatively high level of PGD2 in the focal site, causing pro-inflammatory reactions through DP-independent signaling mechanisms. To examine whether a higher concentration of PGD2 promotes inflammation, we assessed the inflammatory response in transgenic (TG) mice overexpressing H-PGDS [16].
Administration of croton oil to the ears of WT mice induced swelling with increased vascular permeability and neutrophil infiltration into the inflamed ear tissue. Of interest, H-PGDS TG mice showed less ear inflammation than WT mice did in the early phase of the dermatitis, while the inflammation of TG mice was more severe than that of WT mice in the late phase (6 hr). In TG mice, treatment with a DP antagonist reinstated the inhibition of inflammation in the early phase, while treatment with a CRTH2 antagonist inhibited the aggravated inflammation in the late phase of dermatitis. These observations suggested that, in TG mice, DP-mediated signaling is responsible for the anti-inflammatory effect of PGD2 during the early phase of dermatitis, while CRTH2 receptor-mediating signaling is involved in promoting inflammation during the late phase of dermatitis. The half-life of PGD2 is about 50 sec in vivo, and PGD2 is metabolized to 15d-PGJ2 or 9α11β-PGF2α, which have higher affinities for CRTH2 [17]. In the WT mouse ear, the administration of 15d-PGJ2 or 9α11β-PGF2α promoted neutrophil recruitment to the skin, and treatment with a CRTH2 antagonist inhibited it. These metabolites as well as PGD2 itself may stimulate CRTH2 at the site of inflammation.
We tried to identify the PGD2-producing and PGD2-receiving cells in TG mice. Immunostaining of croton oil-treated ears of TG mice demonstrated that both CD45-positive leukocytes and CD31-positive vascular endothelial cells expressed H-PGDS. The data obtained from an experiment in which bone marrow was transplanted between the WT and TG mice suggested that PGD2 derived from cells resident in the inflamed tissue attenuated the early-phase inflammation, while PGD2 produced from hematopoietic cells exacerbated the late-phase inflammation.
Taken together, in the ears of H-PGDS-overexpressing mice, PGD2 from tissue-resident cells at the site of inflammation suppressed dermatitis via activating DP during the early phase of inflammation, while hematopoietic cell-derived PGD2 and presumably its metabolites stimulated CRTH2 and promoted inflammation during the late phase. This was the first report showing that PGD2 exerts opposing effects on inflammation depending on the disease phase in vivo.
PGD2 AND VASCULAR PERMEABILITY
Several processes are required for angiogenesis. Upon inflammation, cytokines and growth factors produced by infiltrating immune cells and tissue-resident cells stimulate endothelial cells and increase vascular permeability. Stimulated endothelial cells proliferate, migrate and then form new blood vessels in non-vascularized areas. To evaluate how DP stimulation inhibits angiogenesis, we performed endothelial permeability assay, proliferation assay, migration assay and tube formation assay using isolated endothelial cells [14]. DP stimulation did not affect endothelial cell migration, proliferation or tube formation, but it strongly suppressed vascular endothelial permeability.
In vivo, not only endothelial barrier formation, but also tissue blood flow is responsible for vascular permeability. There is a possibility that PGD2 affects blood flow in addition to endothelial barrier enhancement. To clarify the effects of PGD2-DP signaling on vascular permeability in detail, in vivo imaging was performed. By microscopy, we could observe that croton oil treatment on mouse ear increased vascular permeability and caused vasodilatation. Stimulation of DP using an agonist suppressed vascular permeability without affecting the croton oil-induced vasodilatation [10].
DP is classified as a Gs protein-coupled receptor. The activation of this receptor induces adenylate cyclase activity and increases the intracellular cyclic adenosine monophosphate (cAMP) concentration, which activates downstream signaling mediated by protein kinase A (PKA) or exchange protein directly activated by cAMP1 (Epac1).
We next investigated the detailed mechanisms underlying the enhancement of the endothelial barrier by PGD2-DP signaling [10]. DP stimulation increased the intracellular cAMP concentration and subsequently activated PKA in endothelial cells. Treatment with a PKA-blocking peptide reversed the DP-mediated endothelial barrier enhancement [15]. In contrast, knockdown of Epac by siRNA treatment did not influence the barrier enhancement.
PKA activates the GTPase Rac1 through the guanine exchange factors Tiam1 and/or Trio, which leads to rearrangement of the cytoskeleton in endothelial cells. Using gene knockdown by siRNA, we revealed that the activation of Tiam1-Rac1 signaling, but not of Trio signaling, is crucial for DP-mediated barrier enhancement. Using immunostaining, we further showed that DP stimulation forms a cortical actin rim and increases vascular endothelial cadherin expression around the cell-cell contact area, which is mediated by Rac1 activation.
These results suggest that PGD2 inhibits vascular permeability to enhance adherence junction formation via the DP/cAMP/PKA/Tiam1-dependent activation of Rac1 and that these phenomena result in the inhibition of angiogenesis.
CONCLUSION
In this review, we summarized data on the pathophysiological role of PGD2 obtained from murine disease models including tumor allograft, chronic enteritis, ALI and dermatitis. PGD2 has both anti- and pro-inflammatory roles at different stages during the progression of inflammation, and these opposing effects are mediated by distinct signaling mechanisms (summarized in Table 1). Although we focused only on H-PGDS-DP signaling, the pathophysiological roles of L-PGDS-derived PGD2, especially in peripheral tissue, and CRTH2-mediating signaling remain elusive. Further studies are needed to clarify these points.
Table 1. The anti-inflammatory role of PGD2 in various disease models.
| Tumor angiogenesis | Colitis and colitis-associated colon cancer |
Acute lung injury | Dermatitis | |
|---|---|---|---|---|
| H-PGDS Expressed cell |
Mast cell | Mast cell | Acute phase: Epithelium/Endothelium Chronic phase: Neutrophil |
Acute phase: Vascular endothelium Chronic phase: Neutrophil |
| H-PGDS Deficiency |
Vascular permeability ↑ Angiogenesis ↑ Inflammatory cell infiltration ↑ Tumor growth ↑ |
Mast cell ↑ Carcinogenesis ↑ |
Inflammation ↑ Vascular permeability ↑ Neutrophil ↑ |
― |
| DP Deficiency |
Vascular permeability ↑ Angiogenesis ↑ Inflammatory cell infiltration Tumor growth ↑ |
― | Inflammation ↑ Vascular permeability ↑ Neutrophil ↑ |
― |
| DP Stimulation | Vascular permeability ↓ Angiogenesis ↓ Inflammatory cell infiltration ↓ Tumor growth ↓ |
Mast cell ↓ Carcinogenesis ↓ |
Inflammation ↓ Neutrophil ↓ Vascular permeability ↓ |
Inflammation ↓ Neutrophil ↓ Vascular permeability ↓ |
| H-PGDS Overexpression | ― | ― | ― | Acute phase: Inflammation ↓ Vascular permeability ↓ Chronic phase: Inflammation ↑ Vascular permeability ↑ Neutrophil ↑ |
Chronic and/or excessive inflammation leads to the onset and progression of various diseases, but the normal inflammatory response is indispensable for tissue repair and regeneration. Again, the pharmacological inhibition of inflammatory responses by NSAIDs does not ameliorate all the manifestations of inflammatory diseases; indeed, NSAID treatment sometimes worsens their manifestations and/or leads to side effects. Thus, it is crucial to understand the detailed signaling and action mechanisms of each contributing factor, including PGs. We hope that this review provides new insights for the further understanding of inflammation.
Acknowledgments
This work was supported by Grant-in-Aid from the Japan Society for the Promotion of Science; The Foundation for Dietary Scientific Research; The Naito Foundation; Kurozumi Medical Foundation; The Cardiovascular Research Fund, Suzuken Memorial Foundation; Nippon Ham Foundation; The Japan Foundation for Pediatric Research; Sapporo Bioscience Foundation.
REFERENCES
- 1.Bertagnolli M. M., Eagle C. J., Zauber A. G., Redston M., Solomon S. D., Kim K., Tang J., Rosenstein R. B., Wittes J., Corle D., Hess T. M., Woloj G. M., Boisserie F., Anderson W. F., Viner J. L., Bagheri D., Burn J., Chung D. C., Dewar T., Foley T. R., Hoffman N., Macrae F., Pruitt R. E., Saltzman J. R., Salzberg B., Sylwestrowicz T., Gordon G. B., Hawk E. T., Investigators A. P. C. Study 2006. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med 355: 873–884. [DOI] [PubMed] [Google Scholar]
- 2.Elinav E., Nowarski R., Thaiss C. A., Hu B., Jin C., Flavell R. A.2013. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 13: 759–771. doi: 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 3.Goldblatt M. W.1935. Properties of human seminal plasma. J. Physiol. 84: 208–218. doi: 10.1113/jphysiol.1935.sp003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamberg M., Fredholm B. B.1976. Isomerization of prostaglandin H2 into prostaglandin D2 in the presence of serum albumin. Biochim. Biophys. Acta 431: 189–193. doi: 10.1016/0005-2760(76)90273-3 [DOI] [PubMed] [Google Scholar]
- 5.Hershko A. Y., Charles N., Olivera A., Alvarez-Errico D., Rivera J.2012. Cutting edge: persistence of increased mast cell numbers in tissues links dermatitis to enhanced airway disease in a mouse model of atopy. J. Immunol. 188: 531–535. doi: 10.4049/jimmunol.1102703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K.2001. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193: 255–261. doi: 10.1084/jem.193.2.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda K., Arima M., Cheng G., Taki S., Hirata H., Eda F., Fukushima F., Yamaguchi B., Hatano M., Tokuhisa T., Fukuda T.2003. Prostaglandin D2 reinforces Th2 type inflammatory responses of airways to low-dose antigen through bronchial expression of macrophage-derived chemokine. J. Exp. Med. 198: 533–543. doi: 10.1084/jem.20022218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwanaga K., Nakamura T., Maeda S., Aritake K., Hori M., Urade Y., Ozaki H., Murata T.2014. Mast cell-derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res. 74: 3011–3019. doi: 10.1158/0008-5472.CAN-13-2792 [DOI] [PubMed] [Google Scholar]
- 9.Kalesnikoff J., Galli S. J.2008. New developments in mast cell biology. Nat. Immunol. 9: 1215–1223. doi: 10.1038/ni.f.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi K., Tsubosaka Y., Hori M., Narumiya S., Ozaki H., Murata T.2013. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler. Thromb. Vasc. Biol. 33: 565–571. doi: 10.1161/ATVBAHA.112.300993 [DOI] [PubMed] [Google Scholar]
- 11.Kostenis E., Ulven T.2006. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol. Med. 12: 148–158. doi: 10.1016/j.molmed.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka T., Hirata M., Tanaka H., Takahashi Y., Murata T., Kabashima K., Sugimoto Y., Kobayashi T., Ushikubi F., Aze Y., Eguchi N., Urade Y., Yoshida N., Kimura K., Mizoguchi A., Honda Y., Nagai H., Narumiya S.2000. Prostaglandin D2 as a mediator of allergic asthma. Science 287: 2013–2017. doi: 10.1126/science.287.5460.2013 [DOI] [PubMed] [Google Scholar]
- 13.Murata T., Aritake K., Matsumoto S., Kamauchi S., Nakagawa T., Hori M., Momotani E., Urade Y., Ozaki H.2011. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc. Natl. Acad. Sci. U.S.A. 108: 19802–19807. doi: 10.1073/pnas.1110011108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata T., Lin M. I., Aritake K., Matsumoto S., Narumiya S., Ozaki H., Urade Y., Hori M., Sessa W. C.2008. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 105: 20009–20014. doi: 10.1073/pnas.0805171105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popoff M. R., Geny B.2009. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim. Biophys. Acta 1788: 797–812. doi: 10.1016/j.bbamem.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 16.Sarashina H., Tsubosaka Y., Omori K., Aritake K., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., Ozaki H., Murata T.2014. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J. Immunol. 192: 459–465. doi: 10.4049/jimmunol.1302080 [DOI] [PubMed] [Google Scholar]
- 17.Sawyer N., Cauchon E., Chateauneuf A., Cruz R. P., Nicholson D. W., Metters K. M., O’Neill G. P., Gervais F. G.2002. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br. J. Pharmacol. 137: 1163–1172. doi: 10.1038/sj.bjp.0704973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno R., Honda K., Inoué S., Hayaishi O.1983. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc. Natl. Acad. Sci. U.S.A. 80: 1735–1737. doi: 10.1073/pnas.80.6.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfe M. M., Lichtenstein D. R., Singh G.1999. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 340: 1888–1899. doi: 10.1056/NEJM199906173402407 [DOI] [PubMed] [Google Scholar]