Abstract
D-allulose is a C-3 epimer of D-fructose and has recently been investigated for its hypoglycemic effects. In the present study, the effects of D-allulose on glucose metabolism were evaluated in healthy dogs administrated sugar or food. The oral administrations of D-allulose decreased plasma glucose concentrations after oral glucose or maltose administration, with a diminished plasma insulin rise. The glucose suppressive effect of D-allulose was also observed after intravenous glucose administrations without increase in plasma insulin concentration. In contrast, D-allulose showed no effect on plasma glucose and insulin concentrations after feeding. The present results suggest that D-allulose administration may be beneficial in dogs with impaired glucose tolerance. Further studies investigating the therapeutic efficacy of D-allulose in diabetic dogs are required.
Keywords: D-allulose, dog, glucose, insulin, rare sugar
D-allulose (D-psicose) is a monosaccharide rarely found in nature. D-allulose is a C-3 epimer of D-fructose, provides no energy [12] and was recently investigated for its hypoglycemic effects [3]. The dietary supplementation of D-allulose decreases plasma glucose and insulin concentrations in healthy rats [11] and suppresses the rise of blood glucose and insulin concentrations in the oral glucose tolerance test in rats with type 2 diabetes [8]. In addition, D-allulose suppresses the elevation of blood glucose and insulin concentrations after the maltodextrin load test in healthy human subjects [9] and postprandial blood glucose concentrations in humans with borderline diabetes [5]. D-allulose may also improve glucose metabolism by decreasing fat accumulation [8, 10, 17, 18]. These lines of evidence suggest that D-allulose can function as a promising anti-diabetic dietary component.
Diabetes mellitus is a common endocrinopathy in dogs. The treatment of the vast majority of diabetic dogs involves the injection of exogenous insulin, because most diabetic dogs lack insulin secretion [13]. In these dogs, insulin-stimulating oral hypoglycemic drugs, such as sulfonylureas, are ineffective [13], and other oral hypoglycemic drugs are also not routinely used because of insufficient efficacy. A high-fiber supplementary diet is often employed to suppress postprandial hyperglycemia and the rise in insulin requirements [14, 15]. Although it can be difficult to achieve good glycemic control in some diabetic dogs, no other effective treatment for canine diabetes is as yet known.
The hypoglycemic effect of D-allulose demonstrated in a previous study would also be beneficial in dogs with diabetes. Recently, we demonstrated the safety of a single dose of D-allulose in dogs [16]. In that study, a high dose administration of D-allulose mildly decreased the concentration of plasma glucose in dogs that had undergone fasting [16]; however, the effect of D-allulose on glucose metabolism has not yet been clarified. D-allulose is expected to suppress blood glucose and insulin concentrations in dogs. In the present study, the effects of D-allulose were investigated in dogs administered sugars or food.
MATERIALS AND METHODS
Animals: This study protocol was approved by the institutional Animal Experiment Committee of Gifu University (approval no. 12075). Six healthy beagle dogs (one male and five females; age, 2.4 ± 0.9 years; and body weight, 13.7 ± 1.5 kg) were used in the present study. In the oral glucose administration experiment, an additional dog (male, 3 years old; body weight, 11.8 kg) was also used. Dogs were used for each experiment repeatedly with a washout period of at least 1 week. All dogs were confirmed healthy by physical examination, complete blood count and biochemical analysis. Food was withheld from dogs overnight before each experiment; however, they were allowed free access to water throughout the experiments.
Oral sugar administration experiments: Seven and six dogs were orally administered 50% glucose (2.0 g/kg) and 50% maltose (2.0 g/kg) solutions, respectively, with oral D-allulose (0.2 g/kg) or equivalent water supplementation. The dose rate of D-allulose was determined referring to similar studies conducted for humans [9]. The same dose rate was used in other experiments. The dose rate of glucose or maltose was determined based on a previous study in dogs [1]. Blood samples were collected from the cephalic vein before and 30, 60, 90 and 120 min after administration of sugars for measurements of glucose and insulin concentrations.
Intravenous glucose administration experiment: Six dogs were orally administered D-allulose solution (0.2 g/kg) or an equivalent volume of water 60 min before the intravenous administration of 50% glucose solution (0.5 g/kg). The dose rate of glucose was determined based on a previous study in dogs [2]. Blood samples were collected from the cephalic vein 60 min and immediately before, and 5, 10, 15, 30 and 60 min after the administration of glucose for measurements of glucose and insulin concentrations.
Feeding experiment: Six dogs were provided a commercial maintenance dry food (Select protein duck and tapioca, Royal Canin Japon, Tokyo, Japan) with D-allulose (0.2 g/kg) or an equivalent volume of water. The amount of food provided was calculated based on half of the daily energy requirement for young adult dogs (50 kcal/kg of body weight0.75) [4]. Blood samples were collected from the cephalic vein before and 1, 2, 3, 4, 6 and 8 hr after feeding for measurements of glucose and insulin concentrations.
Assays: Plasma glucose concentrations were measured using an automated biochemical analyzer (Labospect 003; Hitachi High-Technologies, Tokyo, Japan). Plasma insulin concentration was assayed using the LBIS dog insulin enzyme-linked immunosorbent assay kit (Shibayagi, Shibukawa, Japan) [19].
Statistical analysis: The area under the curves (AUC) of plasma glucose and insulin concentrations were calculated using the trapezoid method. Statistics analyses were conducted in Excel 2011 (Microsoft, Redmond, WA, U.S.A.) with the add-in software Statcel 3 (OMS Publishing, Saitama, Japan). Differences between groups were determined by the Wilcoxon signed-rank test. Values of P<0.05 were considered significant.
RESULTS
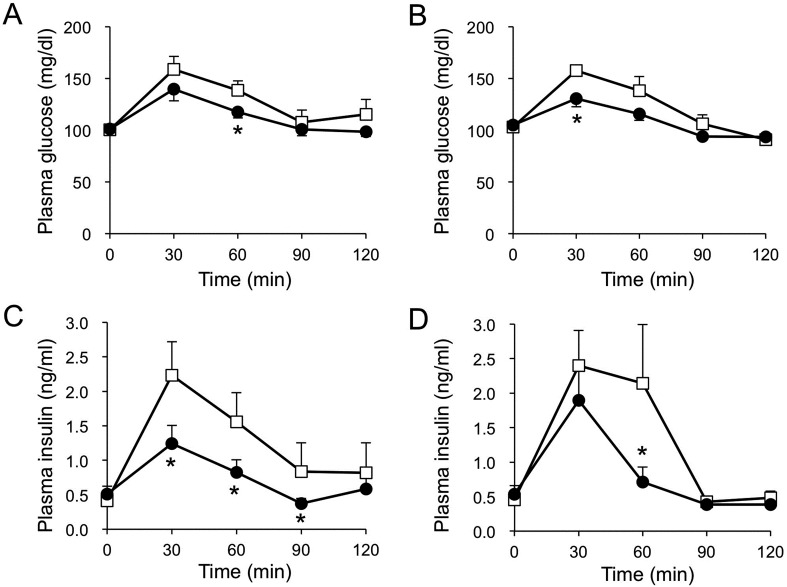
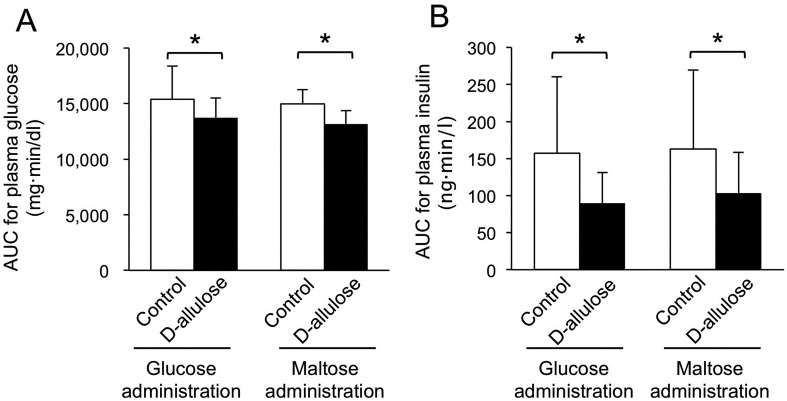
Oral sugar administration experiments: The oral administration of glucose or maltose increased plasma glucose and insulin concentrations (Fig. 1). The administration of D-allulose diminished the rise in plasma glucose after the oral administration of glucose or maltose (P<0.05) (Fig. 1A and 1C). In addition, the concentration of plasma insulin after the oral administration of glucose or maltose was lower after the administration of D-allulose (P<0.05) (Fig. 1B and 1D). The AUCs for plasma glucose and insulin concentrations after the oral administration of glucose were lower (P<0.05) in the D-allulose group than that in the control group (Fig. 2). The AUCs for plasma glucose and insulin concentrations after the oral administration of maltose were lower (P<0.05) in the D-allulose group than that in the control group (Fig. 2).
Fig. 1.
Plasma glucose and insulin concentrations after the oral administration of glucose (A, C) or maltose (B, D) with or without the oral administration of D-allulose in dogs. Closed circles and open squares represent data from dogs administered D-allulose (0.2 g/kg) and control dogs, respectively. Data are shown as means ± standard error of the mean (SEM) (n=7 for the glucose administration experiment and n=6 for the maltose administration experiment). Asterisks represent significant difference (P<0.05) between groups.
Fig. 2.
Area under the curves (AUCs) for plasma glucose (A) and insulin (B) concentrations after the oral administration of glucose or maltose with or without the oral administration of D-allulose in dogs. Data are shown as means ± standard deviation. Asterisks represent significant difference (P<0.05) between groups.
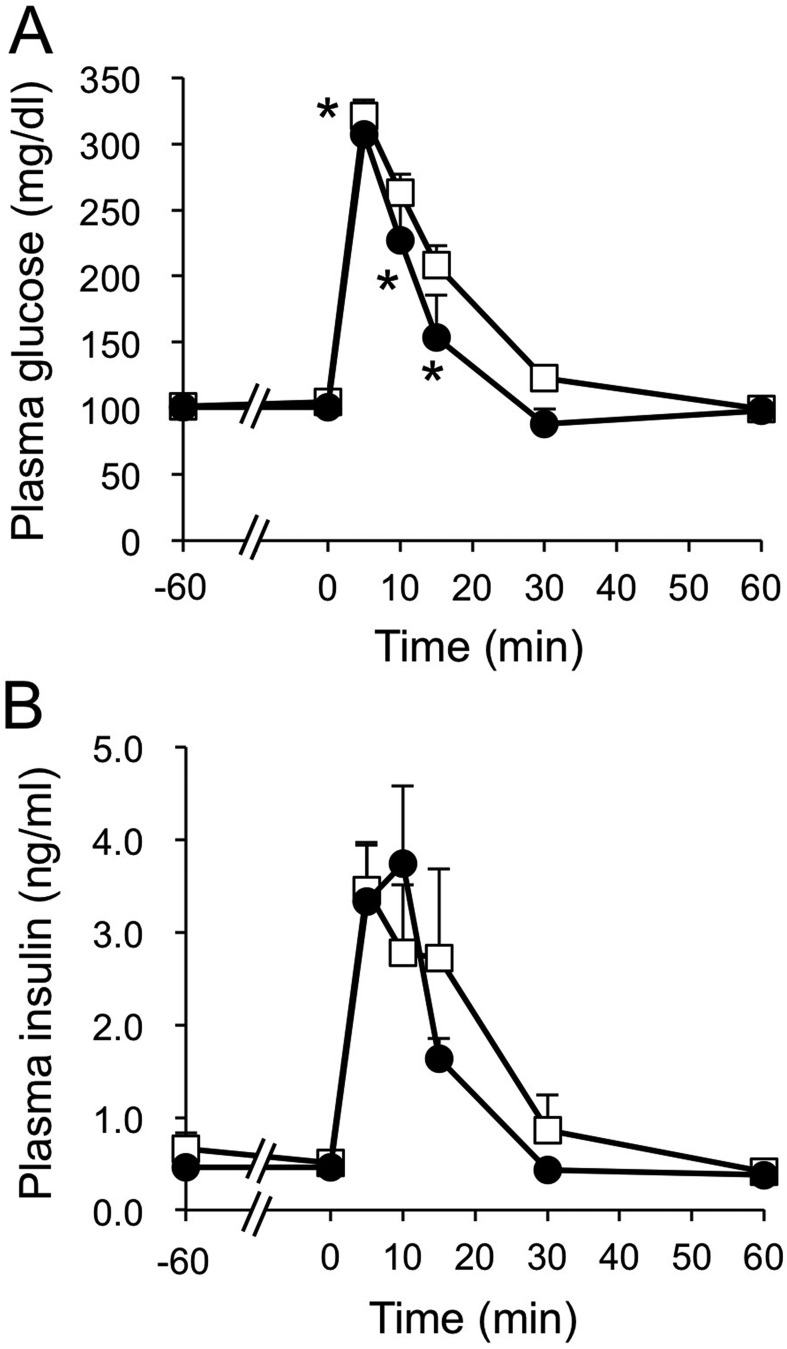
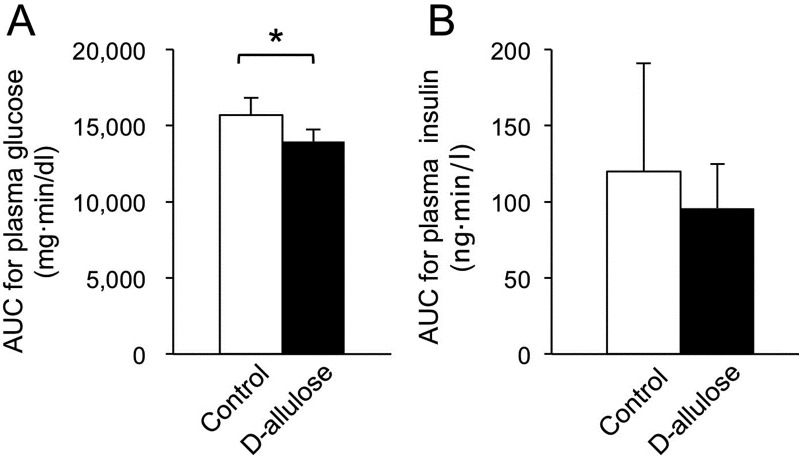
Intravenous glucose administration experiment: The concentration of plasma glucose was lower at 5, 10 and 15 min after the intravenous administration of glucose when D-allulose was administered (P<0.05) (Fig. 3A). On the other hand, there was no significant difference in the concentration of plasma insulin between the control and the D-allulose groups (Fig. 3B). The AUC for plasma glucose concentration after the intravenous administration of glucose was lower (P<0.05) in the D-allulose group than in the control group (Fig. 4A). The AUC for the concentration of plasma insulin after the intravenous administration of glucose for the D-allulose group did not differ significantly from that for the control group (Fig. 4B).
Fig. 3.
Plasma glucose (A) and insulin (B) concentrations after intravenous administration of glucose with or without the oral administration of D-allulose in dogs. Closed circles and open squares represent data from dogs administered D-allulose (0.2 g/kg) and control dogs, respectively. Data are shown as means ± standard error of the mean (SEM) (n=6). Asterisks represent significant difference (P<0.05) between groups.
Fig. 4.
Area under the curves (AUCs) for plasma glucose (A) and insulin (B) concentrations after the intravenous administration of glucose with or without the oral administration of D-allulose in dogs. Data are shown as means ± standard deviation. Asterisks represent significant difference (P<0.05) between groups.
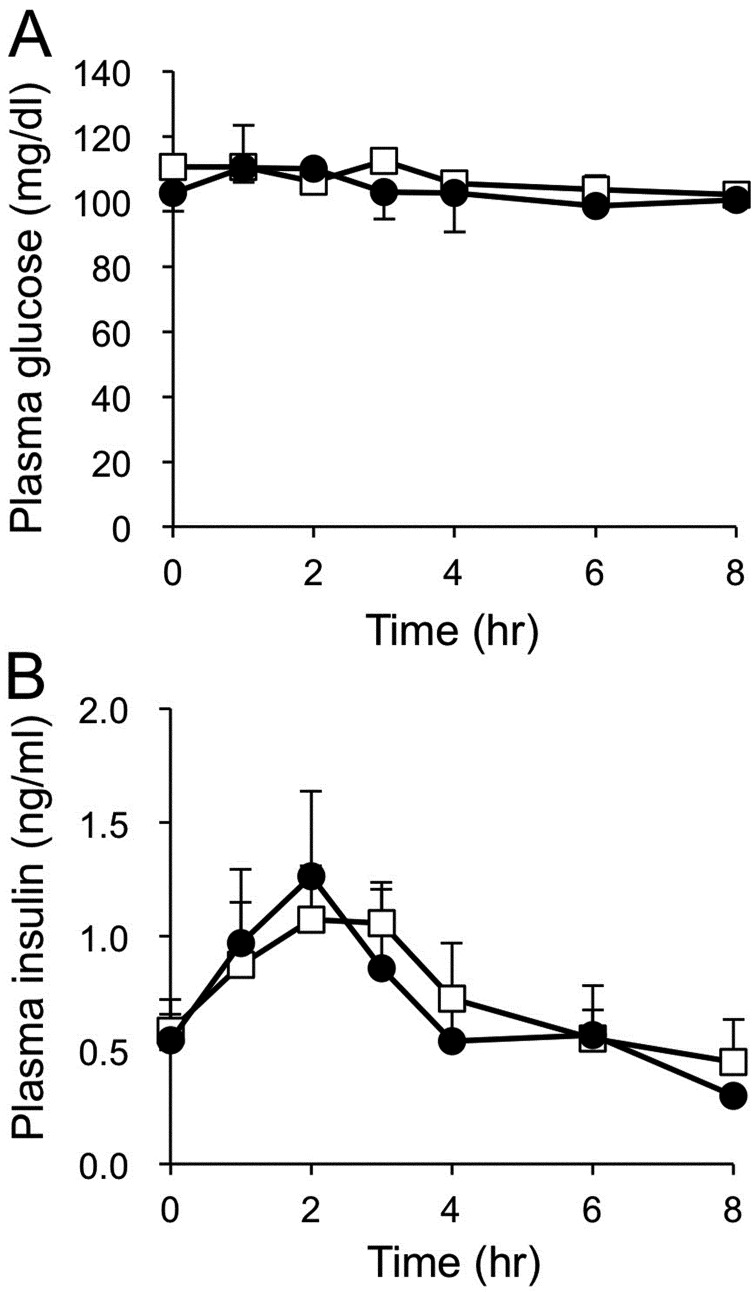
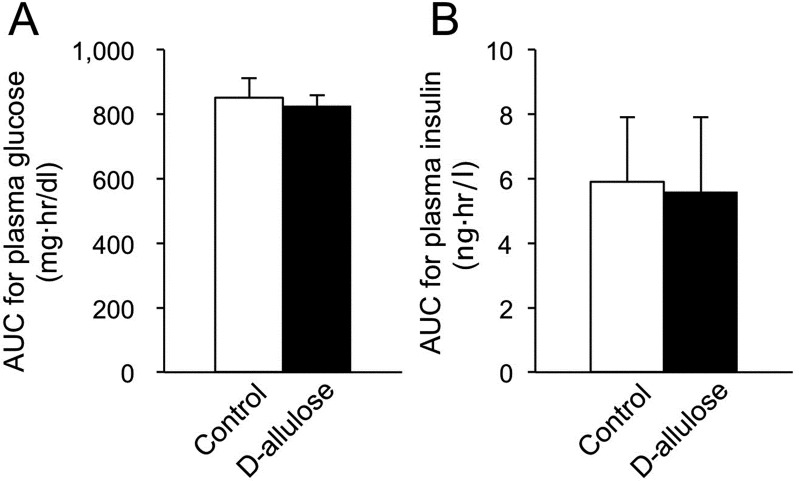
Feeding experiment: The concentration of plasma insulin increased after feeding (Fig. 5B). The concentration of plasma glucose did not fluctuate after feeding (Fig. 5A). The oral administration of D-allulose did not affect these parameters after feeding in the dogs (Fig. 5). There was no significant difference in AUCs for plasma glucose and insulin concentrations between the D-allulose and the control groups (Fig. 6).
Fig. 5.
Plasma glucose (A) and insulin (B) concentrations after feeding with or without oral D-allulose administration in dogs. Closed circles and open squares represent data from dogs administered D-allulose (0.2 g/kg) and control dogs, respectively. Data are shown as means ± standard error of the mean (SEM) (n=6).
Fig. 6.
Area under the curves (AUCs) for plasma glucose (A) and insulin (B) concentrations after feeding with or without oral D-allulose administration in dogs. Data are shown as means ± standard deviation.
DISCUSSION
In the present study, we demonstrated that D-allulose diminished the increase in plasma glucose concentration after the oral administration of glucose or maltose in dogs. The antihyperglycemic effect of D-allulose was not the result of increased plasma insulin concentration. These results were consistent with the result obtained in humans subjected to an oral maltodextrin tolerance test [9]. In rodents, D-allulose has been known to inhibit digestive enzymes, including α-glucosidase and α-amylase [7]. The antihyperglycemic effect after oral administration of maltose in dogs can be partially explained by the inhibition of α-glucosidase in the intestine. However, in the present study, it was difficult to evaluate the effect of D-allulose on α-glucosidase in the canine intestine, because the antihyperglycemic effect was similarly observed after oral administration of glucose. In addition to the inhibition of digestive enzymes, it is reported that D-allulose inhibits the absorption of glucose from the intestine by competition with glucose transporter 2 (GLUT2) on the basolateral membrane of the intestinal epithelial cells [6, 7]. The inhibition of glucose absorption might contribute to the effect of D-allulose on plasma glucose concentrations after the oral administration of glucose or maltose in dogs. The glucose suppressive effect without increased plasma insulin concentration was also observed after the intravenous administration of glucose in dogs. The effect of D-allulose on the digestion or absorption of sugars in the intestine cannot account for the effect of D-allulose in the intravenous glucose administration experiment. In rats, D-allulose has been reported to induce hepatic glucokinase to increase glucose utilization in the liver [8]. This might be one possible mechanism for the glucose lowering effect of D-allulose after the intravenous administration of glucose in dogs. In addition, the increased utilization of glucose in the liver might be one of the factors explaining the glucose lowering effect of D-allulose after the ingestion of sugar in dogs. The diminished responses of insulin after the administrations of sugar are consistent with these theories. A further study is required to clarify the detailed mechanisms of the antihyperglycemic effect of D-allulose in dogs.
In the present study, the effect of D-allulose on concentrations of plasma glucose and insulin after feeding was not significant relative to that after the administration of sugar in dogs. In healthy humans, D-allulose decreased the concentration of postprandial blood insulin, although postprandial blood glucose concentration was not altered [5]. The reason for the discrepancy between studies remains unclear and might be due to a species difference in the metabolism of carbohydrate. However, the carbohydrate content of the meal used in the human study was higher (84.5 g of carbohydrate, 13.3 g of protein and 3.7 g of fat per 435 kcal) [5] than that in the present study (58.5 g of carbohydrate, 25.1 g of protein and 10.8 g of fat per 400 kcal). The difference in carbohydrate content in the diets used might explain the different results obtained. Because the composition or amount of food in our study was determined to represent food that was fed twice daily to pet dogs, postprandial hyperglycemia was not obvious. Our results suggested that D-allulose does not show significant effects on glucose metabolism after a typical feeding in healthy pet dogs. A greater carbohydrate load, which represents a condition of glucose intolerance, may demonstrate an antihyperglycemic effect of D-allulose after feeding in dogs. Actually, D-allulose showed a clearer suppressive effect on postprandial blood glucose in human patients with borderline diabetes [5]. Studies in dogs with abnormal glucose tolerance may be necessary to evaluate the possibility of clinical application of D-allulose to diabetic dogs.
In conclusion, D-allulose showed an antihyperglycemic effect in healthy dogs after the administration of sugar. The present results suggest that D-allulose administration might be beneficial in dogs with impaired glucose tolerance. The antihyperglycemic effect of D-allulose was insulin-independent; thus, D-allulose might help to achieve better glycemic control in diabetic dogs lacking insulin secretion. However, because D-allulose did not show a significant effect on postprandial plasma glucose or insulin concentrations in healthy dogs, a further study investigating the therapeutic efficacy of D-allulose in diabetic dogs is required.
REFERENCES
- 1.Barone G. W., Flanagan T. L., Cornett G., Pruett T. L., Hanks J. B.1991. Glucose metabolism after pancreas autotransplantation. The effect of open duct versus urinary bladder drainage technique. Ann. Surg. 213: 159–165. doi: 10.1097/00000658-199102000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunetto M. A., Sá F. C., Nogueira S. P., Gomes M. O., Pinarel A. G., Jeremias J. T., de Paula F. J., Carciofi A. C.2011. The intravenous glucose tolerance and postprandial glucose tests may present different responses in the evaluation of obese dogs. Br. J. Nutr. 106Suppl 1: S194–S197. doi: 10.1017/S0007114511000870 [DOI] [PubMed] [Google Scholar]
- 3.Chung M. Y., Oh D. K., Lee K. W.2012. Hypoglycemic health benefits of D-psicose. J. Agric. Food Chem. 60: 863–869. doi: 10.1021/jf204050w [DOI] [PubMed] [Google Scholar]
- 4.Debraekeleer J., Gross K. L., Zicker S. C.2010. Feeding young adult dogs: before middle age. pp. 257–272. In: Small Animal Clinical Nutrition 5th ed., (Hand, M. S., Thatcher, C. D., Remillard, R. L., Roudebush, P. and Novotny, B. J. eds.). Mark Morris Institute, Topeka. [Google Scholar]
- 5.Hayashi N., Iida T., Yamada T., Okuma K., Takehara I., Yamamoto T., Yamada K., Tokuda M.2010. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 74: 510–519. doi: 10.1271/bbb.90707 [DOI] [PubMed] [Google Scholar]
- 6.Hishiike T., Ogawa M., Hayakawa S., Nakajima D., O’Charoen S., Ooshima H., Sun Y.2013. Transepithelial transports of rare sugar D-psicose in human intestine. J. Agric. Food Chem. 61: 7381–7386. doi: 10.1021/jf401449m [DOI] [PubMed] [Google Scholar]
- 7.Hossain A., Yamaguchi F., Matsuo T., Tsukamoto I., Toyoda Y., Ogawa M., Nagata Y., Tokuda M.2015. Rare sugar D-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 155: 49–59. doi: 10.1016/j.pharmthera.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Hossain M. A., Kitagaki S., Nakano D., Nishiyama A., Funamoto Y., Matsunaga T., Tsukamoto I., Yamaguchi F., Kamitori K., Dong Y., Hirata Y., Murao K., Toyoda Y., Tokuda M.2011. Rare sugar D-psicose improves insulin sensitivity and glucose tolerance in type 2 diabetes Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biochem. Biophys. Res. Commun. 405: 7–12. doi: 10.1016/j.bbrc.2010.12.091 [DOI] [PubMed] [Google Scholar]
- 9.Iida T., Kishimoto Y., Yoshikawa Y., Hayashi N., Okuma K., Tohi M., Yagi K., Matsuo T., Izumori K.2008. Acute D-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J. Nutr. Sci. Vitaminol. (Tokyo) 54: 511–514. doi: 10.3177/jnsv.54.511 [DOI] [PubMed] [Google Scholar]
- 10.Iida T., Yamada T., Hayashi N., Okuma K., Izumori K., Ishii R., Matsuo T.2013. Reduction of abdominal fat accumulation in rats by 8-week ingestion of a newly developed sweetener made from high fructose corn syrup. Food Chem. 138: 781–785. doi: 10.1016/j.foodchem.2012.11.017 [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T., Izumori K.2006. Effects of dietary D-psicose on diurnal variation in plasma glucose and insulin concentrations of rats. Biosci. Biotechnol. Biochem. 70: 2081–2085. doi: 10.1271/bbb.60036 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo T., Suzuki H., Hashiguchi M., Izumori K.2002. D-psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 48: 77–80. doi: 10.3177/jnsv.48.77 [DOI] [PubMed] [Google Scholar]
- 13.Nelson R. W.2014. Canine diabetes mellitus. pp. 213–257. In: Canine and Feline Endocrinology, 4th ed., (Feldman, E. C., Nelson, R. W., Reusch, C. E., Scott-Moncrieff, C. R. and Behrend, E. N. eds.). Elsevier, St. Louis. [Google Scholar]
- 14.Nelson R. W., Duesberg C. A., Ford S. L., Feldman E. C., Davenport D. J., Kiernan C., Neal L.1998. Effect of dietary insoluble fiber on control of glycemia in dogs with naturally acquired diabetes mellitus. J. Am. Vet. Med. Assoc. 212: 380–386. [PubMed] [Google Scholar]
- 15.Nelson R. W., Ihle S. L., Lewis L. D., Salisbury S. K., Miller T., Bergdall V., Bottoms G. D.1991. Effects of dietary fiber supplementation on glycemic control in dogs with alloxan-induced diabetes mellitus. Am. J. Vet. Res. 52: 2060–2066. [PubMed] [Google Scholar]
- 16.Nishii N., Nomizo T., Takashima S., Matsubara T., Tokuda M., Kitagawa H.2016. Single oral dose safety of D-allulose in dogs. J. Vet. Med. Sci. 78: 1079–1083. doi: 10.1292/jvms.15-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiai M., Nakanishi Y., Yamada T., Iida T., Matsuo T.2013. Inhibition by dietary D-psicose of body fat accumulation in adult rats fed a high-sucrose diet. Biosci. Biotechnol. Biochem. 77: 1123–1126. doi: 10.1271/bbb.130019 [DOI] [PubMed] [Google Scholar]
- 18.Ochiai M., Onishi K., Yamada T., Iida T., Matsuo T.2014. D-psicose increases energy expenditure and decreases body fat accumulation in rats fed a high-sucrose diet. Int. J. Food Sci. Nutr. 65: 245–250. doi: 10.3109/09637486.2013.845653 [DOI] [PubMed] [Google Scholar]
- 19.Sako T., Mori A., Lee P., Goto H., Fukuta H., Oda H., Saeki K., Miki Y., Makino Y., Ishioka K., Mizutani H., Kojima Y., Koikeda S., Arai T.2010. Supplementing transglucosidase with a high-fiber diet for prevention of postprandial hyperglycemia in streptozotocin-induced diabetic dogs. Vet. Res. Commun. 34: 161–172. doi: 10.1007/s11259-010-9342-0 [DOI] [PubMed] [Google Scholar]