Abstract
This study describes the imaging features and characteristics of caval foramen hernias in 7 dogs diagnosed by computed tomography (CT). On lateral radiographs, 6 of 7 dogs showed dome-shaped, broad-based, caudal mediastinal lesions. CT findings included caudal vena cava (CVC) compression (n=7), right lateral (n=6) or medial (n=1) liver lobe involvement, hepatic vein dilation (n=5) and biliary tract involvement (n=1) with partial (n=6) or entire (n=1) liver lobe hernias. A caval foramen hernia should be part of the differential diagnosis when the aforementioned imaging features are detected. CT is considered as a useful tool for diagnosis and evaluation in dogs with a caval foramen hernia.
Keywords: caval foramen hernia, computed tomography, dog, liver, radiography
In a diaphragmatic hernia, some abdominal organs, such as the stomach and liver, enter the thoracic cavity and protrude through the diaphragm foramen, which is congenital or trauma-induced [9, 14]. Although a diaphragmatic hernia occurs mostly with trauma in dogs [14], it can also occur through 3 diaphragmatic apertures: the aortic hiatus, esophageal hiatus and caval foramen [18]. There have been a report about esophageal hiatal hernias in dogs [3], but hernias through the caval foramen have not been well established in dogs.
A caval foramen hernia is a kind of diaphragmatic hernia and rarely reported in dogs. In general, the liver enters the foramen of the caudal vena cava (CVC) at the central tendon of the diaphragm and could compress adjacent structures, such as the CVC, cardiac structures and lung lobes [2, 11]. Although a single case report has been reported in the Japanese literature [20], imaging features have not yet been depicted or established sufficiently.
Medical record databases of three different animal hospitals [Konkuk University Veterinary Medical Teaching Hospital (n=3), Helix Animal Medical Center (n=3) and Busan Animal Medical Center (n=1)] were searched for dogs with a confirmed diagnosis of caval foramen hernia via computed tomography (CT) from January 1, 2012 to December 31, 2015. For study inclusion, dogs had to have medical records including breed, sex, age, body weight and chief complaint. Reports from surgery and histopathology were also reviewed.
Thoracic radiographs were performed in a routine manner (Titan 2000M; Comed Medical Systems Co., Ltd., Seoul, Korea). For CT scan, anesthesia was induced with propofol (6 mg/kg, IV; Provive 1%; Myungmoon Pharmaceutical Co., Seoul, Korea) and was maintained with 1.5% isoflurane (Foran solution; Choongwae Pharma Corporation, Seoul, Korea) in 100% oxygen by endotracheal intubation. CT scans were acquired from all dogs in ventral recumbency on a 4-multi-detector-row (Lightspeed; GE Medical Systems, Milwaukee, WI, U.S.A.) and 16-multi-detector-row system (Brivo CT385; GE Medical Systems). Imaging protocols were 120 kVp, 200 mAs, 1.25-mm slice thickness and scan times of 60 sec for post-contrast scan using a manual breath-hold technique with pressure held at 10 cm H2O. 600 mg iodine/kg iohexol (Omnihexol 300; Korea United Pharmaceutical, Seoul, Korea) was injected manually at a rate of 1 ml/sec with into the cephalic vein.
All acquired images were uploaded and reviewed by two radiologists (J.H. Kim and S.Y. Kim) using commercially available software (Radiant; Medixant, Proznan, Poland). Based on the CT images, the herniated liver size and lobe, hernia direction with respect to the CVC, presence of hepatic vein dilation and abnormalities of the biliary tract were assessed. Defect size was assessed in the sagittal and dorsal planes, and the maximal width and height were measured. Herniated liver size was assessed in 3 orthogonal planes, and the maximum diameter in length, width and height was measured. The herniated liver lobe was clarified, and contrast enhanced patterns both hernia and hepatic parenchyma were assessed on post-contrast series.
Seven dogs met the inclusion criteria. The results of clinical and imaging characteristics are summarized in Table 1. There were 5 breeds represented in the study population: miniature Poodle (n=2), Shih-Tzu (n=2), Yorkshire Terrier (n=1), Pomeranian (n=1) and Pug (n=1). The sex distribution included males (n=1), castrated males (n=3) and females (n=3). Age at the time of diagnosis ranged from 1 year to 12 years (mean ± standard deviation [SD], 8.28 ± 4.33). Body weight ranged from 2.4 kg to 5.1 kg (mean ± SD, 3.33 ± 0.81 kg). The reasons for CT imaging included: lung nodule or mass suspected (n=4, dogs 1, 3, 4, and 7), incidental findings (n=2, dogs 2 and 5) and congenital diaphragmatic hernia suspected (n=1, dog 6). Only a dog (dog 6) underwent surgery, and a partial hernia of the liver through the caval foramen on the central tendon portion was identified.
Table 1. Summarized Signalments and Imaging Features of 7 dogs with Caval Foramen Hernias of the Liver.
| Dog No. | Breed | Sex | Age (years) |
Chief complaint |
Affected liver lobe |
Defect sizea) W × H (mm) |
Hernia size L × W × H (mm) |
Hepatic vein dilation |
Biliary tract involvement |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pomeranian | F | 2 | Lung nodule suspected | RLL | 11.7 × 12.4 | 11 × 22 × 33 | Yes | No |
| 2 | Shih-Tzu | CM | 12 | Glaucoma | RLL | 8.8 × 12.4 | 20 × 14 × 11 | No | No |
| 3 | Miniature Poodle | F | 11 | Lung nodule suspected | RLL | 14.7 × 15.7 | 22 × 19 × 17 | Yes | No |
| 4 | Yorkshire Terrier | CM | 11 | Thoracic mass | RLL | 13.6 × 15.1 | 50 × 35 × 20 | Yes | No |
| 5 | Pug | M | 10 | Left maxillary mass, dyspnea | RML | 16.3 × 17.1 | 13 × 12 × 18 | Yes | Yes |
| 6 | Miniature Poodle | F | 1 | Vomiting, dyspnea | RLL | 14.6 × 12.1 | 30 × 18 × 15 | Yes | No |
| 7 | Shih-Tzu | CM | 11 | Anorexia for 1 week | RLL | 13.9 × 13.3 | 15 × 17 × 12 | -b) | No |
M, male; CM, castrated male; F, female; CVC, caudal vena cava; RML, right medial liver lobe; RLL, right lateral liver lobe; a) defect sizes were measured with computed tomography; b) hard to determine hepatic vein dilation because of multiple metastatic nodules.
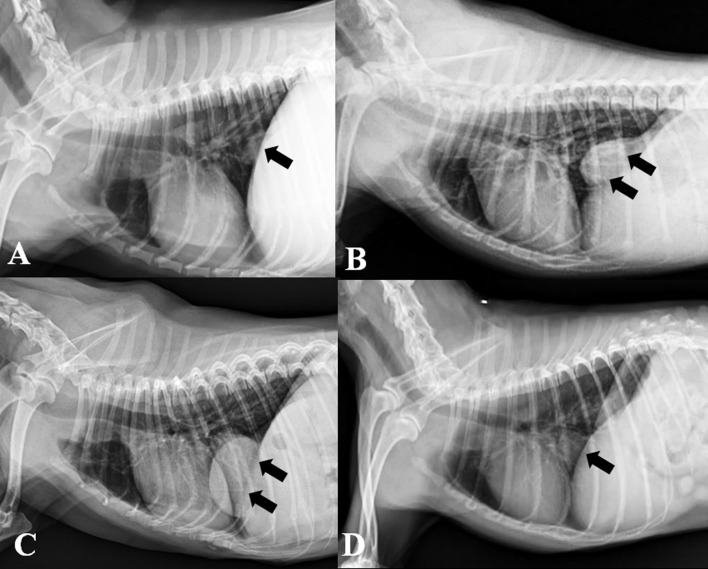
On radiography, all dogs except 1 (dog 2; 6 of 7 dogs) revealed caval foramen hernias on right lateral radiograph. Radiographic findings were a dome-shaped (n=6), broad-based diaphragm (n=6), and caudal (n=5) or caudoventral (n=1) mediastinal nodule/mass patterns (Fig. 1). Because of silhouette effects among the diaphragm, CVC and herniated liver, the ventrodorsal projection was suitable for diagnosis only in 2 of 7 dogs (dogs 4 and 6).
Fig. 1.
Right lateral radiographs of 4 dogs with caval foramen liver hernias are shown. Various-sized pericaval nodules and masses are visible (arrows). Note that all of them show a dome-shape with broad-based on the diaphragm. (A, dog 1; B, dog 3, C: dog 4; D, dog 7)
The CT findings were as follows (Fig. 2): CVC compression (n=7), a right lateral lobe (n=6) or right medial lobe (n=1) hernia with a ventral (n=5), right-ventral (n=1, dog 3) and left-ventral (n=1, dog 5) direction with respect to the CVC. 6 of 7 dogs had a partial, small-volume hernia, but an entire right lateral liver lobe was herniated in 1 dog (dog 4, Supplementary file). All of herniated liver lobes showed iso-attenuation to the hepatic parenchyma on pre- and post-contrast CT images without biliary tract involvement except for 1 dog (dog 5, Fig. 3). The hepatic vein vasculatures could not be assessed in dog 7, which had multiple metastatic nodules on the liver. The width and height of the defect ranged from 8.8 to 14.7 mm (mean ± SD, 14.0 ± 1.9 mm) and 12.1 to 15.7 mm (mean ± SD, 13.4 ± 2.4 mm), respectively.
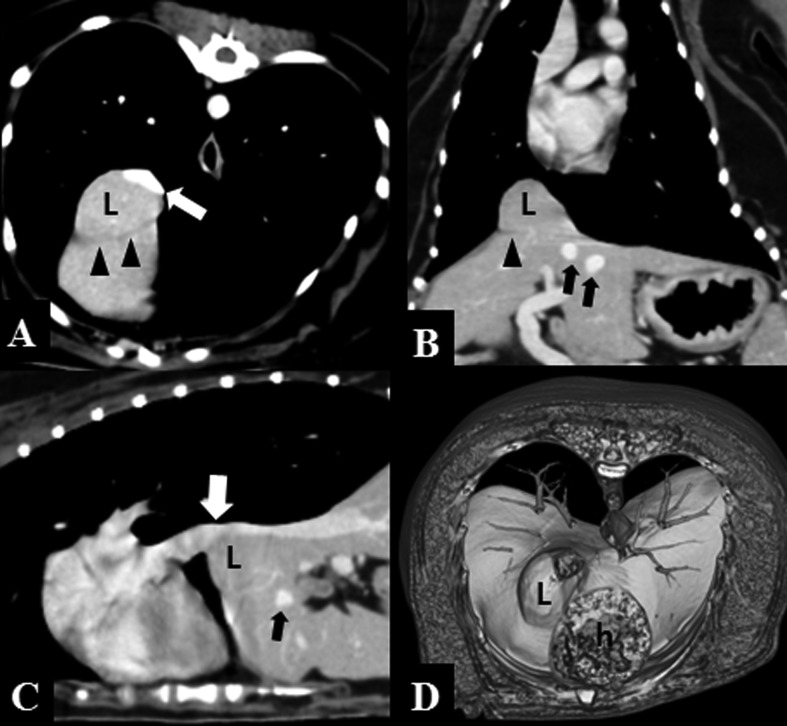
Fig. 2.
Post-contrast CT images of a typical caval foramen liver hernia in dog 3. Partial herniation of the right lateral liver lobe (L) at the central tendon of the diaphragm compresses the adjacent caudal vena cava dorsally (white arrows). Dilation of the main hepatic veins (black arrows) and the intact muscular portion of diaphragm (arrowheads) are visible. (A, transverse plane; B, dorsal plane; C, sagittal plane; D, volume-rendered image, diaphragmatic aspect; h, heart)
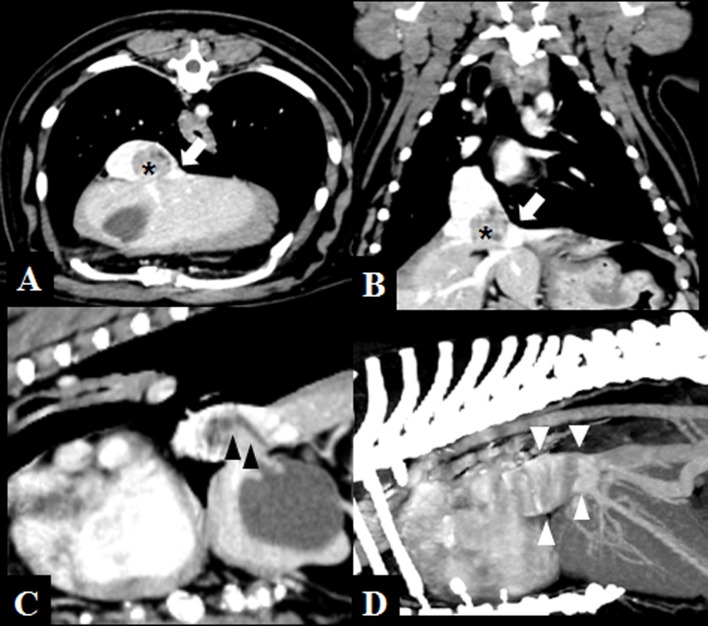
Fig. 3.
Post-contrast CT images of severe caudal vena cava obstruction in dog 5. A heterogeneous intravascular thrombus-like liver hernia is visible (asterisks). The herniated liver lobe severely compresses the caudal vena cava dorsally and induces partial obstruction of the left branch of the hepatic veins (arrows). A cystic duct hernia and mild dilation are visible in C (black arrowheads). Note that post-obstructive dilation of the caudal vena cava is demonstrated in D (white arrowheads). (A, transverse plane; B, dorsal plane; C, sagittal plane; D, maximum intensity projection, sagittal plane)
Several types of diaphragmatic diseases, both congenital and acquired, are familiar to veterinary radiologists, but only a few cases of hernias through the caval foramen have been reported in the human [8, 11] and veterinary literatures [20]. Understandably, the causes and pathogenesis are unknown, and there has not been determined any report that explains whether caval foramen hernia is a congenital or acquired condition in dogs. Based on a human study [11] and there should be muscle fibers radiating into the dorsal border of the caval foramen in normal dogs [5], it is considered a seldom-reported congenital anomaly.
While most dogs had typical radiographic signs on lateral radiographs, the diagnoses were challenging because of the variable sizes and lack of reported imaging features. Traditionally, only limited causes of the caudoventral or caudal mediastinal lesion including peritoneopericardial diaphragmatic hernia, accessory lung lobe mass, diaphragmatic mass and pericardial cyst were reported in dogs [16, 19]. In the present study, four dogs (dogs 1, 4, 5 and 8) were misdiagnosed as having intra-thoracic lesions, such as lung nodules or masses. Even in human medicine, caval foramen hernias have been found incidentally and misdiagnosed as right atrial [2] and thoracic masses [11]. Therefore, there should be caution in the process of diagnosing caval foramen hernia in dogs.
In this study, except for dog 5, most patients demonstrated right lateral liver lobe involvement in the hernia. It is considered likely to be affected, because the right medial and lateral lobes are the nearest hepatic structures to the caval foramen [5]. Interestingly, the affected liver lobe is considered to be important, and there seemed to be no correlation between hernia size and severity. Different from other diaphragmatic hernias, because of the anatomical proximity of the hernia to the CVC, a small-volume hernia could be clinically important. Moreover, because the gall bladder and cystic duct are located between the right medial and quadrate lobes [5], the clinical importance, such as biliary tract involvement, could be increased if the right medial lobe is affected.
The degrees of the CVC compression were variable in 7 dogs. Even though there have been no references to the clinical importance of CVC compression in dogs, various associated diseases have been reported in human medicine [7, 15]. For example, Budd-Chiari-like syndrome refers to hepatic venous outflow obstruction, resulting in portal hypertension and peritoneal effusion [13]. It results from any disease that could cause obstruction or compression of the hepatic vein, CVC and right atrium [12]. In the veterinary field, Budd-Chiari-like syndrome occurs with intra-luminal obstruction, extraluminal compression, right-side heart failure, a kinked CVC and massive diaphragmatic hernia [1, 8, 12, 13]. Almost no dog in this study had peritoneal effusion or hepatic congestion, but 1 dog (dog 5) that had a small-volume and left medial liver lobe hernia showed severe CVC compression with post-obstructive CVC dilation and hepatic vein congestion, which could cause Budd-Chiari-like syndrome. Thus, a caval foramen hernia, even if it looks like a small-volume hernia, should be considered as a cause of Budd-Chiari-like syndrome in dogs.
There have been many reports about congenital diaphragmatic disease including diaphragmatic eventration in dogs [4, 6, 10]. Unlikely in other diseases, diaphragmatic eventration is considered as the first differential to caval foramen hernia in terms of small diaphragmatic bulging on lateral radiographs, small breeds are predisposed, and the abdominal organ did not tend to prolapse into the thoracic cavity [4]. However, it has been known as a condition where the diaphragmatic muscle is malpositioned with subtotal diaphragmatic tears that do not occur at the central tendon or in the pericaval area because the fibrous tissue is stronger than the muscular portion of the diaphragm [4]. In addition, on radiographs, diaphragmatic eventration seems not to have a pericaval pattern, but rather a caudoventral mediastinal mass pattern [3] or entire hemidiaphragm displacement [17] that definitely differs from caval foramen hernia.
The clinical relevance of a caval foramen hernia has not yet been demonstrated, and in the present study, 5 dogs were diagnosed without associated clinical signs. However, in human medicine, there was a life-threatening pulmonary embolism caused by CVC obstruction with a herniated liver lobe [2]. In addition, in concurrence with a previous report [20], 1 young dog (dog 6) that had indistinct clinical signs including anorexia and vomiting recovered well after surgery. This presents the possibility that a caval foramen hernia could induce clinical signs or a life-threatening complication. In this regard, it should be emphasized that a CT examination should be performed in dogs to estimate any risk factors and accurately evaluate the condition.
Several limitations need to be considered in this study. First, the small number of animals in the study population was a limitation. Because of this, it was hard to derive predisposed breed, a statistical assessment or any categorized results. Second, almost all dogs were senile and more than 10 years old, which made it difficult to rule out underlying disease and hard to determine the clinical relevance. In addition, because the disease had been found incidentally in almost all dogs, most owners declined surgical treatment or a histopathologic investigation. Lastly, as this study reviewed only anatomical aspects via radiographs and CT, the hemodynamic effects of CVC compression could not be evaluated, such as pulsed-wave hepatic vein flow.
In conclusion, hernia of the liver through the caval foramen should be part of the differential diagnosis when a dome-shaped, broad-based, caudal or caudoventral lesion detected on a lateral radiograph, especially in small breed dogs. CT examination is considered as a useful tool for diagnosis, evaluation and surgical planning in dogs with a caval foramen hernia. Although it is a rare condition, various abnormalities, such as CVC compression, hepatic vein dilation and biliary involvement, should be considered.
Supplementary Material
REFERENCES
- 1.Baig M. A., Gemmill T., Hammond G., Patterson C., Ramsey I. K.2006. Budd-Chiari-like syndrome caused by a congenital hiatal hernia in a shar-pei dog. Vet. Rec. 159: 322–323. doi: 10.1136/vr.159.10.322 [DOI] [PubMed] [Google Scholar]
- 2.Benitez Lazzarotto A., O’Rourke N. A., Fitzgerald B. T., Wong D., Scalia G. M.2016. Hernia of the diaphragmatic caval foramen causing right atrial “mass”, caval obstruction and pulmonary embolism. Int. J. Cardiol. 207: 215–216. doi: 10.1016/j.ijcard.2016.01.166 [DOI] [PubMed] [Google Scholar]
- 3.Callan M. B., Washabau R. J., Saunders H. M., Kerr L., Prymak C., Holt D.1993. Congenital esophageal hiatal hernia in the Chinese shar-pei dog. J. Vet. Intern. Med. 7: 210–215. doi: 10.1111/j.1939-1676.1993.tb01009.x [DOI] [PubMed] [Google Scholar]
- 4.Choi J., Kim H., Kim M., Yoon J.2009. Imaging diagnosis--positive contrast peritoneographic features of true diaphragmatic hernia. Vet. Radiol. Ultrasound 50: 185–187. doi: 10.1111/j.1740-8261.2009.01514.x [DOI] [PubMed] [Google Scholar]
- 5.Evans H. E., de Lahunta A.2013. Miller’s Anatomy of the Dog. pp. 222–223. In: the Diaphragm., 4th ed. Elsevier Health Sciences, Amsterdam. [Google Scholar]
- 6.Evans S. M., Biery D. N.1980. Congenital peritoneopericardial diaphragmatic hernia in the dog and cat: a literature review and 17 additional case histories. Vet. Radiol. Ultrasound 21: 108–116. doi: 10.1111/j.1740-8261.1980.tb00589.x [DOI] [Google Scholar]
- 7.Kandpal H., Sharma R., Gamangatti S., Srivastava D. N., Vashisht S.2008. Imaging the inferior vena cava: a road less traveled. Radiographics 28: 669–689. doi: 10.1148/rg.283075101 [DOI] [PubMed] [Google Scholar]
- 8.Langs L. L.2009. Budd-Chiari-like syndrome in a dog due to liver lobe entrapment within the falciform ligament. J. Am. Anim. Hosp. Assoc. 45: 253–256. doi: 10.5326/0450253 [DOI] [PubMed] [Google Scholar]
- 9.Litman L. M.2001. Traumatic diaphragmatic hernia in a clinically normal dog. Can. Vet. J. 42: 564–566. [PMC free article] [PubMed] [Google Scholar]
- 10.Lojszczyk-Szczepaniak A., Komsta R., Debiak P.2011. Retrosternal (Morgagni) diaphragmatic hernia. Can. Vet. J. 52: 878–883. [PMC free article] [PubMed] [Google Scholar]
- 11.Ng C. S. H., Lee T. W., Wan S., Yim A. P. C.2006. Caval foramen hernia masquerading as a thoracic mass. Can. J. Surg. 49: 64–65. [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa C. T., Schoeman J. P., Dvir E.2012. Budd-chiari-like syndrome associated with a pheochromocytoma invading the right atrium in a dog. Isr. J. Vet. Med. 67: 180–185. [Google Scholar]
- 13.Schoeman J. P., Stidworthy M. F.2001. Budd-Chiari-like syndrome associated with an adrenal phaeochromocytoma in a dog. J. Small Anim. Pract. 42: 191–194. doi: 10.1111/j.1748-5827.2001.tb01801.x [DOI] [PubMed] [Google Scholar]
- 14.Slatter D. H.2003. Textbook of Small Animal Surgery, Volume 1. pp. 471–486. In: the Diaphragm, Elsevier Health Sciences, Amsterdam. [Google Scholar]
- 15.Smillie R. P., Shetty M., Boyer A. C., Madrazo B., Jafri S. Z.2015. Imaging evaluation of the inferior vena cava. Radiographics 35: 578–592. doi: 10.1148/rg.352140136 [DOI] [PubMed] [Google Scholar]
- 16.Thrall D. E.2013. Textbook of Veterinary Diagnostic Radiology. pp. 550–563. In: the Mediastinum, 6th ed. Elsevier Health Sciences, Amsterdam. [Google Scholar]
- 17.Tiryaki T., Livanelioğlu Z., Atayurt H.2006. Eventration of the diaphragm. Asian J. Surg. 29: 8–10. doi: 10.1016/S1015-9584(09)60285-2 [DOI] [PubMed] [Google Scholar]
- 18.Tobias K. M., Johnston S. A.2013. Veterinary Surgery: Small Animal: 2-Volume Set. pp. 1380–1390. In: Diaphragmatic Hernias, Elsevier Health Sciences, Amsterdam. [Google Scholar]
- 19.Tobias S., Victoria J.2008. BSAVA Manual of Canine and Feline Thoracic Imaging. pp. 177–199. In: the Mediastinum., Blackwell Wiley, Hoboken. [Google Scholar]
- 20.Yuichi N., Kazuaki T., Tsuyoshi Y., Yoshihisa Y.2009. Caval Foramen Hernia in a Dog. J. Anim. Clin. Med. 17: 81–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.