Abstract
Introduction
There is broad consensus that high-grade basal proteinuria and failure to achieve remission of proteinuria are key determinants of adverse renal prognosis in patients with primary membranous nephropathy. Since current regimens are not ideal due to short- and long-term toxicity and propensity to relapse after treatment withdrawal, we developed a treatment protocol based on a novel combination of rituximab and cyclosporine that targets both the B-cell and T-cell limbs of the immune system. Herein, we report pilot study data on proteinuria and changes in autoantibody levels and renal function that offer a potentially effective new approach to treatment of severe membranous nephropathy.
Methods
Thirteen high-risk patients defined by sustained high-grade proteinuria (mean 10.8 g/d) received combination induction therapy with rituximab plus cyclosporine for 6 months, followed by a second cycle of rituximab and tapering of cyclosporine during an 18-month maintenance phase.
Results
Mean proteinuria decreased by 65% at 3 months and by 80% at 6 months. Combined complete or partial remission was achieved in 92% of patients by 9 months; 54% achieved complete remission at 12 months. Two patients relapsed during the trial. All patients with autoantibodies to PLA2R achieved antibody depletion. Renal function stabilized. The regimen was well tolerated.
Discussion
We report these encouraging preliminary results for their potential value to other investigators needing prospectively collected data to inform the design and power calculations of future randomized clinical trials. Such trials will be needed to formally compare this novel regimen to current therapies for membranous nephropathy.
Keywords: cyclosporine, membranous nephropathy, nephrotic syndrome, rituximab
Primary membranous nephropathy (MN) is an autoimmune disorder caused by antibodies to constitutive antigens of glomerular podocytes.1, 2 Cell surface antigen–antibody complexes are capped into aggregates and shed from podocytes where they bind to and accumulate along the external lamina of the glomerular basement membrane. Complement is activated by the immune complexes and is a key factor leading to the glomerular proteinuria.
The natural history of MN is variable3, 4, 5 and likely depends on ambient levels of circulating pathogenic autoantibodies. Remissions can occur spontaneously,4, 5 presumably by restoration of autoregulation of normal antibody production, or can be gained by treatment-induced suppression of pathogenic autoantibodies. Approximately one-quarter of patients with MN undergo spontaneous remission, while the vast majority are prone to persistent high-grade proteinuria, approximately one-half of whom are at risk of progression to renal failure.6
Given the potential toxicities of traditional immunomodulatory drugs, decisions regarding therapy must take into account the natural history of the disease and objective efficacy of the various therapeutic options balanced against the risks of protracted nephrotic syndrome (NS) and loss of renal function, as well as risks of drug toxicities. Current guidelines support limiting the use of immunosuppressive treatment to patients who are considered at medium and high risk of progression to end-stage kidney disease (ESKD) based on clinical observations acquired over time.6, 7, 8, 9 Accepted treatment options include combination therapy with glucocorticoids and a cytotoxic alkylating agent or calcineurin inhibitors (CNIs).9 Cytotoxic-based regimens are often considered as first-line therapy for patients at high risk of progression,9, 10 but potential short- and long-term adverse effects of cytotoxic drugs (bone marrow suppression, infertility, as well as infection and malignancy diathesis with greater cumulative exposure) greatly influence therapeutic decisions.11, 12 CNIs lead to earlier reductions in proteinuria but are associated with high relapse rates (occurring in almost 50% within a year of drug withdrawal);13, 14, 15, 16 these considerations usually lead to prolonged therapy with its attendant risks, particularly nephrotoxicity. In the continued search for new treatments that might offer higher therapeutic indices, there has been growing enthusiasm for use of the B cell–depleting agent rituximab for MN based on the central role in disease pathogenesis of IgG autoantibodies to M-type phospholipase A2 receptor (PLA2R),17 and other glomerular antigens.18, 19 Encouraging results from case series and uncontrolled pilot trials of rituximab have been reported.20, 21, 22, 23, 24 However, small series reported to date show mostly delayed and partial remissions of NS, as well as a propensity to relapse after single courses of rituximab.
In an effort to overcome these unresolved issues and limitations of conventional therapies for MN, we initiated a prospective single-arm pilot study to investigate whether “induction” treatment with the combination of rituximab plus a 6-month course of cyclosporine followed by a “maintenance” course of rituximab might lead to earlier, more complete and durable clinical and immunologic remissions of MN than either agent alone. We hypothesized that cyclosporine and rituximab would act synergistically, as they have different effects on the immune system (T and B cells, respectively) and on the podocyte, and distinct onset of action (early vs. delayed, respectively) as well as duration of action. We envisioned that such pilot studies were necessary to acquire data that would inform the design and power calculations for testing rituximab-based combination therapies in the future. We considered that the preliminary results regarding safety and efficacy of this regimen were informative and merited early publication.
Materials and Methods
Patients aged 18 years and older with biopsy-proven MN were eligible to participate in this study. Patients were required to have persistent nephrotic-range proteinuria (>3.5 g/d proteinuria) after a minimum observation phase of 6 months and at least 2 months of treatment with renin–angiotensin system blockade. Presence of PLA2R autoantibody in serum or in glomerular deposits was not required for inclusion. Exclusion criteria included estimated glomerular filtration rate (eGFR) <40 ml/min per 1.73 m2 (determined by the 2009 CKD-EPI creatinine equation25), prior treatment with CNI for ≥6 months, any previous treatment with rituximab, pregnancy, nursing mothers, or subjects not practicing birth control. Patients with an active infection, diabetes, or a likely secondary cause of MN were excluded. The NIDDK Institutional Review Board approved the protocol. All participants provided informed consent as per the Declaration of Helsinki for Medical Research Involving Human Subjects. The study was performed at the NIH Clinical Center in Bethesda, Maryland. Rituximab was provided by Genentech through its Investigator Sponsored Trials program. Genentech did not participate in study design, data collection, or analysis or writing of the report.
Run-in Period
Potential participants were managed with standard supportive therapy for a minimum of 6 months prior to study enrollment (“observation phase”) in order to assess for spontaneous recovery. During this phase, they received a regimen of angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), or both, along with adjunctive antihypertensives if necessary to achieve target systolic blood pressure of <130 mm Hg, statins for control of lipids, dietary sodium restriction, and loop diuretics to control edema. Patients were eligible for enrollment in the treatment trial after the observation phase if they had persistent nephrotic-range proteinuria that did not show evidence of decline from baseline. Earlier initiation of immunosuppression was allowed if the patient suffered from a significant complication of the NS, such as a thrombotic event.
Immunosuppressive Regimen
Experimental treatment consisted of “induction” with rituximab plus oral cyclosporine followed by “maintenance” rituximab. Both cyclosporine and rituximab were initiated on day 1 of the formal trial period. Cyclosporine (Gengraf) was initiated at a dose of 3 mg/kg/d, given in divided equal doses at 12-hour intervals. The dose was adjusted according to 12-hour trough blood concentrations to achieve a concentration of 125 to 190 μg/l and to avoid toxicity. The first cycle of rituximab was given at a dose of 1000 mg i.v. on day 1 and day 15. After 6 months of therapy during the induction phase, cyclosporine was tapered at a rate of 50 mg/d every 3 weeks to discontinuance during the maintenance phase. Therefore, the duration of the taper could vary among patients (i.e., occurring over 9 to 21 weeks) depending on the total dose of cyclosporine that each individual was taking during the induction phase. All patients were retreated with a second cycle of rituximab (same dose and 15-day interval) when the following criteria were fulfilled: a minimum of 6 months lapsed since the first dose of rituximab and CD19+ B-cell count was ≥5 cells/μl (confirmed on 2 values at least 2 weeks apart). Depending on the pace of B-cell recovery in each individual, the second cycle of rituximab could be administered at any point during the cyclosporine taper. Treatment with the second cycle of rituximab was independent of the remission status.
To reduce the frequency and severity of rituximab infusion reactions, patients were premedicated with oral acetaminophen (1000 mg), oral diphenhydramine hydrochloride (50 mg), and 100 mg methylprednisolone i.v. before each infusion. Prophylactic antibacterial or antiviral agents were not routinely initiated, with the following exception: patients with a history of hepatitis B exposure (HBV surface antigen–negative/core antibody–positive, HBV DNA–negative) were treated prophylactically with lamivudine. Prophylactic anticoagulation was not initiated.
To avoid confounding the interpretation of renal outcomes, dose escalations of drugs that block the renin–angiotensin–aldosterone system were not permitted once immunosuppressive treatment was initiated, but dose reductions were permitted if clinically indicated. Addition of other antihypertensive agents were permitted as needed to achieve target blood pressure control. Patients were followed for a minimum of 24 months, the duration of the trial. Extended follow-up beyond the end of the trial continues regardless of remission status.
Assessments
Clinical and laboratory parameters were collected at study entry, at 6 weeks, and then at 3-month intervals until the end of the formal trial period. Blood pressure, weights, complications of the NS, and side effects of therapy were registered at every visit. Laboratory parameters included complete blood counts, electrolytes, lipid panels, serum albumin, and serum immunoglobulins. Cyclosporine levels were measured by immunoassay. Quantification of T, B, and natural killer cells was performed on whole blood (red blood cell lysis method) using a BD FACS Canto flow cytometer (Becton, Dickinson and Company, Franklin Lakes, New Jersey). Protein excretion was assessed by 24-hour urine collections and was considered accurate when 24-hour creatinine excretion was consistent with baseline values. Spot urine protein–creatinine ratios were also collected. Additional urinary studies included routine urinalysis and direct microscopic examination of urine sediment (to assess for dysmorphic red blood cells, white blood cells, casts, and fat droplets). An inactive urine sediment was defined as <3 red blood cells per high-power field, <5 white blood cells per high-power field, and absence of casts. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used for eGFR. Circulating anti-PLA2R antibodies were determined using a commercially available enzyme-linked immunosorbent assay kit (EUROIMMUN US, Mountain Lakes, NJ) that contained PLA2R1-coated microplates (described in Supplementary Methods online). Titer values higher than 20 relative units (RU)/ml are positive; titers of 14 to 20 RU/ml are considered borderline positive.
Outcome Measures
The primary outcome was the safety profile of the regimen including serious adverse events and drug-related adverse events. A serious adverse event was defined as any adverse event or reaction that results in death, is life-threatening, results in hospital admission or extends the length of an existing hospital stay, results in persistent or serious disability or incapacity, or, based on appropriate medical judgment, may jeopardize the subject’s health and may require medical or surgical intervention to prevent one of the outcomes outlined above. Adverse events were graded based on the NCI Common Terminology Criteria for Adverse Events version 4.0. Secondary outcomes included the proportion of subjects that achieved a complete or partial remission at 3, 6, 9, 12, 18, and 24 months, time to remission, and the proportion of patients with relapse. Remission status was based on degree of proteinuria measured with 24-hour urinary collection and confirmed with a second collection at least 4 weeks apart. If there was an unexplained discrepancy in repeat proteinuria values such that it changed remission status at a particular time point, the higher of the 2 proteinuria values was selected. Complete remission was defined as proteinuria ≤0.3 g per 24 hours, partial remission as proteinuria ≤3.5 g per 24 hours and a >50% reduction from baseline proteinuria, and non-response as <50% reduction in baseline proteinuria or worsening of proteinuria. Relapse was defined as reappearance of proteinuria to ≥3.5 g per 24 hours and at least 50% higher than the lowest post-treatment value in those who previously achieved a partial or complete remission.
Statistical Methods
This is a pilot study of the safety and feasibility of the treatment regimen. Thus, all efficacy analyses are deemed as hypothesis-generating rather than testing an a priori hypothesis. Data are summarized using counts and percentages for categorical variables and means ± SD, medians, and, at times, interquartile ranges and percentage change in values relative to baseline for continuous variables. Descriptive statistics include only values for the first 13 patients who have data complete for the 24-month trial period. If a patient met criteria for relapse, they were treated “off protocol” with rituximab and continued to be followed to gather information about safety. However, efficacy data for these patients from that point forward were not included in the analysis. For purposes of calculating percentages, where applicable (i.e., percentage of remissions), all 13 patients are included in the denominator. For drawing inferences about percentages of participants experiencing partial or complete remission, we employ Clopper–Pearson exact binomial confidence intervals. The percent change in urine protein was modeled without transformation (a longitudinal model with general mean profile over time and random intercepts and slopes capturing within-individual dependence); thus, without relying on normal approximations, 95% confidence intervals were estimated using a data resampling method (2.5% and 97.5% percentile values from 1000 bootstrap replicates). Where applicable, we employ a statistical significance level of 0.05 for P value. For drawing inferences about trends over the first 24 months, we employ tests and models based on linear regressions for each individual, with distinct rates of change during the “induction” phase (baseline to 6 months) and “maintenance” phase (from 6 to 24 months) with the exception of percent change from baseline urine protein; see Supplementary Methods for greater detail. All summaries, tests, and models are calculated using statistical software SAS version 9.3 (SAS Institute Inc., Cary, NC) and R version 3.0 (www.r-project.org).
Results
Fifty-two patients were screened for eligibility to participate in the experimental treatment phase of the trial. Sixteen patients met entry criteria and enrolled in the treatment phase by December 2014. Data on 13 patients who completed 24 months of follow-up were analyzed in this report. Mean (± SD) follow-up was 41 ± 11 months (range, 24–56). Demographic and clinical attributes of patients at study enrollment (initiation of protocol immunosuppression drugs) are presented in Table 1. Participants were mostly male and Caucasian and had a mean age of 50 years (range, 21–72). Mean time from most recent renal biopsy to study enrollment was 11 months (range, 6–24 months). Four patients had received treatment with other immunosuppressive agents (alkylating agents, mycophenolate mofetil, steroids) prior to trial enrollment but were resistant or relapsed. At enrollment, all patients were severely nephrotic with mean protein excretion of 10.8 g per 24 hours, and had marked hypoalbuminemia with mean serum albumin of 1.8 mg/dl (range, 0.9–2.7 g/dl). Six patients were treated with ACEi monotherapy, 3 were treated with ARB, 2 were on a combination of ACEi and ARB, and 2 patients could not tolerate this class of drugs due to hypotension. Seven patients had persistent hypertension despite escalating doses of ACEi and/or ARB and required addition of other immunosuppression antihypertensive agents prior to initiation of study drugs.
Table 1.
Demographics and clinical characteristics at study enrollment
| Variable | |
|---|---|
| Number of patients | 13 |
| Sex % | |
| Male/Female | 56/44 |
| Age at diagnosis (yr) | 49.9 ± 13.4 |
| Race or ethnic group (%) | |
| White/Black/Asian/Hispanic | 68/13/13/6 |
| Serum creatinine (mg/dl) | 1.36 ± 0.14 |
| eGFR CKD-EPI (ml/min/1.73 m2) | 62 ± 23 |
| Proteinuria (grams/24 h) | 10.8 ± 2.8 |
| Serum albumin (g/dl) | 1.8 ± 0.5 |
| Hypertension | 67% |
| Urinary abnormalities (% patients) | |
| Dipstick | |
| Hemoglobin ≥1+ | 81% |
| Glucosuria ≥1+ | 23% |
| Sediment (microscopic examination) | |
| Dysmorphic RBCs/acanthocytes | 68% |
| RBC casts | 0% |
| Fatty casts | 43% |
| Fat droplets | 100% |
Study enrollment defined as initiation of protocol immunosuppressive drugs. Data presented as mean ± SD for continuous variables.
eGFR, estimated glomerular filtration rate; RBC, red blood cell.
Clinical Outcome
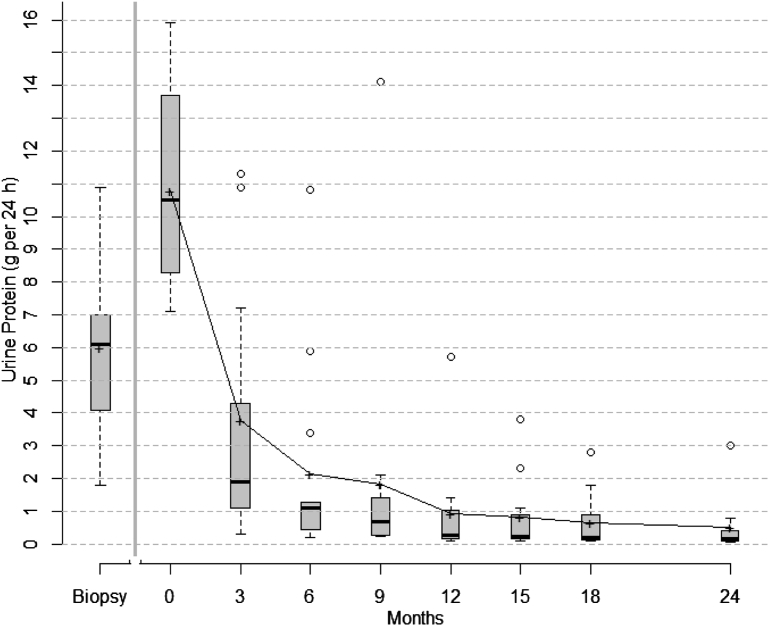
Changes in proteinuria for individual patients and percentages of remissions over time are shown in Table 2. During the observation phase, proteinuria increased in all patients from 6 g per 24 hours at time of renal biopsy to 10.8 g per 24 hours at the start of protocol immunosuppressive treatment. After initiation of immunosuppressive study drugs, mean proteinuria progressively decreased (Table 2 and Figure 1). Overall, there was a 65% reduction in mean proteinuria from baseline values within 3 months of starting therapy (95% confidence interval: 53%–80%) and an 80% reduction of proteinuria from baseline values within 6 months (95% confidence interval: 68%–92%). Eight patients (61%) experienced rapid reduction in proteinuria, achieving either partial or complete remission by 3 months. By 6 months, 85% of patients had achieved remission prior to receiving the second cycle of rituximab. By 12 months, more than half of patients (54%) achieved complete remission and maintained it for the duration of the trial period.
Table 2.
Changes in urinary protein excretion (g per 24 hours) in individual patients from time of diagnosis to study enrollment to 24 months and percentages of remission achieved over time
| Patient number | Proteinuria (g/24 h) at time of diagnosis by renal biopsy (pre-enrollment) | Proteinuria (g/24 h) at start of protocol treatment (enrollment) | 3 mo | 6 mo | 9 mo | 12 mo | 15 mo | 18 mo | 24 mo |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.8 | 10.6 | 10.9 | 10.8 | 14.1 | 5.7 | 3.8 | 1.8 | 0.80 |
| 2 | 8.3 | 10.5 | 1.1 | 0.20 | 0.30 | 0.10 | 0.20 | 0.10 | 0.20 |
| 3 | 9.1 | 14.1 | 11.3 | 3.4 | 2.1 | 1.4 | 1.1 | 0.80 | 0.52 |
| 4 | 6.9 | 13.9 | 1.1 | 0.98 | 0.35 | 0.21 | 0.1 | 0.10 | 0.10 |
| 5 | 5.3 | 8.0 | 1.9 | 1.27 | 0.68 | 0.70 | 0.67 | 1.0 | 3.5 (R) |
| 6 | 7.0 | 9.8 | 7.2 | 5.9 | 2.1 | 7.4 (R) | 2.7 | 1.6 | 0.69 |
| 7 | 6.1 | 9.6 | 1.23 | 0.22 | 0.24 | 0.15 | 0.18 | 0.17 | 0.10 |
| 8 | 10.9 | 11.2 | 4.3 | 1.23 | 0.26 | 0.28 | 0.16 | 0.16 | 0.13 |
| 9 | 3.5 | 15.9 | 2.9 | 1.2 | 1.4 | 1.34 | 2.3 | 2.8 | 3.0 |
| 10 | 1.8 | 8.3 | 4.0 | 0.44 | 0.27 | 0.26 | 0.21 | 0.24 | 0.05 |
| 11 | 6.0 | 7.3 | 1.5 | 0.85 | 0.85 | 0.21 | 0.23 | 0.15 | 0.17 |
| 12 | 4.1 | 13.7 | 1.1 | 1.1 | 0.78 | 0.59 | 0.66 | 0.39 | 0.37 |
| 13 | 6.2 | 7.1 | 0.30 | 0.20 | 0.23 | 0.10 | 0.10 | 0.11 | 0.10 |
| Mean proteinuria, g/24 h (± SD) | 6±2.6 | 10.8±2.8 | 3.8±3.7 | 2.1±3.1 | 1.8±3.8 | 0.9±1.6 | 0.8±1.1 | 0.7±0.9 | 0.5±0.9 |
| % PR | - | - | 54% [25,81] | 62% [32,86] | 54% [25,81] | 31% [9,61] | 31% [9,61] | 38% [14,68] | 31% [9,61] |
| % CR | - | - | 7% [0.2,36] | 23% [5,54] | 38% [14,68] | 54% [25,81] | 54% [25,81] | 54% [25,81] | 54% [25,81] |
| Total remissions, % | - | - | 61% [32,86] | 85% [55,98] | 92% [64,100] | 85% [55,98] | 85% [55,98] | 92% [64,100] | 85% [55,98] |
Proteinuria values based on 24-hour urine collection. Individual patient values shown as well as mean values and percentages of remission at each time point with 95% confidence intervals. Mean proteinuria increased during the observation phase (period from diagnosis to enrollment) despite initiation and escalation of angiotensin antagonists. Initiation of protocol immunosuppressive drugs led to decrease in proteinuria at all time points. There were 2 relapses (R) during the trial period. After relapse, patients were treated off protocol with rituximab. Efficacy data from that point forward were not included in the analysis; their proteinuria values (i.e., mean proteinuria) are included in the table to show response. However, for purposes of calculating percentages of remissions, all patients are included in the denominator even if relapsed.
CR, complete remission; PR, partial remission.
Figure 1.
Box plots of urinary protein excretion from time of diagnosis (biopsy) to study initiation/enrollment (time 0) to 24 months. Proteinuria increased during the observation phase (from diagnostic biopsy to time 0). After initiation of therapy, there was a rapid reduction in proteinuria within 3 months. The top and bottom of the box are the estimated 75th and 25th percentiles, respectively. The horizontal lines and “+” signs within each box represent the median and mean values, respectively. The vertical dashes denote the largest as well as the smallest data point that is within 1.5 times the interquartile range (75th to 25th percentile) above the 75th percentile or below the 25th; data points outside of this range are denoted by open circles. After relapse, patients were treated off protocol. Outcomes and efficacy data of relapsed patients from that point forward were not included in the analysis.
Reduction in proteinuria was accompanied by a progressive increase in mean serum albumin levels (Table 3). There was a concomitant improvement in serum IgG levels with most achieving near-normal levels by 3 months. Complete remission of proteinuria was associated with an inactive urine sediment and absence of hematuria and glucosuria. Trends in other relevant laboratory and clinical parameters are shown in Table 3.
Table 3.
Laboratory and clinical parameters from study enrollment to 24 months
| Study enrollment | 3 mo | 6 mo | 9 mo | 12 mo | 15 mo | 18 mo | 24 mo | |
|---|---|---|---|---|---|---|---|---|
| Serum albumin (g/dl) (Ref. range: 3.5–5.2) |
1.8±0.5 | 3.2±0.6 | 3.5±0.7 | 3.6±0.7 | 3.8±0.6 | 3.8±0.4 | 3.8±0.5 | 3.9±0.4 |
| Cholesterol (mg/dl)a | 267±97 | 215±61 | 201±59 | 191±52 | 189±54 | 162±23 | 184±34 | 172±47 |
| Serum IgG (mg/dl) (Ref. range: 700–1600) |
465±146 | 720±109 | 844±133 | 820±172 | 862±206 | 1021±183 | 973±150 | 1009±117 |
| Serum IgM (mg/dl) (Ref. range: 40–230) |
85.3±62.1 | 79.6±62.4 | 86.3±51.4 | 62±39.0 | 73.5±55.7 | 73.6±61.8 | 75.7±47.5 | 66.4±52.8 |
| White blood cells (K/μl) | 5.7±1.7 | 5.6±1.8 | 6.6±3.4 | 5.7±2.0 | 6.0±2.0 | 5.9±2.4 | 6.3±2.6 | 6.0±3.1 |
| Hemoglobin (g/dl) | 11.4±1.8 | 11.5±1.6 | 11.1±1.4 | 11.7±1.2 | 12.1±1.3 | 12.2±1.4 | 12.5±1.4 | 12.3±1.1 |
| CD19 (cells/μl)b (Ref. range: 61–321) |
184 (139–230) | 0 (0–0) | 4 (2–57) | 0 (0–2) | 1 (0–5) | 3 (0–68) | 42 (15–144) | 151 (37–277) |
| Systolic blood pressure (mm Hg)c | 125.3±17 | 133.5±14 | 133.7±11 | 123.2±11 | 124.2±15 | 115.0±13 | 114.4±11 | 116.9±10 |
| Diastolic blood pressure (mm Hg)c | 75.6±9 | 79.7±8 | 77.0±7 | 72.4±8 | 69.5±10 | 64.5±6 | 69.2±11 | 67.4±7 |
Data are presented as mean ± SD with the exception of CD19 counts, which are presented as median (interquartile range).
Twelve of thirteen patients on statin at initial evaluation.
Median duration of CD19+ B cell depletion (defined as <5 cells/μl) was 172 days (range, 99–254 days) after the first cycle of rituximab. All patients received the second cycle of rituximab between 6 and 8 months after the first cycle, which was based on the timing of B-cell repletion in each individual patient, as discussed in Methods.
New-onset or worsening hypertension occurred in 54% of patients during treatment, necessitating addition or dose escalation of antihypertensive medications (except renin–angiotensin–aldosterone system antagonists) to maintain pre-enrollment blood pressure levels.
Nine patients had detectable circulating autoantibodies against PLA2R in pretreatment serum as determined by enzyme-linked immunosorbent assay, and 1 patient had very low titers by Western blot (Table 4). One patient had circulating autoantibodies to thrombospondin type 1 domain–containing 7A proteins (THSD7A) as determined by Western blot. Median (interquartile range) baseline anti-PLA2R titer in seropositive patients was 261 RU/ml (143–576). Patients with baseline anti-PLA2R titers above the median had a mean baseline proteinuria of 12.6 ± 2.9 g per 24 hours; those with anti-PLA2R titers at or below the median had mean baseline proteinuria of 9.8 ± 2.5 g per 24 hours. Mean baseline proteinuria in seronegative patients was 10.1 ± 3 g per 24 hours. During treatment, all patients seropositive for anti-PLA2R or anti-THSD7A became seronegative and achieved remission, regardless of baseline antibody titer. Time to remission of proteinuria relative to immunologic remission (lag time) is shown in Table 4. Three patients had re-emergence of anti-PLA2R antibodies during the trial period (discussed in Relapse section later in the text).
Table 4.
Changes in anti-PLA2R autoantibody titers in individual membranous nephropathy protocol patients from study enrollment (baseline) to 24 months
| Pt. # |
Study enrollment (baseline) Anti-PLA2R |
3 mo |
6 mo |
9 mo |
12 mo |
18 mo |
24 mo |
Time to clinical remission (best achieved) status (mo) |
|
|---|---|---|---|---|---|---|---|---|---|
| Achieved partial remission | Partial remission | Complete remission | |||||||
| 1 | 3260 | 621 | 70 | 55.8 | 2.8b | 2.6b | 7.0b | 18 | NA |
| 3 | 730.9 | 46.07 | 3.6b | mb | 1.2b | 0.86b | 0.97b | 6 | NA |
| 5 | Negativea | – | – | – | – | – | 142c | 3 | NA |
| 6 | 295.6 | 5.4b | 19b | 7.3b | 23.9c | 1.7b | 0.6b | 9 | NA |
| 9 | 576.1 | 0.7b | 0.63b | 0.65b | 1.08b | 17.29b | 60.66c | 3 | NA |
| 12 | 78.5 | 1.8b | 2.4b | 2.1b | 1.4b | 1.8b | 1.8b | 3 | NA |
| Achieved complete remission | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 162.7 | 2.4b | 1.45b | 0.7b | 0.8b | 1.5b | 0.8b | 3 | 6 |
| 7 | 260.5 | 0.88b | 1.01b | 0.83b | 0.89b | 0.83b | 1.3b | 3 | 6 |
| 10 | 137.7 | 1.1b | 0.84b | 0.63b | 0.66b | 1.3b | 0.87b | 6 | 9 |
| 13 | 142.6 | 1.4b | 1.5b | 1.5b | 1.5b | 1.08b | 1.1b | 3 | 3 |
| 4 | Negative | – | – | – | – | – | – | 3 | 12 |
| 8 | THSD7A+ | – | – | – | – | – | – | 6 | 9 |
| 11 | Negative | – | – | – | – | – | – | 3 | 12 |
PLA2R antibody titer determined by enzyme-linked immunosorbent assay (ELISA); titer >20 RU/ml is considered seropositive. Subgrouping based on best achieved remission status (partial vs. complete). Nine patients were PLA2R-seropositive at baseline by ELISA, and 1 patient (apatient 5) had very low levels as detected by Western blot. During treatment, all seropositive patients became seronegative and achieved remission. This includes subject 8, who was positive for anti-THSD7A at baseline and had negligible levels at 3 months and undetectable levels by Western blot for the remaining time points through 24 months.
Timing of anti-PLA2R seronegative status in previously seropositive patients.
Return of detectable anti-PLA2R antibody after previously achieving antibody depletion. These patients experienced clinical relapse and were retreated “off protocol.” Patient 6 experienced remission after retreatment that coincided with anti-PLA2R titer becoming negative again. Time to best achieved clinical remission is shown to allow comparisons between timing of immunologic and timing of clinical remission (lag time).
Changes in Renal Function
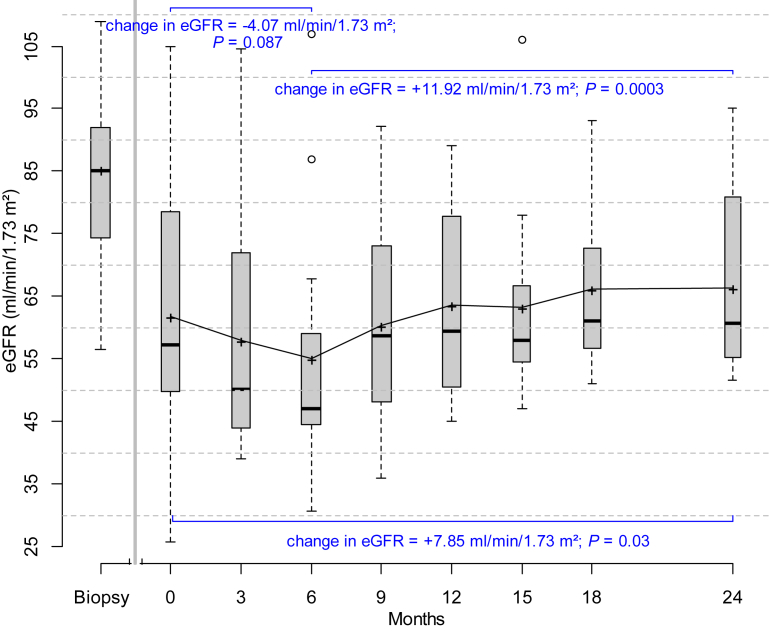
During the run-in observation phase (prior to initiation of protocol immunosuppressive treatment), there was a modest decline in renal function. Mean serum creatinine increased from 0.93 ± 0.06 mg/dl to 1.36 ± 0.14 mg/dl, eGFR decreased from 85 ml/min per 1.73 m2 to 62 ml/min per 1.73 m2, and the slope of decline in eGFR was −1.89 ml/min per 1.73 m2 per month (Figure 2 and Supplementary Table 1). Initiation of renin–angiotensin–aldosterone system antagonism may partially account for this observation. In 5 patients, serum creatinine increased by at least 40% during the run-in phase. The momentum and progressive nature of renal function decline in these patients appeared most compatible with immune-mediated kidney injury as alternative etiologies for acute kidney injury were ruled out. Initiation of “induction” therapy with cyclosporine plus rituximab was associated with further (but non-significant) decline in renal function with the nadir occurring at approximately 6 months. These changes were temporally associated with achieving target drug levels of cyclosporine (mean cyclosporine level: 154 ±25 μg/l) during the induction phase. Tapering of cyclosporine during the maintenance phase was associated with improvement in GFR. By 24 months, there was improvement in renal function compared to baseline (enrollment) values (Figure 2 and Supplementary Table 1). The overall change in eGFR from the beginning to the end of the trial (0 to 24 months) was +7.85 ml/min per 1.73 m2.
Figure 2.
Box plots showing changes in eGFR (CKD-EPI) from the time of diagnosis (biopsy) to study initiation/enrollment (time 0) to 24 months. Estimates of changes in eGFR over various intervals are based on mixed-effects models. During the observation phase (defined as time from biopsy to start of study drugs at time 0), there was a decline in renal function; the slope of decline in eGFR was –1.89 ml/min per 1.73 m2 per month. Initiation of induction therapy was associated with further decline in renal function that improved as cyclosporine was tapered and discontinued during the maintenance phase (starting at month 7). The change in eGFR during the induction phase was –4.07 ml/min per 1.73 m2 and +11.92 ml/min per 1.73 m2 during the maintenance phase (7–24 months). By 24 months, there was improvement in renal function compared to enrollment (time 0) values. The top and bottom of the box are the estimated 75th and 25th percentiles. The horizontal lines and “+” signs within each box represent the median and mean values, respectively. The vertical dashes denote the largest as well as the smallest data point that is within 1.5 times the interquartile range (75th to 25th percentile) above the 75th percentile or below the 25th; data points outside of this range are denoted by open circles. P values compare 0 versus 6 months, 6 months versus 24 months, and 0 versus 24 months.
Relapse
Relapse occurred in 2 patients (patients 5 and 6) during the 24-month trial period (Table 2). Both had achieved partial remission prior to relapse. A third patient (patient 9) relapsed after completion of the trial period. Relapse was associated with re-emergence of anti-PLA2R antibody in these patients (Table 4).
Adverse Events
The experimental immunosuppressive regimen of cyclosporine and rituximab was well tolerated. Adverse events were mostly clinically insignificant (Table 5). Acute infusion reactions associated with the first dose of rituximab occurred in 6 (37%) patients but were mild and easily manageable. Five episodes of late-onset neutropenia (neutrophil count ≤1 × 109 cells/l, at least 4 weeks following rituximab) occurred in 3 patients. There were no episodes of febrile neutropenia. There were 2 hospitalizations, 1 for diverticulitis and 1 for costochondritis.
Table 5.
Adverse events
| System | Adverse event: no. of patients (%) |
|---|---|
| Hematologic | Neutropenia
|
| Infectious | Upper respiratory tract infection: 5 (38%) Sinusitis: 2 (15%) Influenza: 1 (7%) |
| Rheumatologic | Gout: 2 (15%) Elevated creatine phosphokinase, grade 1: 3 (23%) |
| Neurologic | Dysesthesias (hands and/or feet), grade 1: 3 (23%) Tremor, grade 1: 1 (7%) Headache: 3 (23%) |
| Gastrointestinal | Hyperbilirubinemia, grade 1: 3 (23%) Other liver test abnormalities (alanine aminotransferase), grade 1: 2 (15%) Dyspepsia: 2 (15%) |
| Cardiovascular | New-onset hypertension requiring therapy: 2 (15%) Worsening of hypertension requiring additional therapy: 5 (39%) |
| Metabolic | Hyperkalemia, grade 2: 1 (7%); grade 1: 2 (15%) Hypomagnesemia, grade 1: 3 (23%) Hyperglycemia: 1 (7%) |
| Other | Increased hair growth or coarser hair: 4 (30%) Gingival hyperplasia: 1 (7%) |
Grading based on National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Discussion
We present preliminary results of a prospective phase 2 pilot trial using a combination of rituximab plus 6 months of cyclosporine as “induction” therapy followed by a maintenance phase with rituximab in primary MN. The concept of the trial is based on the paradigm used in lupus, vasculitis, and cancer treatment in which there is a more intensive induction phase, the goal of which is to achieve remission, followed by a maintenance phase to reduce the risk of relapse. The combination regimen takes advantage of the different mechanisms of action of each agent on the immune system (T and B cells) and on the podocytes, as well as their variable onset of action, to achieve both immunologic and clinical remission during the induction phase. Cyclosporine inhibits T-cell activation26 and has well-known effects on the podocyte cytoskeleton that contribute to the earlier antiproteinuric effects.27 Rituximab suppresses production of pathogenic antibodies by B cells28 and may also have direct effects on podocyte function and the actin cytoskeleton,29, 30 but its antiproteinuric effects tend to be delayed. The rationale for retreating with a second cycle of rituximab during the maintenance phase is to maintain remission and prevent relapse as the cyclosporine is tapered and discontinued.
Patients in this pilot study were considered to be at moderate to high risk of progression to ESKD because they demonstrated persistent (and increasing) high-grade proteinuria with or without decline in GFR during the observation phase. To date, all patients experienced either a partial or a complete remission with therapy. Remission rates, particularly the high percentage of complete remissions (54% by 12 months), are encouraging compared with those observed using currently accepted treatments that include alkylating agents or CNIs. The combination regimen leads to a higher proportion of sustained complete remissions than either rituximab or cyclosporine alone (Table 6). The significance of achieving remission of proteinuria, especially complete, for long-term renal prognosis is underscored in an analysis of a large cohort of MN patients in the Toronto Registry31 followed over 5 years. No patients who attained complete remission reached ESKD, and only 9% of patients who achieved partial remission developed ESKD. In contrast, 29% of patients who did not achieve a remission progressed to ESKD.
Table 6.
Summary of remission and relapse rates using various immunosuppressive regimens compared to protocol induction/maintenance regimen for treatment of membranous nephropathy
| Treatment regimens | 6 mo remissions |
12 mo remissions |
24 mo remissions |
Relapse rates during trial period (%) | |||
|---|---|---|---|---|---|---|---|
| % CR | % PR | % CR | % PR | % CR | % PR | ||
| Cyclosporine (6–24 mo) + steroids13, 15 | 0–7 | 50–68 | 7–10 | 39–40 | 7–40 | 32–40 | 13–48 |
| Tacrolimus (18 mo)14, 45 | 12–23 | 37–44 | 26–34 | 44–48 | 32 | 44 | 44–47 |
| Rituximab20, 21, 22, 23, 41, 46, 47 | 0 | 29–63 | 0–18 | 43–63 | 20–27 | 45–60 | 6–29 |
| Alkylating agent alternating with steroids for 6 mo48, 49, 50, 51 | 10–15 | 45–50 | 15–28 | 35–65 | 30–40 | 30–50 | 10–31 |
| Oral cytoxan for 12 mo + steroids for 6 mo52 | NA | NA | NA | NA | 17 | 77 | 28 |
| MMF ± steroids53, 54, 55 | 5 | 21 | 5 | 31 | NA | NA | 29–57 |
| Adrenocorticotropic hormone (synthetic) for 12 mo56 | 19 | 44 | 38 | 50 | NA | NA | 21 |
| Protocol regimen Cyclosporine + rituximab | 23 | 62 | 54 | 31 | 54 | 31 | 15 |
Percentage of complete remissions (CR) and partial remissions (PR) achieved in patients with membranous nephropathy at various time points using other immunosuppression regimens versus the protocol induction/maintenance regimen.
MMF, mycophenolate mofetil; NA, data not available at these time points.
The timing of remissions also deserves to be highlighted: 61% of patients achieved either partial or complete remission by 3 months, leading to shorter exposure to the metabolic, infectious, and prothrombotic consequences of the NS compared to treatment with rituximab monotherapy or with alkylating-based regimens. Thromboembolic complications are among the most concerning nonrenal consequences of MN accompanied by hypoalbuminemia.32, 33 Prophylactic warfarin anticoagulation extending for the duration of the “at-risk” period is considered for patients with severe hypoalbuminemia (<2.0 g/dl)34 but may be associated with bleeding and renal injury35 and necessitates frequent monitoring. We chose not to provide prophylactic anticoagulation in this trial in light of the aforementioned safety concerns. However, our combination immunosuppressive regimen was effective in shortening the critical “at-risk” period, as mean serum albumin levels increased from less than 2 g/dl at baseline to greater than 3 g/dl within 3 months.
Our definition of remission status for this trial was based on measurement of protein excretion. It is anticipated that future definitions of remission will incorporate both clinical and immunologic parameters (i.e., anti-PLA2R antibody), which may provide a more accurate assessment of response to therapies and potential for disease progression than is provided by proteinuria alone.36, 37, 38, 39, 40 The protocol treatment was effective in inducing immunologic remission. All patients who were positive for anti-PLA2R or anti-THSD7A achieved complete antibody depletion during the trial. This likely accounts for the high rate of observed remissions. Disappearance of antibodies has important implications, as it has been associated with good long-term outcomes. Bech et al. reported that almost 60% of patients who are seronegative following treatment remain in remission for 5 years, whereas patients who remain seropositive usually do not maintain remission.36 The efficacy of this regimen on antibody depletion is similar to that reported using a cyclophosphamide-containing regimen36, 40 but represents improvement over results reported with rituximab monotherapy in which 25% of patients with PLA2R-related disease never achieve antibody depletion.38, 41 In accordance with previous studies, changes in antibody levels preceded the reduction in proteinuria.38, 41, 42 Numerous investigators have described a pattern of steep fall in antibody titers after initiation of immunosuppression followed by a more gradual reduction in proteinuria over months to years.36 This time lag between immunologic and observed clinical remission was shortened with our experimental regimen compared with other immunosuppressive regimens due to earlier reductions in proteinuria. Anti-PLA2R titers dropped substantially after immunosuppression initiation such that more than 75% of patients achieved immunologic remission by 3 months and 61% of patients achieved clinical remission by that time point.
No patients reached a doubling of serum creatinine or dialysis during the trial period or during extended follow-up. The protocol treatment was associated with an early but non-significant decline in renal function during the induction phase that improved as cyclosporine was tapered and discontinued during the maintenance phase. These observations of reversible reduction in GFR are in line with the known acute hemodynamic effects of CNIs. By 24 months, there was improvement in renal function compared to baseline (enrollment) values, suggesting that this combination immunosuppressive regimen may attenuate and possibly reverse the decline in renal function that occurred during the observation period. The data on monotherapy with CNIs and effects on renal survival in MN are conflicting. One randomized trial reported that cyclosporine slowed the rate of renal function decline in patients with progressive MN,43 whereas 2 studies showed faster progression of disease.10, 44 The shorter duration of cyclosporine treatment (6 months) and lower doses used in our combination immunosuppressive regimen compared with other trials may partly account for the differences in renal outcomes.
Relapse rates within the 24-month trial period (15%) and during extended follow-up (23%) are in line with those reported with alkylating agents, and represent marked improvement over the high relapse rates seen after CNI withdrawal. Table 6 compares relapse rates among different immunosuppression regimens.
This combination regimen was well tolerated, and no new or unexpected safety signals were observed. The relatively rapid improvement in hypogammaglobulinemia associated with treatment and avoidance of corticosteroids may have accounted for the low infection rates in our study compared to those observed with cytotoxic-based regimens.
Our trial has inherent limitations. It has a single-arm design and limited sample size. However, the purpose of this study was to gather both safety and efficacy data in patients at high risk of progression and in those with declining renal function. These preliminary data are necessary to design and power future controlled studies. We were rigorous in our attempt to identify those at highest risk of progression who are more difficult to treat and tend to suffer more adverse effects with therapy. Continued enrollment of additional patients and longer-term follow-up are required to confirm these results and assess long-term safety of this combination regimen, relapse-free survival, and impact on hard renal end points such as ESKD.
The combination of short-term cyclosporine and rituximab in this induction and maintenance regimen appears to be effective in achieving both immunologic and clinical remission in MN patients at high risk of poor outcomes. This regimen is associated with a high proportion of complete remissions. We have demonstrated tolerability and a favorable safety profile even in patients with declining renal function. Advantages of this regimen are the relatively short exposure to cyclosporine, less exposure to the complications of the NS given the more rapid onset of remissions, and avoidance of the short- and long-term toxicity associated with steroids and alkylating agents. We believe that these preliminary findings support formal comparison of this regimen with currently accepted immunosuppressive regimens in a randomized controlled trial.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The study was supported by the Intramural Research Program within the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. We are grateful to Dr. Michael Ring, who helped in data organization, entry, and handling. We are also grateful to Paige Coles, who assisted in the enzyme-linked immunosorbent assay and Western blot assessment of anti-PLA2R and anti-THSD7A antibodies (supported by RO1-DK097053). Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology in Philadelphia, Pennsylvania (11–16 November 2014).
Footnotes
Supplementary Methods
Table S1. Changes in renal function from diagnosis to study enrollment (baseline) and during treatment
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Changes in renal function from diagnosis to study enrollment (baseline) and during treatment
References
- 1.Glassock R.J. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56:157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Ronco P., Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet. 2015;385:1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 3.Glassock R.J. Diagnosis and natural course of membranous nephropathy. Semin Nephrol. 2003;23:324–332. doi: 10.1016/s0270-9295(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 4.Polanco N., Gutierrez E., Rivera F. Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol Dial Transplant. 2012;27:231–234. doi: 10.1093/ndt/gfr285. [DOI] [PubMed] [Google Scholar]
- 5.Polanco N., Gutierrez E., Covarsi A. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol. 2005;16:1188–1194. doi: 10.1681/ASN.2005010028. [DOI] [PubMed] [Google Scholar]
- 7.Cattran D.C., Pei Y., Greenwood C.M. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51:901–907. doi: 10.1038/ki.1997.127. [DOI] [PubMed] [Google Scholar]
- 8.Pei Y., Cattran D., Greenwood C. Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int. 1992;42:960–966. doi: 10.1038/ki.1992.374. [DOI] [PubMed] [Google Scholar]
- 9.Beck L., Bomback A.S., Choi M.J. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–441. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Howman A., Chapman T.L., Langdon M.M. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381:744–751. doi: 10.1016/S0140-6736(12)61566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Brand J.A., van Dijk P.R., Hofstra J.M., Wetzels J.F. Cancer risk after cyclophosphamide treatment in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9:1066–1073. doi: 10.2215/CJN.08880813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faurschou M., Sorensen I.J., Mellemkjaer L. Malignancies in Wegener's granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35:100–105. [PubMed] [Google Scholar]
- 13.Cattran D.C., Appel G.B., Hebert L.A. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 14.Praga M., Barrio V., Juarez G.F., Luno J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. doi: 10.1038/sj.ki.5002215. [DOI] [PubMed] [Google Scholar]
- 15.Naumovic R., Jovanovic D., Pavlovic S. Cyclosporine versus azathioprine therapy in high-risk idiopathic membranous nephropathy patients: a 3-year prospective study. Biomed Pharmacother. 2011;65:105–110. doi: 10.1016/j.biopha.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Chen M., Li H., Li X.Y. Tacrolimus combined with corticosteroids in treatment of nephrotic idiopathic membranous nephropathy: a multicenter randomized controlled trial. Am J Med Sci. 2010;339:233–238. doi: 10.1097/MAJ.0b013e3181ca3a7d. [DOI] [PubMed] [Google Scholar]
- 17.Beck L.H., Jr., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtas C., Bruschi M., Candiano G. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:1394–1400. doi: 10.2215/CJN.02170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomas N.M., Beck L.H., Jr., Meyer-Schwesinger C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remuzzi G., Chiurchiu C., Abbate M. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 21.Ruggenenti P., Chiurchiu C., Brusegan V. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851–1857. doi: 10.1097/01.asn.0000071511.35221.b3. [DOI] [PubMed] [Google Scholar]
- 22.Fervenza F.C., Cosio F.G., Erickson S.B. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 23.Fervenza F.C., Abraham R.S., Erickson S.B. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segarra A., Praga M., Ramos N. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083–1088. doi: 10.2215/CJN.06041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiber S.L., Crabtree G.R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 27.Faul C., Donnelly M., Merscher-Gomez S. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maloney D.G. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366:2008–2016. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 29.Reiser J., Fornoni A. Rituximab: a boot to protect the foot. J Am Soc Nephrol. 2014;25:647–648. doi: 10.1681/ASN.2013121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornoni A., Sageshima J., Wei C. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Science Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002231. 85ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troyanov S., Wall C.A., Miller J.A. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–1205. doi: 10.1111/j.1523-1755.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 32.Lionaki S., Derebail V.K., Hogan S.L. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour S.J., Greenwald A., Djurdjev O. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 34.Lee T., Biddle A.K., Lionaki S. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420. doi: 10.1038/ki.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodsky S.V., Nadasdy T., Rovin B.H. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80:181–189. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bech A.P., Hofstra J.M., Brenchley P.E., Wetzels J.F. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9:1386–1392. doi: 10.2215/CJN.10471013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanigicherla D., Gummadova J., McKenzie E.A. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 38.Beck L.H., Jr., Fervenza F.C., Beck D.M. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofstra J.M., Debiec H., Short C.D. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoxha E., Thiele I., Zahner G. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruggenenti P., Debiec H., Ruggiero B. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoxha E., Harendza S., Zahner G. An immunofluorescence test for phospholipase-A-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 43.Cattran D.C., Greenwood C., Ritchie S. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 44.Pisoni R., Grinyo J.M., Salvadori M. Cyclosporine versus conservative therapy in patients with idiopathic membranous nephropathy and deteriorating renal function. J Am Soc Nephrol. 2000;11 0514A (abstract) [Google Scholar]
- 45.Caro J., Gutierrez-Solis E., Rojas-Rivera J. Predictors of response and relapse in patients with idiopathic membranous nephropathy treated with tacrolimus. Nephrol Dial Transplant. 2015;30:467–474. doi: 10.1093/ndt/gfu306. [DOI] [PubMed] [Google Scholar]
- 46.Cravedi P., Sghirlanzoni M.C., Marasa M. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33:461–468. doi: 10.1159/000327611. [DOI] [PubMed] [Google Scholar]
- 47.Ruggenenti P., Cravedi P., Chianca A. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponticelli C., Zucchelli P., Passerini P. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989;320:8–13. doi: 10.1056/NEJM198901053200102. [DOI] [PubMed] [Google Scholar]
- 49.Ponticelli C., Zucchelli P., Passerini P. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–1604. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 50.Ponticelli C., Altieri P., Scolari F. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 51.Jha V., Ganguli A., Saha T.K. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 52.du Buf-Vereijken P.W., Branten A.J., Wetzels J.F. Cytotoxic therapy for membranous nephropathy and renal insufficiency: improved renal survival but high relapse rate. Nephrol Dial Transplant. 2004;19:1142–1148. doi: 10.1093/ndt/gfh036. [DOI] [PubMed] [Google Scholar]
- 53.Chan T.M., Lin A.W., Tang S.C. Prospective controlled study on mycophenolate mofetil and prednisolone in the treatment of membranous nephropathy with nephrotic syndrome. Nephrology (Carlton) 2007;12:576–581. doi: 10.1111/j.1440-1797.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 54.Branten A.J., du Buf-Vereijken P.W., Vervloet M., Wetzels J.F. Mycophenolate mofetil in idiopathic membranous nephropathy: a clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis. 2007;50:248–256. doi: 10.1053/j.ajkd.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Dussol B., Morange S., Burtey S. Mycophenolate mofetil monotherapy in membranous nephropathy: a 1-year randomized controlled trial. Am J Kidney Dis. 2008;52:699–705. doi: 10.1053/j.ajkd.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Ponticelli C., Passerini P., Salvadori M. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in renal function from diagnosis to study enrollment (baseline) and during treatment