Abstract
One Holstein cow housed with 21 other cows exhibited clinical signs of pyrexia, anorexia and diarrhea along with severe hemoglobinuria. Hematological and biochemical analyses conducted before and after antibiotic therapy indicated severe hemolytic anemia and disruption of hepatic function. A general improvement in conditions was observed after an 11-day program of treatment comprising a regular dose of antibiotics and prescribed supportive therapies. A tentative diagnosis of bacillary hemoglobinuria was made based on the clinical and clinico-pathologic features on day 7. A molecular diagnosis was made by a PCR amplification of the flagellin gene of Clostridium haemolyticum using DNA extracted from the whole blood. The cow was diagnosed with the first recorded occurrence of bacillary hemoglobinuria of Holstein cattle in Japan.
Keywords: bacillary hemoglobinuria, Clostridium haemolyticum, flagellin gene, Holstein cattle
Bacillary hemoglobinuria (BHU; red water disease) in cattle is an acute, toxemic and highly fatal clostridial disease exhibiting the clinical signs of hemoglobinuria, fever and jaundice caused by infection of Clostridium haemolyticum [7, 8]. The major toxin produced by C. haemolyticum is a phospholipase C, known as β-toxin, which possesses disease-producing hemolytic activity. β-toxin causes necrosis of hepatocytes and hemolysis of erythrocytes, as a result, increased serum activities of hepatic enzymes and hemoglobinuria [3]. Although prompt treatment with both high doses of specific antibiotics and antitoxic serum is recommended [7, 8], the illness is of such a short duration that in most cases, affected cattle are found dead in the pasture without prior observation of clinical signs [8].
The diagnosis of BHU in living or dead cattle involves microbiological analysis for detection of C. haemolyticum in blood or tissue samples. However, it has been reported that isolation of C. haemolyticum is both time-consuming and very difficult [10, 13]. Indeed, C. haemolyticum cultures require strict anaerobic conditions, and clinical specimens are often contaminated with other anaerobic bacteria (including environmental clostridia from the soil) which grow faster than the pathogenic bacteria in culture media. Because the clinical course of BHU is rapid and the outcome is almost invariably fatal [2, 15], early treatment by administration of a large dose of specific antibiotics before full diagnosis has been recommended as a response to the appearance of typical clinical signs [1, 7, 8]. These practical limitations of sampling also make it difficult to establish an accurate diagnosis for suspected cases of BHU, once the animals have completely recovered after antibacterial therapy.
Recently, Takagi et al. [12] reported the first case of BHU in a Japanese Black cow and its complete recovery following administration of a large dose of antibiotics. Subsequently, Shinozuka et al. [11] reported the second occurrence of BHU in Japanese Black cows and their complete recovery following administration of a large dose of antibiotics. Hence, all previous available reports concerning the occurrence of BHU in Japan were about Japanese Black cows in herds managed under pasture feeding conditions [11, 12]. Furthermore, to the best of our knowledge, there has been no documented report on the occurrence of BHU in Holstein cattle. In the case study presented here, BHU is reported in a Holstein cow from a dairy herd housed and fed in Japan, which successfully recovered with the help of a regular dose of antibiotics.
In February 2016, a dairy farmer reported that a non-pregnant 4-year-old Holstein dairy cow, which was housed in tie-stalls with 21 other cows on a farm in Yamaguchi prefecture, Japan, had exhibited a decline in activities and loss of appetite during the previous one-week period. On the same day, clinical examination revealed excretion of red wine-like urine, pyrexia (39.7°C), anorexia, diarrhea (dark brown excretion), absence of rumen movement and pale (but not jaundiced) mucous membranes. A blood sample was collected from the jugular vein in a 10 ml-heparinized tube for laboratory examination. Based on these clinical signs, a provisional clinical diagnosis of bacillary pyelonephritis or cystitis was made, and the animal was treated intravenously (IV) with cefazolin (Cefamezin; cefazolin sodium salt, Intervet, Tokyo, Japan) at a dose of 5 mg/kg body weight (BW) (the recommended dosage, 5 mg/kg BW) and with IV Ringer’s solution as a supportive therapy.
Hematological examination revealed severe hemolytic anemia. As shown in Table 1, the erythrocyte count, hemoglobin concentration and hematocrit value were all decreased markedly with a macrocytic hypochromic change, while the leukocyte count was slightly increased. Biochemical analyses indicated disruption of hepatic tissues as shown by increased activities of aspartate aminotransferase (AST) and γ-glutamyltransferase (GGT). Despite the continuation of both the antibiotic and supportive therapies for five days, the clinical signs were not improved. Consequently, the antibiotic treatment (cefazolin) was replaced with Vetecillin (a mixture of ampicillin sodium salt and cloxacillin sodium salt, Meiji Seika, Tokyo, Japan) administered IV at a dose of 12 mg/kg BW (the recommended dosage, 8–12 mg/kg BW), for the following five days. On day 7, a presumptive diagnosis of BHU was made based on the specific clinical signs (including severe hemoglobinuria), and a blood sample for the diagnosis of BHU by PCR was collected.
Table 1. Hematological and biochemical data from a Holstein cow with bacillary hemoglobinuria.
| Day 1 | Day 8 | Day 15 | Reference range | |
|---|---|---|---|---|
| WBC (×103/μl) | 13.1* | 21.0* | 8.2 | 4.0–12.0 |
| RBC (×106/μl) | 1.73* | 0.85* | 1.61* | 5.0–10.0 |
| Ht (%) | 8.6* | 7.0* | 14.3* | 24–46 |
| Hb (g/dl) | 3.6* | 2.4* | 4.0* | 8–15 |
| MCV (fl) | 49.7 | 82.4* | 88.8* | 40–60 |
| MCHC (g/dl) | 41.9* | 34.3 | 28.0* | 30–36 |
| TP (g/dl) | 8.0 | 7.6 | 7.1 | 5.7–8.1 |
| A/G | 0.74* | 0.72* | ND | 0.79–1.21 |
| AST (U/l) | 473* | UM | 90* | 35–80 |
| GGT (U/l) | 46* | 200* | 124* | 6–17 |
| BUN (mg/dl) | 18.5 | 14.6 | 3.7* | 6–27 |
| Cre (mg/dl) | ND | 0.7 | 0.7 | 1–2 |
| Glu (mg/dl) | 72 | 76* | 60 | 45–75 |
| T-Cho (mg/dl) | 174 | 141 | 75 | 65–220 |
| Na (mmol/l) | 141 | 137* | 144 | 138–152 |
| Cl (mmol/l) | 101 | 95* | 100 | 97–111 |
| Ca (mg/dl) | 9.2 | 9.0 | 8.9 | 8.5–12 |
WBC, leukocyte count; RBC, erythrocyte count; Ht, hematocrit value; Hb, hemoglobin concentration; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; TP, total protein concentration; AST, aspartate aminotransferase activity; GGT, γ-glutamyltransferase activity; BUN, blood urea nitrogen concentration; Cre, creatinine concentration; Glu, glucose concentration; T-Cho, total cholesterol concentration. *Data outside the reference range. ND: not determined. UM: unmeasurable due to severe hemolysis.
On day 8, blood and urine samples were obtained from this cow for evaluating the effectiveness of the course of therapies. The subsequent hematological and urinary tests still demonstrated severe anemia and disruption of hepatic function (Table 1). Additionally, hemoglobinuria was detected at a moderate level (2+) using a urine test strip (Multistix; Siemens, Tokyo, Japan). However, no hemotrophic parasite was observed by morphological examination of the erythrocytes on blood smears. On day 9, ultrasound examination of the bladder was conducted, but no abnormalities (e.g. cystitis) were observed. After the series of antibiotic treatments, appetite and rumen motility gradually recovered (by day 8), and normal excretions of both urine and feces were observed on day 9. On day 11, the cow was evaluated as being in remission, and the antibiotic therapy was finished. On day 15, a general health check was conducted, and a blood sample was taken in order to evaluate the remission. The apparent clinical improvements were confirmed by the recoveries of appetite and rumen movements and the normal excretions of urine and feces. The hematological results indicated that hemolysis and hepatic disruption had ceased. Moreover, the related parameters were mid-course to the original values, although most of the parameters were still out of the normal reference range (Table 1). Based on these observations, it was considered that the cow had recovered completely from the sickness. During the clinical course, culture examination and analysis of β-toxin were not performed using the cow’s blood and urine specimens.
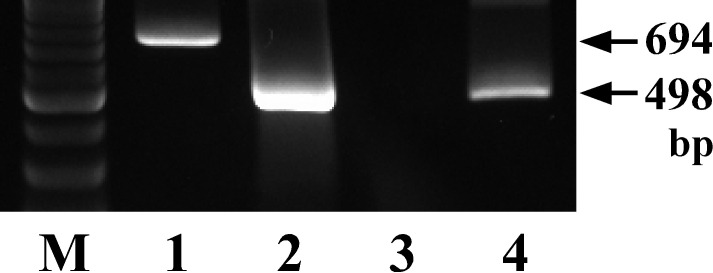
Using the blood sample collected on day 7, molecular diagnosis of C. haemolyticum infection was conducted using a nested PCR procedure targeting the flagellin gene as previously reported [12]. The result of the nested PCR procedure is shown in Fig. 1. The result indicated that a PCR band was observed with a size similar to that obtained using control DNA from a standard strain ATCC 9650T of C. haemolyticum [9]. There was no amplified band observed in blood from healthy cows used as negative controls (data not shown). The result suggested that the case was infected with C. haemolyticum.
Fig. 1.
PCR amplification of DNA from a standard strain ATCC 9650T of Clostridum haemolyticum (lane 1: first PCR, lane 2: second PCR) and a whole blood sample obtained from a Holstein cow with bacillary hemoglobinuria (lane 3: first PCR, lane 4: second PCR) using a nested PCR method targeting the flagellin gene. Lane M shows the molecular size markers. bp: base pairs.
In Japan, the first occurrence of BHU was reported in Japanese Black cows in Kagoshima prefecture in 2009 [12], and the second occurrence was also reported in Japanese Black cows as well in Hiroshima prefecture in 2011 [11]. Before that, there were several reports on bovine cases suspected with BHU in various regions in Japan, which were not diagnosed definitively and microbiologically [6]. More recently, several other cases of BHU-like infection have occurred in Japanese Black cattle in other regions in Japan (personal communication, OY). These reports and information suggest that BHU cannot be described as a rare infectious disease among cattle in Japan. In cattle herds fed on natural pastures (especially in Japanese Black cattle), animals can intake spores of C. haemolyticum from the spore-contaminated pasture. However, to our knowledge, the present case study reports the first occurrence of BHU in Holstein dairy cattle in Japan. This report suggests the possibility that BHU can occur everywhere in Japan in cattle fed not only on pasture but also under the environment of a tie-stall housed feeding management system, although the route of contamination and infection was not known in the present case.
Our previous reports of BHU in Japanese Black cattle suggested that the dose, route and timing of antibiotic administration at the first treatment were critical for achievement of an effective blood concentration of the antibiotic against C. haemolyticum and eventual recovery from the illness [11, 12]. The actual cases that recovered successfully had required an early presumptive diagnosis of BHU after observation of similar clinical signs and sudden death in preceding cases, and a prompt administration of a large dose of antibiotics. Interestingly, the present Holstein cow successfully recovered as a sporadic case with the help of a regular dose of antibiotics. The reasons for these different pathological conditions between our previous cases [11, 12] and the present case are not known.
Under natural conditions in endemic areas, bacterial invasion originates in the alimentary tract after ingestion of contaminated material, and fascioliasis can later act as a triggering factor that creates focal anaerobic conditions in the liver for the germination of dormant spores [5, 7, 8, 14]. Our previous BHU cases occurred in cattle herds fed on fascioliasis-contaminated pastures [11, 12]. However, based on the epidemiological examination, fascioliasis was absent in the Holstein cattle herd including the present case. Moreover, based on moderate increased levels of AST and GGT and the absence of signs of jaundice at the period of onset in the present case, the damage in the hepatic tissue was not as severe when compared with that observed in previous reported cases [11, 12]. Indeed, in the previous cases, the affected cattle exhibited considerably higher AST activity (>1,000 U/l) at the period of onset. Therefore, the difference in hepatic damage due to the differences in the infection status regarding fascioliasis between the previous cases and the present case may be one of the possible causes for the different pathologic conditions of C. haemolyticum infection. Other possible causes may include a difference in virulence due to different strains of C. haemolyticum and different sensitivities of cattle breeds against C. haemolyticum and/or β-toxin.
Apart from the different pathological conditions between Japanese Black cattle and Holstein cattle, one important reason for the observed occurrences of BHU in cattle in Japan may be that no vaccine for C. haemolyticum has been produced and used in Japan. However, vaccines are used in cattle worldwide (including a toxoid of the β-toxin), and vaccination is recommended for prevention and control of BHU, especially in fluke-infested areas [4, 7, 8]. Therefore, the production and usage of a vaccine may be recommended for the prevention and control of BHU in Japan.
Our previous nested PCR technique targeting the flagellin gene is useful for rapid and accurate detection of C. haemolyticum in suspected cases of BHU even about three weeks after initiation of antibiotic therapy [11]. The reason why the PCR analysis detects the flagellin gene of the bacteria for such a long period after antibiotic therapy may be that components of dead bacteria still continue to circulate in the blood of cattle once infected by the bacteria. In the present case, a whole blood sample collected from the sick cow on day 7 after initiation of antibiotic therapy was used. The results of the present case confirm both the validation of this nested PCR method and its practical usefulness for the diagnosis of BHU regardless of antibiotic administration.
In conclusion, this is the first report in Japan of a Holstein cow with BHU caused by C. haemolyticum infection. The past and present cases of BHU reported in Japanese cattle herds suggest that BHU is not a rare illness among cattle in Japan and that it is not restricted to fascioliasis-contaminated cattle herd fed on natural pasture. Therefore, it is necessary to add BHU to a differential diagnosis list when encountering animals with acute and severe hemoglobinuria.
REFERENCES
- 1.Crowe S. P., Moss E. W.1989. Alberta. Bacillary hemoglobinuria in a beef herd. Can. Vet. J. 30: 681. [PMC free article] [PubMed] [Google Scholar]
- 2.Harwood D. G., Watson E. N.2004. Clostridial infection in young cattle-an update. Cattle Pract. 12: 219–226. [Google Scholar]
- 3.Hauer P. J., Yeary T. J., Rosenbusch R. F.2004. Cloning and molecular characterization of the beta toxin (phospholipase C) gene of Clostridium haemolyticum. Anaerobe 10: 243–254. doi: 10.1016/j.anaerobe.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 4.Hjerpe C. A.1990. Bovine vaccines and herd vaccination programs. Vet. Clin. North Am. Food Anim. Pract. 6: 167–260. doi: 10.1016/S0749-0720(15)30903-8 [DOI] [PubMed] [Google Scholar]
- 5.Janzen E. D., Orr J. P., Osborne A. D.1981. Bacillary hemoglobinuria associated with hepatic necrobacillosis in a yearling feedlot heifer. Can. Vet. J. 22: 393–394. [PMC free article] [PubMed] [Google Scholar]
- 6.Nakao A.1988. Sudden death with hemoglobinuria and hepatic focal necrosis in a cow. J. Hokkaido Vet. Med. Assoc. 32: 133–155(in Japanese; the title was translated into English by the corresponding author, OY). [Google Scholar]
- 7.Oliver O., Staempfli H.1999. Bacillary hemoglobinuria, braxy, and black disease. pp. 386–387. In: Current Veterinary Therapy 4: Food Animal Practice (Howard, J. L. ed.), WB Saunders Company Ltd., Philadelphia. [Google Scholar]
- 8.Radostits O. M., Gay C. C., Blood D. C., Hinchcliff K. W.2000. Diseases caused by bacteria −II. pp. 767–769. In: Veterinary Medicine, 9th ed. (Radostits O. M., Gay C. C., Blood D. C. and Hinchcliff K. W., eds.), WB Saunders, Philadelphia. [Google Scholar]
- 9.Sasaki Y., Kojima A., Aoki H., Ogikubo Y., Takikawa N., Tamura Y.2002. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet. Microbiol. 86: 257–267. doi: 10.1016/S0378-1135(02)00002-0 [DOI] [PubMed] [Google Scholar]
- 10.Sasaki Y., Takikawa N., Kojima A., Norimatsu M., Suzuki S., Tamura Y.2001. Phylogenetic positions of Clostridium novyi and Clostridium haemolyticum based on 16S rDNA sequences. Int. J. Syst. Evol. Microbiol. 51: 901–904. doi: 10.1099/00207713-51-3-901 [DOI] [PubMed] [Google Scholar]
- 11.Shinozuka Y., Yamato O., Hossain M. A., Higaki T., Ishikawa I., Ichiba S., Takagi M.2011. Bacillary hemoglobinuria in Japanese black cattle in Hiroshima, Japan: a case study. J. Vet. Med. Sci. 73: 255–258. doi: 10.1292/jvms.10-0231 [DOI] [PubMed] [Google Scholar]
- 12.Takagi M., Yamato O., Sasaki Y., Mukai S., Fushimi Y., Yoshida T., Mizukami K., Shoubudani T., Amimoto K., Chuma T., Shahada F., Endo Y., Deguchi E.2009. Successful treatment of bacillary hemoglobinuria in Japanese Black cows. J. Vet. Med. Sci. 71: 1105–1108. doi: 10.1292/jvms.71.1105 [DOI] [PubMed] [Google Scholar]
- 13.Uzal F. A., Belak K., Rivera E., Robles C. A., Feinstein R. E.1992. Bacillary haemoglobinuria diagnosis by the peroxidase-antiperoxidase (PAP) technique. Zentralbl. Veterinarmed. B. 39: 595–598. [DOI] [PubMed] [Google Scholar]
- 14.Vanness G. B.1964. Ecology of bacillary hemoglobinuria. J. Am. Vet. Med. Assoc. 144: 492–496. [PubMed] [Google Scholar]
- 15.Vine N., Fayers J., Harwood D.2006. Bacillary haemoglobinuria in dairy cows. Vet. Rec. 159: 160. doi: 10.1136/vr.159.5.160 [DOI] [PubMed] [Google Scholar]