Abstract
A 4-year-old female Siamese crocodile (Crocodylus siamensis) housed at a zoo died without any prior clinical signs. During necropsy, numerous scattered, well-demarcated, yellowish-white, firm nodules were observed throughout the liver and lungs. Microscopic examination with periodic acid-Schiff staining revealed granulomatous inflammation in the liver and lungs. Liver granulomas were characterized by the presence of a connective tissue barrier and hyphae, and the centers of the granulomas showed signs of necrosis. Lung samples showed characteristics similar to those observed in the liver samples. The fungus was identified as Aspergillus fumigatus based on its appearance on Sabouraud dextrose agar, microscopic examination with lactophenol cotton blue staining and genetic sequencing. Therefore, zoo veterinarians should pay close attention to fungal infections in captive animals.
Keywords: Aspergillus fumigatus, Crocodylus siamensis, fungus, siamese crocodile
Members of the genus Aspergillus are ubiquitous, and their spores are widely distributed [5]. Aspergillus spp. cause disease by invading body tissues and producing toxins [16]. In reptiles, systemic aspergillosis often originates from the respiratory and gastrointestinal tracts and is commonly associated with opportunistic saprophytic fungi [21]. In previous cases, aspergillosis was found in reptiles kept in environments with suboptimal conditions, such as improper temperatures, inappropriate humidity levels, poor hygiene and chronic stressors [5, 15, 16, 21, 23].
In crocodiles, fungal dermatitis and pneumonia are frequently associated with Fusarium spp., Candida spp. and Aspergillus spp. [16]. Fungal pneumonia associated with A. fumigatus and A. ustus has been reported in juvenile American alligators (Alligator mississippiensis) [6, 8]. Systemic fungal infections caused by F. solani have been described in captive saltwater crocodiles (Crocodylus porosus) and freshwater crocodiles (Cr. johnstoni) [3, 16, 17]. This is the first report of systemic aspergillosis causing the sudden death of a Siamese crocodile in Korea.
A 4-year-old female Siamese crocodile (Cr. siamensis) housed at a zoo died without any prior clinical signs. The animal was housed with a 3-year-old male Siamese crocodile in an enclosed facility at Daejeon O-World Theme Park, located in the middle of Korea (36°17ʹN, 127°23ʹE), and she was fed a diet of chicken and pork.
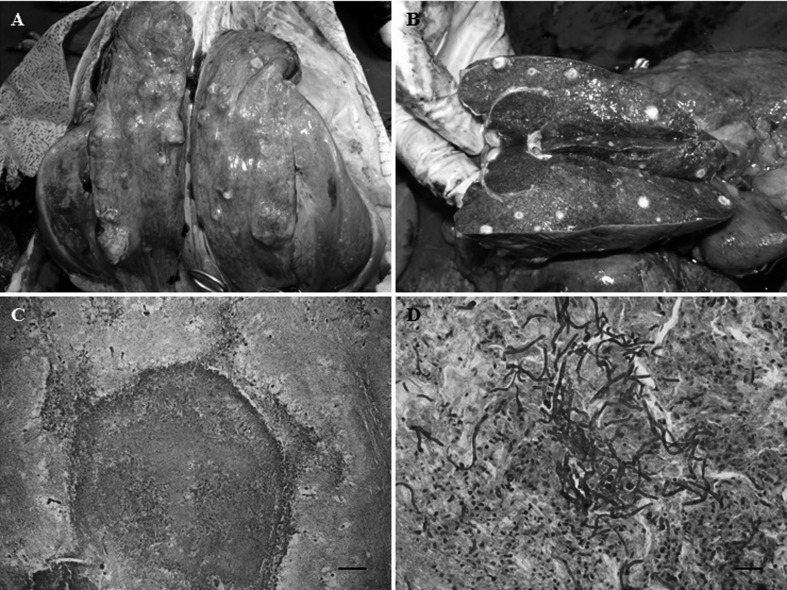
During necropsy, multifocal nodules were observed in the lung lobes (Fig. 1A). The examination of the cut surface of the liver parenchyma indicated the presence of scattered white nodules of approximately 1 cm in diameter (Fig. 1B). In the stomach, there were several ulcerations with greenish watery exudates. The remaining organs did not show any remarkable findings.
Fig. 1.
Gross appearance of the lungs and liver in the diseased Siamese crocodile at necropsy and samples of the lungs and liver stained with periodic acid-Schiff. (A) Multiple scattered nodules in the lung lobes. (B) Many white nodules (approximately 1 cm in diameter) in the parenchyma of the dissected liver. (C) Well-demarcated granuloma in the liver (×40). Bar=200 µm. (D) Hyphae and inflammatory cells in the liver parenchyma (×400). Bar=50 µm.
Lung and liver samples were submitted to the zoo laboratory for microbiological examination, and additional samples were fixed in 10% neutral buffered formalin for 2 weeks. Paraffin sections were prepared and stained with hematoxylin and eosin (H&E) for histopathological examination and with periodic acid-Schiff (PAS) for differential diagnosis of fungal infection. The sampled organs were cultured on blood agar and MacConkey agar at 37°C for 12–18 hr. In addition, a culture was performed on Sabouraud dextrose agar (SDA) at 37°C for 5 days under aerobic conditions to cultivate the fungi [10].
The histopathological examination of the liver and lungs was unremarkable. When the liver was examined with PAS staining, the proliferation of fungal hyphae within the parenchyma and granulomas were observed (Fig. 1C). The granulomas were well demarcated and surrounded by fibrous connective tissue, and the centers of the granulomas displayed signs of necrosis. The fungal elements consisted of long, branched, septate hyphae and were surrounded by heterophils, macrophages, epitheloid cells and multinucleated giant cells (Fig. 1D). Similar granulomatous changes were found in the lungs.
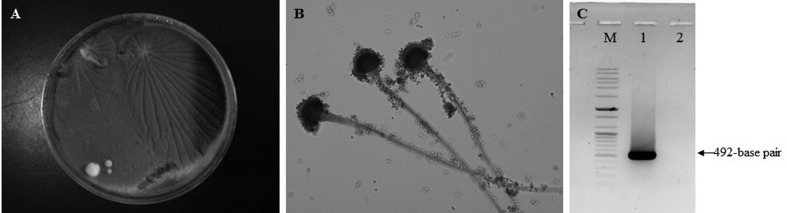
Cultures of lung and liver samples produced velvet-like, bluish-green fungal colonies on SDA (Fig. 2A); however, bacterial colonies were not observed in the culture on blood agar or MacConkey agar. Microscopic examination of samples stained with lactophenol cotton blue revealed that each fungus had a columnar conidial head and phialidae on the upper half, which are typical of A. fumigatus (Fig. 2B) [22].
Fig. 2.
Species identification of Aspergillus fumigatus cultured from the lesions was based on the appearance of colonies, microscopic observation and nucleotide sequencing. (A) Colonies cultured on Sabouraud dextrose agar appear velvet-like and bluish-green. (B) Dome-shaped colonies stained with lactophenol cotton blue show a columnar conidial head and phialidae on the upper half, which are typical of A. fumigatus (×200). (C) Electrophoresis gel showing the 492-bp amplicon of an A. fumigatus β-tubulin gene fragment. Lanes: M, 100-bp DNA ladder; 1, A. fumigatus in this study; 2, a negative sample.
In addition to the examination of morphological features, genetic sequencing was also used for species identification. DNA of A. fumigatus cultured on SDA was extracted by using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany), and PCR was performed to amplify the β-tubulin gene of A. fumigatus as described in a previous study [1]. Agarose gel electrophoresis of PCR products showed a 492-bp amplicon of A. fumigatus β-tubulin gene fragment (Fig. 2C), and the amplified PCR products were sent to Solgent (Daejeon, Korea) for nucleotide sequencing. Use of the Basic Local Alignment Search Tool revealed a high degree of homology between this sequence and those of A. fumigatus deposited in GenBank database: 98.8% (DQ438512) to 100% (KJ527255). On the basis of morphological assessments and genetic sequencing results, the causative agent was identified as A. fumigatus.
Only a few studies have reported aspergillosis in free-living reptiles, but numerous cases have been reported in captive chelonians and crocodiles [5, 7]. Most cases of systemic aspergillosis in reptiles were diagnosed at necropsy [5]. The prognosis of aspergillosis is poor, particularly when it involves a major organ or the infection has spread to multiple locations [5, 21].
The species of many fungal genera, such as Aspergillus, Penicillium, Basidiobolus, Fusarium and Mucor, have been isolated from the intestine and feces of healthy reptiles [13, 16]. A. niger, A. fumigatus, Pseudallescheria boydii, Penicillium spp., Paecilomyces spp. and Cladosporium spp. (all of which belong to the normal fungal population of the lungs) have been isolated from pulmonary lesions [11, 16]. These species usually cause opportunistic infections, but can occasionally act as primary pathogens [19]. Typically, fungal infections are due to immunosuppression, overexposure to fungal spores or stressors within the captive environment [14, 21]. In field situations, inadequate husbandry practices, poor sanitation, overcrowding, stressful conditions and heating system failure were identified as potential predisposing factors [21] that might have suppressed the immune system and caused a secondary systemic fungal infection. Because reptiles are ectotherms, their core body temperatures are more prone to fluctuations compared to those of mammals or birds. The wider variation in body temperature may favor the development of mesophilic fungi in reptiles [16].
In the present study, A. fumigatus, which causes aspergillosis, was identified by the appearance of the colonies on SDA, by microscopic examination of samples stained with lactophenol cotton blue and by genetic sequencing. Microscopic observation revealed that the fungal spores were dome-shaped, which is characteristic of A. fumigatus and A. terreus, and does not radiate as in A. niger, A. flavus and A. glaucus [22]. The color of the colonies on SDA was blue-green, which is consistent with the presence of A. fumigatus, and was not brown, as in A. terreus [22]. However, identification of A. fumigatus can be difficult, because the morphological features within that species vary and depend on growth conditions [1, 2]. Usually, the recommended treatment for systemic aspergillosis in reptiles has been a systemic antifungal chemotherapeutic, such as amphotericin B, fluconazole, mystatin, itraconazole, ketoconazole, fluorocytosine or terbinafine [5]. Because A. fumigatus resembles other species within the genus, such as A. lentulus and A. udagawae, that have low susceptibility to multiple antifungal drugs [2], it is important to identify the species by using molecular techniques.
This study and previous studies [12, 18, 20] revealed that the skin, respiratory tract and the gastrointestinal tract, are the usual routes of entry for the fungi that cause aspergillosis in reptiles. In this case, stress, improper temperature and inappropriate humidity may have predisposed the crocodile to systemic aspergillosis by facilitating mycotic invasion.
In this study, we described for the first time the sudden death of a Siamese crocodile due to systemic aspergillosis in Korea. Usually, A. fumigatus is not considered as a primary pathogen in crocodiles. However, aspergillosis in captive animals, including monkeys, vultures and penguins, has been reported in several studies [4, 9, 10]. Therefore, zoo veterinarians should pay close attention to fungal infections in captive animals and to the environmental conditions under which they are kept.
REFERENCES
- 1.Balajee S. A., Gribskov J. L., Hanley E., Nickle D., Marr K. A.2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 4: 625–632. doi: 10.1128/EC.4.3.625-632.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balajee S. A., Nickle D., Varga J., Marr K. A.2006. Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot. Cell 5: 1705–1712. doi: 10.1128/EC.00162-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buenviaje G. N., Ladds P. W., Melville L., Manolis S. C.1994. Disease-husbandry associations in farmed crocodiles in Queensland and the Northern Territory. Aust. Vet. J. 71: 165–173. doi: 10.1111/j.1751-0813.1994.tb03381.x [DOI] [PubMed] [Google Scholar]
- 4.Chege S., Howlett J., Al Qassimi M., Toosy A., Kinne J., Obanda V.2013. Opportunistic infection of Aspergillus and bacteria in captive Cape vultures (Gyps coprotheres). Asian Pac. J. Trop. Biomed. 3: 401–406. doi: 10.1016/S2221-1691(13)60084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Figueroa O., Mitchell M. A.2006. Gastrointestinal anatomy and physiology. pp. 145–162. In: Reptile Medicine and Surgery, 2nd ed. (Mader, D. R. ed.), Elsevier, St. Louis. [Google Scholar]
- 6.Hall N. H., Conley K., Berry C., Farina L., Sigler L., Wellehan J. F., Jr, Roehrl M. H., Heard D.2011. Computed tomography of granulomatous pneumonia with oxalosis in an American alligator (Alligator mississippiensis) associated with Metarhizium anisopliae var anisopliae. J. Zoo Wildl. Med. 42: 700–708. doi: 10.1638/2011-0027.1 [DOI] [PubMed] [Google Scholar]
- 7.Heatley J. J., Mitchell M. A., Williams J., Smith J. A., Tully T. N.2001. Fungal periodontal osteomyelitis in a chameleon, Furcifer pardalis. J. Herpetological Med. Surg. 11: 7–12. [Google Scholar]
- 8.Jasmin A. M., Carroll J. M., Baucom J. N.1968. Pulmonary aspergillosis of the American alligator (Alligator mississippiensis). Am. J. Vet. Clin. Path. 2: 93. [Google Scholar]
- 9.Jurczynski K., Gruber-Dujardin E., Widmer D., Kaup F. J., Mätz-Rensing K.2012. Invasive aspergillosis in a putty-nosed monkey (Cercopithecus nictitans) with adrenocortical Cushing’s syndrome. J. Med. Primatol. 41: 172–175. doi: 10.1111/j.1600-0684.2012.00538.x [DOI] [PubMed] [Google Scholar]
- 10.Kim K. T., Cho S. W., Son H. Y.2004. Aspergillus fumigatus infection in Jackass penguin (Spheniscus demersus). Korean J. Vet. Res. 44: 615–619(Korean with English abstract). [Google Scholar]
- 11.Lovely C. J., Leslie A. J.2008. Normal intestinal flora of wild Nile crocodiles (Crocodylus niloticus) in the Okavango Delta, Botswana. J. S. Afr. Vet. Assoc. 79: 67–70. doi: 10.4102/jsava.v79i2.246 [DOI] [PubMed] [Google Scholar]
- 12.Miller D. L., Radi Z. A., Stiver S. L., Thornhill T. D.2004. Cutaneous and pulmonary mycosis in green anacondas (Euncectes murinus). J. Zoo Wildl. Med. 35: 557–561. doi: 10.1638/03-096 [DOI] [PubMed] [Google Scholar]
- 13.Núñez-Otaño N. B., Piña C. I., Portelinha T. C. G., Arambarri A. M.2013. Cloacal mycobiota in wild females of Caiman latirostris (Crocodylia: Alligatoridae). Rev. Mex. Biodivers. 84: 722–726. doi: 10.7550/rmb.32425 [DOI] [Google Scholar]
- 14.Orosz S. E.2000. Overview of aspergillosis: pathogenesis and treatment options. Semin. Avian Exotic Pet Med. 9: 59–65. doi: 10.1053/AX.2000.4618 [DOI] [Google Scholar]
- 15.Pace L. W., Wirth N. R., Foss R. R., Fales W. H.1994. Endocarditis and pulmonary aspergillosis in a horse. J. Vet. Diagn. Invest. 6: 504–506. doi: 10.1177/104063879400600423 [DOI] [PubMed] [Google Scholar]
- 16.Paré J. A., Sigler L., Rosenthal K. L., Mader D. R.2006. Microbiology: fungal and bacterial diseases of reptiles. pp. 217–238. In: Reptile Medicine and Surgery, 2nd ed. (Mader, D. R. ed.), Elsevier, St. Louis. [Google Scholar]
- 17.Pawaiya R. V. S., Sharma A. K., Swarup D., Somvanshi R.2011. Pathology of mycotic gastritis in a wild Indian freshwater/marsh crocodile (Mugger; Crocodylus palustris): a case report. Vet. Med. 56: 135–139. [Google Scholar]
- 18.Peden W. M., Richard J. L., Trampel D. W., Brannian R. E.1985. Mycotic pneumonia and meningoencephalitis due to Aspergillus terreus in a neonatal snow leopard (Panthera uncia). J. Wildl. Dis. 21: 301–305. doi: 10.7589/0090-3558-21.3.301 [DOI] [PubMed] [Google Scholar]
- 19.Rødland E. K., Mattingsdal M., Olstad O. K., Ovstebø R., Kierulf P., Muller F., Frøland S. S.2008. Expression of genes in normal human monocytes in response to Aspergillus fumigatus. Med. Mycol. 46: 327–336. doi: 10.1080/13693780701874507 [DOI] [PubMed] [Google Scholar]
- 20.Roh Y. S., Park H., Cho A., Islam M. R., Chekarova I., Ejaz S. E., Lim C. W., Kim B.2010. Granulomatous pneumonia in a captive freshwater crocodile (Crocodylus johnstoni) caused by Mycobacterium szulgai. J. Zoo Wildl. Med. 41: 550–554. doi: 10.1638/2009-0237.1 [DOI] [PubMed] [Google Scholar]
- 21.Schumacher J.2003. Fungal diseases of reptiles. Vet. Clin. North Am. Exot. Anim. Pract. 6: 327–335, vi. doi: 10.1016/S1094-9194(03)00013-6 [DOI] [PubMed] [Google Scholar]
- 22.Sigler L., Verweij P. E.2003. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi. pp. 1726–1760. In: Manual of Clinical Microbiology, 8th ed. (Murray, P. R., Baron, E. J., Jorgensen, J. H., Landry, M. L. and Pfaller, M. A. eds.), ASM press, Washington, DC. [Google Scholar]
- 23.Slocombe R. F., Slauson D. O.1988. Invasive pulmonary aspergillosis of horses: an association with acute enteritis. Vet. Pathol. 25: 277–281. doi: 10.1177/030098588802500405 [DOI] [PubMed] [Google Scholar]