Abstract
Introduction
The survival benefit from simultaneous liver-kidney transplantation (SLK) over liver transplant alone (LTA) in recipients with moderate renal dysfunction is not well understood. Moreover, the impact of deceased donor organ quality in SLK survival has not been well described in the literature.
Methods
The Scientific Registry of Transplant Recipients was studied for adult recipients receiving LTA (N = 2700) or SLK (N = 1361) with moderate renal insufficiency between 2003 and 2013. The study cohort was stratified into 4 groups based on serum creatinine (<2 mg/dl versus ≥2 mg/dl) and dialysis status at listing and transplant. The patients with end-stage renal disease and requiring acute dialysis more than 3 months before transplantation were excluded. A propensity score matching was performed in each stratified group to factor out imbalances between the SLK and LTA regarding covariate distribution and to reduce measured confounding. Donor quality was assessed with liver donor risk index. The primary outcome of interest was posttransplant mortality.
Results
In multivariable propensity score-matched Cox proportional hazard models, SLK led to decrease in posttransplant mortality compared with LTA across all 4 groups, but only reached statistical significance (hazard ratio 0.77; 95% confidence interval, 0.62–0.96) in the recipients not exposed to dialysis and serum creatinine ≥ 2 mg/dl at transplant (mortality incidence rate per patient-year 5.7% in SLK vs. 7.6% in LTA, P = 0.005). The decrease in mortality was observed among SLK recipients with better quality donors (liver donor risk index < 1.5).
Discussion
Exposure to pretransplantation dialysis and donor quality affected overall survival among SLK recipients.
Keywords: deceased donor quality, dual organ allocation, propensity score matching, simultaneous liver-kidney transplantation, UNOS
Since the inception of model for end-stage liver disease (MELD) based on organ sharing, there has been a substantial increase in the number of simultaneous liver-kidney transplantations (SLK). Abnormal serum creatinine (Scr) and dialysis dependence significantly impact wait-list mortality for liver transplant candidates as impaired kidney function is heavily weighted in the MELD score calculation.1, 2 The guidelines for dual organ distribution have changed several times since 2007, and the allocation process currently allows a kidney to be offered to any liver candidate based on medical necessity and local transplant physician opinion.2, 3, 4, 5 These changes in the distribution system have resulted in different organ utilization practices among transplant centers.2
SLK remains to be the ideal treatment option for patients with decompensated cirrhosis and end-stage renal disease (ESRD)6, 7, 8 as the reported survival is similar to liver transplant alone (LTA) recipients without renal dysfunction. However, the recommended SLK criteria do not provide clear guidelines concerning transplant recipients with acute kidney injury (AKI) requiring renal replacement therapy (<3 months) and AKI superimposed on chronic kidney disease (CKD). The parameters for optimal stratification of these transplant candidates (SLK vs. LTA) remain unclear, and conflicting data have failed to describe the patient survival benefit among transplant recipients with moderate renal dysfunction.6, 9, 10
It is well known that donor and recipient characteristics predict graft survival.11, 12 Standardized measures for assessing donor quality (liver donor risk index [L-DRI]) and the recipient’s pretransplant mortality risk (MELD score) have helped optimize transplant survival, which is essential given the scarce organ supply.13 However, few studies have evaluated the impact of organ quality on mortality especially among high-risk donors (L-DRI > 1.5) for patients seeking SLK allocation.14, 15
In this study, we aimed to evaluate the survival benefit of SLK over LTA among propensity score (PS)-matched liver transplant recipients with moderate renal dysfunction. We stratified our study cohort into 4 groups defined by kidney function and dialysis status at listing and transplantation. The predictive models estimating mortality were performed within each stratum. We also examined donor quality on posttransplant mortality in SLK recipients, as measured by L-DRI.
Materials and Methods
Study Cohort and Design
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system is a national database that includes data on all donors, wait-listed candidates, and transplant recipients in the USA, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration and US Department of Health and Human Services provide oversight to the activities of the OPTN and SRTR contractors.
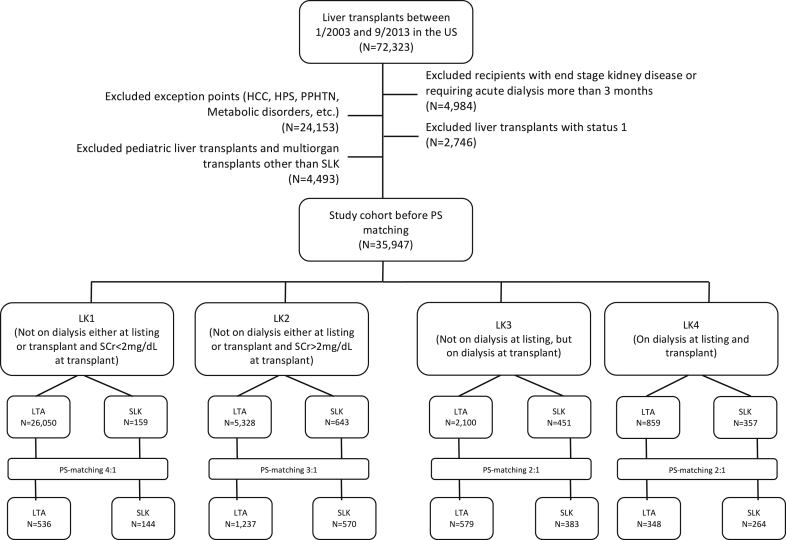
This study was a retrospective cohort analysis of the OPTN/United Network for Organ Sharing Registry and included all adults who underwent liver transplantation between January 2003 and September 2013 in the USA (N = 72,323). Figure 1 illustrates the selection of study cohort. Exclusion criteria included the following: (i) liver transplant recipients who received exception points for any reason (e.g., hepatocellular cancer), as these patients tend not to get SLK except in the case of primary hyperoxaluria; (ii) status 1 recipients (acute liver failure cases); (iii) pediatric patients (younger than 18); (iv) multi-organ transplantations (other than SLK); (v) diagnosis of ESRD before transplantation; and (iv) acute dialysis that needs more than 3 months.
Figure 1.
Strobe chart showing the selection of the study cohort. HCC, hepatocellular cancer; HPS, hepatopulmonary syndrome; LTA, liver transplant alone; PPHTN, portapulmonary hypertension; PS matching, propensity score matching; Scr, serum creatinine; SLK, simultaneous liver-kidney transplantation.
We stratified the recipients by their transplant type (SLK being the primary exposure) into 4 different groups based on Scr and dialysis status at listing and at the time of transplant: LK1, not on dialysis either at listing or transplant, and Scr < 2 mg/dl at transplant; LK2, not on dialysis either at listing or transplant, and Scr ≥ 2 mg/dl at transplant; LK3, not on dialysis at listing but on dialysis at transplant; LK4, on dialysis both at listing and at transplant. The cutoff value for Scr was chosen from the recommendations of recent transplant consensus conferences. Scr < 2 mg/dl (approximately estimated glomerular filtration rate [eGFR] ≥ 30 ml/min per 1.73 m2, using the Modification of Diet in Renal Disease Study 4-variable equation) versus Scr ≥ 2 mg/dl (approximate eGFR < 30 ml/min per 1.73 m2), and the Chronic Liver Failure-Sequential Organ Failure scoring defines renal failure as a Scr of ≥ 2.3, 4, 16, 17 The aim of our study is to find the differences between SLK and LTA regarding posttransplant survival. For a meaningful comparison, it is essential to consider imbalances between the SLK and LTA regarding the covariate pattern. We adjusted for imbalances among covariates by developing PS matching within each stratum (LK1–LK4). A total of 4061 patients were included in the final analysis. Because of a sample size imbalance between the LTA and SLK recipients within each LK group, we adopted different matching ratios in the PS analysis (4:1 in the LK1 group, 3:1 in the LK2 group, and 2:1 in the LK3 and LK4 groups).
Patients were followed up from the date of liver transplant to posttransplant death, loss to follow-up, or end of study period (30 September 2013). The primary outcome of interest was posttransplant mortality. We also investigated the effect of deceased donor quality on posttransplant mortality in SLK patients, as measured by L-DRI categories (L-DRI < 1, 1–1.5, 1.5–2, and >2). The L-DRI score was derived from 6 donor characteristics (age, cause of death, race, donation after cardiac death, graft [whole vs. partial split], donor height [cm]) and 2 transplant factors (location of donor service area [local, regional, and national] and cold ischemia time [hour]). Most L-DRI scores range from 0 to 3.5, and lower scores represent better quality liver (especially L-DRI < 1.2).
Statistical Analysis
Donor and recipient characteristics were described using mean and SD or frequencies. Comparisons between groups were made using the t-test, Kruskal-Wallis test, or χ2 test. Patient survival rates were estimated using the Kaplan-Meier product limit method. The log-rank test was used for comparison of the unadjusted patient survival curves. Cox proportional hazard regression models were used to estimate the hazard ratios associated with patient mortality risk. P value < 0.05 was considered statistically significant. Statistical analyses were performed with Stata/MP13 (StataCorp LP, College Station, TX). The magnitude of missing data in the PS-matched cohort was minuscule (1%–2% among covariates, specifically in ascites and encephalopathy variables) and all models were directly comparable. That is why we practically considered this study as complete case analysis.
PS is a balancing score representing the probability of receiving an SLK versus LTA given the patient’s covariate pattern.18 After PS is estimated, the next step involves matching exposure versus control (SLK vs. LTA) patients based on predicted PSs. Within each stratum (LK1–LK4), we used nearest-neighbor Mahalanobis metric matching with a bias correction term (http://www.stata.com/manuals13/teteffectsnnmatch.pdf) to match and allocate patients to the respective groups. The 11 covariates used in the PS analysis included the recipient’s age, gender, race, body mass index, diabetes, underlying liver disease etiology, Scr (mg/dl) at transplant (if a patient is on dialysis, Scr is omitted from the PS analysis), serum bilirubin (mg/dl) at transplant, international normalized ratio at transplant, time to transplant (wait-list duration in months), and the L-DRI score. Statistical inference was based on the analysis within strata defined by kidney function and/or dialysis status using multivariable PS-matched Cox proportional hazard models adjusted for other covariates including ABO type, encephalopathy, ascites, estimated slope of creatinine from wait-listing to transplantation (for the LK1 and LK2 groups only), hospitalized in ICU before transplant (on mechanical ventilation), and transplant year.
Results
Characteristics of the Study Cohort
Selected demographics and clinical characteristics for LTA and SLK before PS matching are shown in Table 1. Compared with LTA patients, SLK recipients had lower GFR at transplant (62.9 ± 32.5 ml/min per 1.73 m2 vs. 26.9 ± 17.2 ml/min per 1.73 m2), more likely required dialysis (8.6% vs. 50%), and tend to have a higher MELD score at transplant (24.2 ± 9.2 vs. 30.9 ± 8.4). After PS matching, the LK groups were well balanced on the distribution of covariates (Supplementary Tables S1a–S1d). The median waiting time to transplantation was approximately 2 months in all groups except the LK4 group (<1 month). The recipients in the LK3 and LK4 groups had more severe disease on the level of liver and kidney dysfunction.
Table 1.
Characteristics of liver transplant candidates before propensity score matching by transplant type (UNOS data between 2003 and 2013 in the USA)
| N | LTA | SLK | P value | |
|---|---|---|---|---|
| N = 35,947 | 34,337 | 1610 | ||
| Age (yr)a | 35,947 | 52.6 ± 10 | 54.3 ± 9.6 | <0.001 |
| Gender (female, %)a | 35,947 | 33.9 | 34.1 | 0.84 |
| Race (AA, %)a | 35,947 | 8.9 | 15.3 | <0.001 |
| Recipient DM (%)a | 35,947 | 23.5 | 37 | <0.001 |
| Liver disease (%)a | 35,947 | <0.001 | ||
| HCV infection | 27.8 | 28.6 | ||
| ETOH and NASH | 23.6 | 23.4 | ||
| Cholestatic | 10.9 | 7 | ||
| Others | 37.6 | 40.1 | ||
| BMIa | 35,919 | 28.3 ± 5.8 | 27.4 ± 5.8 | <0.001 |
| Scr (mg/dl) at transplant (if not on dialysis)a | 34,403 | 1.5 ± 1 | 3 ± 1.2 | <0.001 |
| eGFR at transplant (ml/min per 1.73 m2)b | 34,403 | 62.9 ± 32.5 | 26.9 ± 17.2 | <0.001 |
| INR at transplanta | 35,947 | 2 ± 1.2 | 1.9 ± 0.9 | <0.001 |
| Total bilirubin (mg/dl) at transplanta | 34,258 | 10.7 ± 12 | 12.4 ± 14.5 | <0.001 |
| On dialysis at transplant (%) | 35,947 | 8.6 | 50 | <0.001 |
| MELD at transplant | 35,944 | 24.2 ± 9.2 | 30.9 ± 8.4 | <0.001 |
| Liver DRIa | 30,982 | 1.4 ± 0.4 | 1.2 ± 0.3 | <0.001 |
| Median time to transplant (25th and 75th percentiles), moa | 35,946 | 1.9 (0.4, 6.8) | 1.3 (0.4, 5.2) | 0.001 |
± Denotes 1 SD.
AA, African American; BMI, body mass index; DM, diabetes; DRI, donor risk index; eGFR, estimated glomerular filtration rate; ETOH and NASH, alcohol and nonalcoholic steatohepatitis; HCV, hepatitis C virus; INR, international normalized ratio; LTA, liver transplant alone; MDRD, Modification of Diet in Renal Disease Study; MELD, model for end-stage liver disease; N, sample size; Scr, serum creatinine; SLK, simultaneous liver-kidney transplantation; UNOS, United Network for Organ Sharing.
The variables used in the propensity score matching.
The MDRD 4-variable equation to eGFR, calculated for the recipient who was not on dialysis.
Outcomes
Mortality
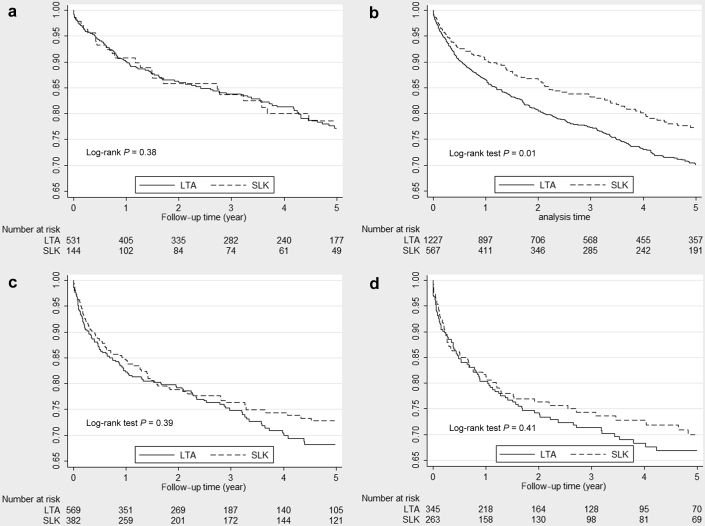
For the study cohort (after the PS matching), unadjusted Kaplan-Meier curves for patient survival for LTA versus SLK recipients are illustrated in Figure 2a–d. The SLK recipients in the LK2 group had better patient survival compared with the LTA patients (77% vs. 70% at 5 years, P = 0.01). Causes of death (reported for liver recipients) and its observed frequencies by the LK groups and posttransplant outcomes through the end of follow-up time are shown in Table 2. Mortality incidence rate per patient-year was lower in SLK recipients across all LK groups, and the mortality incidence rate ratio (SLK/LTA: 0.75, 0.60–0.92, P value = 0.005) reached statistical significance in only the LK2 group.
Figure 2.
(a) Unadjusted Kaplan-Meier patient survival curves for liver transplant alone (LTA) versus simultaneous liver-kidney transplantations (SLK) recipients not on dialysis either at listing or transplant, and serum creatinine <2 mg/dl at transplant (LK1), after the propensity score matching (UNOS data between 2003 and 2013 in the USA). (b) Unadjusted Kaplan-Meier patient survival curves for LTA versus SLK recipients not on dialysis either at listing or transplant and serum creatinine ≥2 mg/dl at transplant (LK2), after the propensity score matching (UNOS data between 2003 and 2013 in the USA). (c) Unadjusted Kaplan-Meier patient survival curves for LTA versus SLK recipients not on dialysis at listing but on dialysis at transplant (LK3), after the propensity score matching (UNOS data between 2003 and 2013 in the USA). (d) Unadjusted Kaplan-Meier patient survival curves for LTA versus SLK recipients on dialysis at listing and transplant (LK4), after the propensity score matching (UNOS data between 2003 and 2013 in the USA). UNOS, United Network for Organ Sharing.
Table 2.
Causes (reported for liver transplant recipients) and observed frequencies of death and posttransplant outcomes after the propensity score matching
| Liver transplant recipients not on dialysis either at listing or transplant, and Scr < 2 mg/dl at transplant (LK1) |
Liver transplant recipients not on dialysis either at listing or transplant, and Scr ≥ 2 mg/dl at transplant (LK2) |
Liver transplant recipients not on dialysis at listing but on dialysis at transplant (LK3) |
Liver transplant recipient on dialysis both at listing and transplant (LK4) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTA | SLK | P | LTA | SLK | P | LTA | SLK | P | LTA | SLK | P | |
| N | 536 | 144 | 1237 | 570 | 579 | 383 | 348 | 264 | ||||
| Posttransplant mortality | ||||||||||||
| Incidence rate per patient-year (%) | 5.7 | 4.7 | 7.6 | 5.7 | 9.2 | 7.4 | 10 | 8.4 | ||||
| Incidence rate ratio (95% CI) | 0.81 (0.50–1.28) | 0.38 | 0.75 (0.60–0.92) | 0.005 | 0.81 (0.61–1.05) | 0.10 | 0.84 (0.61–1.16) | 0.28 | ||||
| Time to death (mo) | 31 ± 32 | 19 ± 20 | 0.08 | 27 ± 30 | 30 ± 32 | 0.33 | 19 ± 27 | 24 ± 31 | 0.19 | 16 ± 11 | 16 ± 10 | 0.99 |
| Causes of death reported for liver transplantation (%) | 0.09∗ | 0.09∗ | 0.10∗ | 0.06∗ | ||||||||
| Graft failure | 14.4 | 11.1 | 13 | 11.1 | 9.4 | 13.1 | 12.5 | 7.4 | ||||
| CVS | 13.6 | 14.8 | 18.6 | 17.1 | 22.7 | 16.7 | 17.9 | 7.4 | ||||
| Hemorrhage | 6.8 | 0 | 5.1 | 0.9 | 4.7 | 0.0 | 6.3 | 5.9 | ||||
| Infection | 14.4 | 33.3 | 21.5 | 25.6 | 31.3 | 27.4 | 23.2 | 41.2 | ||||
| Malignancy | 14.4 | 11.1 | 9.8 | 9.4 | 7.8 | 7.2 | 9.8 | 8.8 | ||||
| Renal failure | 5.1 | 14.8 | 9.5 | 4.3 | 7 | 3.6 | 11.6 | 4.4 | ||||
| Other | 14.4 | 11.1 | 8.8 | 16.3 | 8.6 | 13.1 | 12.5 | 11.8 | ||||
| Unknown | 17 | 3.7 | 13.7 | 15.4 | 8.6 | 19.1 | 6.3 | 13.4 | ||||
| Posttransplant ESRD | ||||||||||||
| Incidence rate per 1000 patient-days | 1.1 | 1.5 | 1.3 | 0.8 | 1.4 | 1.7 | 2.6 | 0.9 | ||||
| Incidence rate ratio | 1.36 (0.62–2.79) | 0.38 | 0.63 (0.43–0.89) | 0.01 | 1.22 (0.74–2.00) | 0.40 | 0.34 (0.16–0.69) | 0.001 | ||||
| Kidney transplantation post-LTA or post-SLK, n (%) | 6 (1.1) | 3 (2.1) | 0.37 | 36 (2.9) | 5 (0.9) | 0.01 | 18 (3.1) | 6 (1.6) | 0.13 | 11 (3.2) | 7 (2.6) | 0.71 |
| Liver retransplantation | 20 (3.7) | 4 (2.8) | 0.58 | 50 (4) | 14 (2.5) | 0.09 | 23 (4) | 11 (2.9) | 0.37 | 10 (2.9) | 8 (3) | 0.91 |
CI, confidence interval; CVS, cardiovascular system; ESRD, end-stage renal disease; LTA, liver transplant alone; Scr, serum creatinine; SLK, simultaneous liver-kidney transplantation.
P value for trend (type III P value).
In multivariable PS-matched Cox proportional hazard models (Table 3), SLK resulted in a decrease in posttransplant mortality compared with LTA across all 4 LK groups, but only reached statistical significance (hazard ratio, 0.77; 95% confidence interval, 0.62–0.96) in the recipients who were not exposed to dialysis and Scr ≥ 2 mg/dl at transplant (LK2).
Table 3.
Cox proportional hazard models for mortality among patients undergoing simultaneous liver-kidney transplant versus liver transplant alone by strata defined by renal function and dialysis status
| Model | Liver transplant recipients not on dialysis either at listing or transplant, and Scr < 2 mg/dl at transplant (LK1) |
Liver transplant recipients not on dialysis either at listing or transplant, and Scr ≥ 2 mg/dl at transplant (LK2) |
Liver transplant recipients not on dialysis at listing but on dialysis at transplant (LK3) |
Liver transplant recipient on dialysis both at listing and transplant (LK4) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Before propensity score matching | ||||||||
| Missing data, % (missing/total N) | 0.9 (234/26,209) | 1.4 (82/5971) | 2.5 (63/2551) | 1.5 (18/1216) | ||||
| Unadjusted model | 0.83 (0.56–1.22) | 0.34 | 0.83 (0.70–0.99) | 0.04 | 0.92 (0.76–1.13) | 0.42 | 1.04 (0.82–1.32) | 0.73 |
| After propensity score matching | ||||||||
| Missing data, % (missing/total N) | 0.4 (3/680) | 1.5 (27/1807) | 2.1 (20/962) | 2.1 (13/612) | ||||
| Model adjusted for other covariatesa | 0.88 (0.56–1.39) | 0.59 | 0.77 (0.62–0.96) | 0.02 | 0.89 (0.68–1.16) | 0.39 | 0.89 (0.64–1.23) | 0.48 |
CI, confidence interval; HR, hazard ratio; N, sample size; Scr, serum creatinine.
Other covariates: ABO type, encephalopathy category, ascites category, estimated slope of creatinine from wait-listing to transplantation (for the LK1 and LK2 groups), hospitalized to ICU before transplant (on mechanical ventilation), and transplant year.
Renal Outcomes and Rate of ESRD
Compared with SLK recipients, posttransplant ESRD was more prevalent in the LTA transplants, except LK1 group (Table 2). In SLK recipients, ESRD incidence rate per 1000 patient-days and the incidence rate ratio (SLK/LTA) were lower and reached statistical significance in the LK2 and LK4 groups. Kidney transplantation after LTA or SLK was uncommon across all LK groups (<3.2% of recipients).
Subgroup Analysis in SLK Recipients According to L-DRI Categories
Figure 3 shows the unadjusted Kaplan-Meier curves for patient survival according to L-DRI categories in SLK recipients. Higher L-DRI scores were associated with higher mortality. In a multivariable Cox proportional hazard model adjusted for other covariates, posttransplant mortality significantly increased with more top L-DRI categories (1.5–2 and >2 categories). For comparison, L-DRI categories of 1.5 to 2 and L-DRI > 2 approximately exemplify higher quintiles of kidney donor profile index, 60% to 80%, and 80% to 100%, respectively (Table 4).
Figure 3.
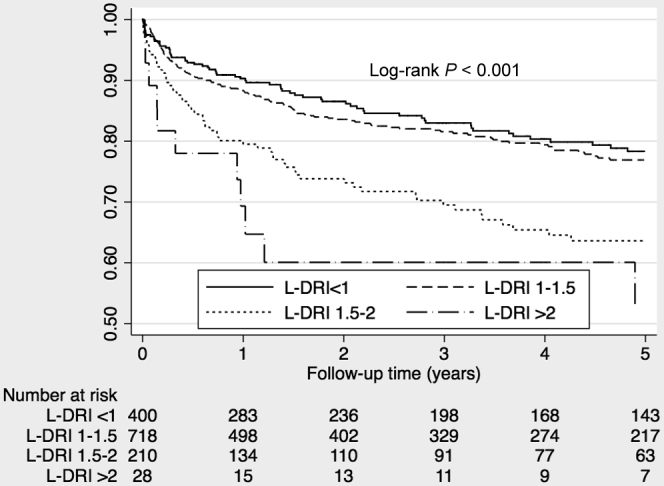
Kaplan-Meier survival curves in SLK recipients in the USA between January 2003 and September 2013, by L-DRI categories (after the PS matching). L-DRI, liver donor risk index; PS matching, propensity score matching; SLK, simultaneous liver-kidney transplantation.
Table 4.
Multivariable Cox proportional hazard model for posttransplant mortality in simultaneous liver-kidney transplant recipients by liver-donor risk index (L-DRI) categories, after the propensity score matching
| N = 1341 | Hazard ratioa | 95% confidence interval | P value |
|---|---|---|---|
| L-DRI (reference L-DRI < 1) | |||
| 1–1.5 | 1.07 | 0.81–1.42 | 0.64 |
| 1.5–2 | 2.00 | 1.44–2.79 | <0.001 |
| >2 | 3.75 | 2.03–6.93 | <0.001 |
Adjusted for the recipient factors (age, body mass index, race, gender, diabetes), waiting time, the cause of liver disease, blood type, model for end-stage liver disease at transplant encephalopathy at transplant, ascites on at transplant, mechanical ventilation/ICU stay and transplant year, and transplant type.
Discussion
The prevalence of pretransplant renal dysfunction in liver transplant recipients ranges between 15% and 30%.19, 20 In addition, posttransplant ESRD portends inferior outcomes for LTA recipients (5-year patient survival in LTA: 80% in recipients without renal dysfunction vs. 40% in recipients after onset of ESRD).21, 22, 23 Short-term acute renal replacement therapy (<3 months), in pre-LTA period, is associated with relatively small (8.9%) nonrenal recovery at 6 months after transplantation. The 5-year cumulative risk of new-onset ESRD after LTA fluctuates between 3% and 18% depending on “renal risk index” decile. Renal risk index is a validated prognostic tool predicting post-LTA ESRD risk based on 14 different factors at the time of transplantation7, 24 (https://rri.med.umich.edu).
Effect of Renal Function and Dialysis on the Survival Benefit of SLK Over LTA
In the literature, single center reports have been underpowered because of small sample sizes,10, 20, 25, 26 and comprehensive registry analyses have been limited due to center-specific heterogeneity in transplant recipient selection and differences in defining AKI versus CKD. Moreover, dialysis status and duration are not well reported in the OPTN/UNOS or the SRTR datasets.6, 9, 27, 28, 29 Unsurprisingly, these studies have yielded conflicting results concerning the independent protective effect of SLK on posttransplant survival. In a recent publication, Sharma et al.5 analyzed the SRTR data between 2002 and 2009 to study survival benefit of SLK (N = 4283) over LTA (N = 1326) in a PS- and prognostic score-matched study cohort. For patients not on dialysis, SLK offered 3.7 months’ survival benefit whereas only 1.4 months in recipients on dialysis compared with LTA.5
Our study is one of the largest contemporary OPTN/UNOS registry analyses in the post-MELD era that examines the survival benefit of SLK and LTA when candidates are stratified into 4 groups according to the level of renal insufficiency and dialysis status at listing and transplant. We placed more emphasis on patient selection and established the PS matching to allow for meaningful comparisons between SLK and LTA recipients. The results of our study showed that significant survival benefit (23% decrease in relative risk of mortality) of SLK was limited to recipients not exposed to dialysis but still had moderate renal dysfunction (Scr ≥ 2 mg/dl) at the time of transplant. We suspect that this observed reduction in posttransplant mortality rate is likely related to a lower posttransplant ESRD incidence rate. The posttransplant mortality rate exceeds the rate of ESRD in patients exposed to dialysis before transplant (LK3 and LK4) where the priority is primarily to obtain optimal quality of liver to minimize mortality.
Compared with SLK recipients, posttransplant ESRD was significantly higher among LTA patients in the LK2 (moderate CKD) and LK4 groups (AKI requiring dialysis at listing and transplant), probably representing the lower chance of renal recovery. However, the LTA patients with shorter dialysis exposure (LK3 group) experienced similar ESRD and mortality incidence rate compared with the SLK patients suggesting significant renal recovery posttransplantation.
Effect of Organ Quality on Mortality
There are limited data on the effect of donor quality on mortality in liver transplant recipients when comparing SLK with LTA. In their single-center study, Levitsky et al.30 examined the rate of renal recovery post-SLK (N = 155) and found that patient and kidney survival at 1 year was worse among SLK recipients who received expanded criteria donors (59.5% for both outcomes) compared with those who had non-ECD donors (89% and 81%, respectively).30 In our study cohort, before the PS matching across all groups, LTA and SLK recipients were transplanted with significantly better quality organs (L-DRI ≤ 1.2) and SLK patients had lower liver disease severity (lower MELD scores) at transplant. We observed that posttransplant mortality significantly increased with lower quality donors (L-DRI > 1.5 or equivalent of kidney donor profile index > 60%). This SLK survival benefit with higher quality organs was described in another registry analysis.5
This study analysis generates several intertwined ethical questions on fairness in kidney allocation: (i) granting higher quality kidneys to SLK recipients; (ii) unequal access to kidneys (shorter waiting time for SLK, even though a kidney recipient may get equal benefit from the organ); (iii) permitting higher priority to SLK recipients (multi-organ transplants receive precedence over all kidney alone candidates, including highly sensitized candidates and children); and (iv) violation of the UNOS Final Rule (§ 121.8 Allocation of organs based on objective and measurable medical criteria), as transplant centers currently decide who gets SLK.
Given the shortcoming of the current allocation system, in June 2016, the (OPTN/UNOS) Boards of Directors approved a policy launching specific medical eligibility criteria for candidates pursuing SLK transplants. The new criteria include the following: (i) AKI requiring dialysis support or having GFR < 25 ml/min for more than 6 weeks; (ii) established CKD with GFR < 35 ml/min at listing with preceding CKD stage 3 (eGFR < 60 ml/min at least 3 consecutive months); (iii) metabolic diseases (primary hyperoxaluria, atypical hemolytic uremic syndrome with factor H mutation, familial systemic amyloidosis, or methylmalonic aciduria); and (iv) allocation rules (expedited access to deceased donor kidney transplantation for LTA recipients with persistent dialysis need or GFR < 20 ml/min within the first year of liver transplantation).31 Implementation of the policy may take more than a year, and its potential effect on SLK allocation is yet to be seen. Some researchers32, 33 predict a reduction in kidney allograft distribution (approximately 19% decrease) to SLK transplants after the implementation of this policy, whereas others predict an increase in kidney utilization (twice as much in SLK number, sharp increase from 550 to 920 annually).34
Kidney Utilization Conundrum in Liver Transplant Candidates With Renal Dysfunction in the Post-MELD Era
Kidney disease severity is one of the major determinants of wait-list1, 35 and postliver transplant mortality.21, 22, 36 Nevertheless, it is heavily weighted in the current MELD allocation system (overestimating mortality risk),37 which assumes that liver transplant candidates with identical MELD scores achieve similar survival benefit regardless of differences in the individual components of the MELD score.15, 38 However, Sharma et al. demonstrated that, comparing mortality rates between wait-list and postliver transplant, higher Scr at the similar level of MELD scores was associated with decreased survival benefit (no significant advantage of liver transplantation for Scr > 2.5 mg/dl) across different MELD score categories (15–17 and 24–40 at transplant).39 They also observed similar results in recipients on dialysis at transplant (no survival benefit for the MELD score > 27). These observations suggest that the MELD-based allocation may be shifting mortality risk from the wait-list to the posttransplant period in liver recipients with renal dysfunction. Remarkably, the results of our study, when matched with severity of renal and liver disease, support previous reports concerning the inverse relationship between dialysis exposure and the survival advantage with SLK over LTA despite adding a renal transplant to the equation.39, 40, 41
Strength and Limitations
Our study has limitations that are inherent in observational studies. Although we adjusted for various covariates at baseline, we cannot rule out the possibility of residual confounding by unmeasured confounders. Also, we acknowledge potential misclassification about kidney disease etiology, severity, and duration of renal insufficiency. Additional limitations include potential selection bias due to missing data, finally the inability to generalize beyond the group SLK and LTA patients who have similar PSs. On the other hand, we have used a relatively large sample size, carefully stratified groups based on Scr and dialysis status at listing and transplant, and balanced covariates using the PS matching.
In conclusion, survival benefit with SLK is significantly affected by pretransplant dialysis exposure. Donor quality strongly affects this benefit, and these data fail to support the use of kidney allografts with L-DRI ≥ 1.5 in SLK recipients.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the Scientific Registry of Transplant Recipients or the US Government. This research is partly supported by the UT Southwestern O’Brien Kidney Research Core Center (Grant number: P30DK079328).
Footnotes
Tables S1a–S1d. Selected patient characteristics before and after propensity score matching according to renal function and dialysis status by transplant type (LK1–LK4).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Selected patient characteristics before and after propensity score matching according to renal function and dialysis status by transplant type (LK1–LK4).
References
- 1.Wiesner R.H., McDiarmid S.V., Kamath P.S. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 2.Nadim M.K., Davis C.L., Sung R. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant. 2012;12:3119–3127. doi: 10.1111/j.1600-6143.2012.04176.x. [DOI] [PubMed] [Google Scholar]
- 3.Davis C.L., Feng S., Sung R. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7:1702–1709. doi: 10.1111/j.1600-6143.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 4.Eason J.D., Gonwa T.A., Davis C.L. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK) Am J Transplant. 2008;8:2243–2251. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P., Shu X., Schaubel D.E. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pre-transplant renal dysfunction. Liver Transpl. 2016;22:71–79. doi: 10.1002/lt.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke J.E., Warren D.S., Singer A.L. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85:935–942. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P., Goodrich N.P., Zhang M. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135–1142. doi: 10.2215/CJN.09600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Gonzalez E., Meneu-Diaz J.C., Garcia I. Simultaneous liver-kidney transplantation for adult recipients with irreversible end-stage renal disease. Arch Surg. 2004;139:1189–1193. doi: 10.1001/archsurg.139.11.1189. [DOI] [PubMed] [Google Scholar]
- 9.Martin E.F., Huang J., Xiang Q. Recipient survival and graft survival are not diminished by simultaneous liver-kidney transplantation: an analysis of the united network for organ sharing database. Liver Transpl. 2012;18:914–929. doi: 10.1002/lt.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz R., Kunitake H., Wilkinson A.H. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. 2006;141:735–741. doi: 10.1001/archsurg.141.8.735. discussion 741–742. [DOI] [PubMed] [Google Scholar]
- 11.Feng S., Goodrich N.P., Bragg-Gresham J.L. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 12.Rao P.S., Schaubel D.E., Guidinger M.K. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 13.Akkina S.K., Asrani S.K., Peng Y. Development of organ-specific donor risk indices. Liver Transpl. 2012;18:395–404. doi: 10.1002/lt.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volk M.L., Lok A.S., Pelletier S.J. Impact of the model for end-stage liver disease allocation policy on the use of high-risk organs for liver transplantation. Gastroenterology. 2008;135:1568–1574. doi: 10.1053/j.gastro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Schaubel D.E., Sima C.S., Goodrich N.P. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–425. doi: 10.1111/j.1600-6143.2007.02086.x. [DOI] [PubMed] [Google Scholar]
- 16.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Moreau R., Jalan R., Gines P. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 18.D'Agostino R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Thuluvath P.J., Guidinger M.K., Fung J.J. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 20.Brennan T.V., Lunsford K.E., Vagefi P.A. Renal outcomes of simultaneous liver-kidney transplantation compared to liver transplant alone for candidates with renal dysfunction. Clin Transplant. 2015;29:34–43. doi: 10.1111/ctr.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonwa T.A., McBride M.A., Anderson K. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 22.Nair S., Verma S., Thuluvath P.J. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 23.Gonwa T.A., Mai M.L., Melton L.B. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 24.Sharma P., Goodrich N.P., Schaubel D.E. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol. 2013;24:2045–2052. doi: 10.1681/ASN.2013040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northup P.G., Argo C.K., Bakhru M.R. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010;16:440–446. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
- 26.Hibi T., Sageshima J., Molina E. Predisposing factors of diminished survival in simultaneous liver/kidney transplantation. Am J Transplant. 2012;12:2966–2973. doi: 10.1111/j.1600-6143.2012.04121.x. [DOI] [PubMed] [Google Scholar]
- 27.Fong T.L., Khemichian S., Shah T. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94:411–416. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt T.M., Kumer S.C., Al-Osaimi A. Combined liver-kidney and liver transplantation in patients with renal failure outcomes in the MELD era. Transpl Int. 2009;22:876–883. doi: 10.1111/j.1432-2277.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 29.Mindikoglu A.L., Raufman J.P., Seliger S.L. Simultaneous liver-kidney versus liver transplantation alone in patients with end-stage liver disease and kidney dysfunction not on dialysis. Transplant Proc. 2011;43:2669–2677. doi: 10.1016/j.transproceed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levitsky J., Baker T., Ahya S.N. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2949–2957. doi: 10.1111/j.1600-6143.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- 31.Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/news/board-actions-june-2016/. Accessed June 30, 2016.
- 32.Formica R.N., Aeder M., Boyle G. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant. 2016;16:758–766. doi: 10.1111/ajt.13631. [DOI] [PubMed] [Google Scholar]
- 33.Formica R.N., Jr. Simultaneous liver-kidney allocation: let's not make perfect the enemy of good. Am J Transplant. 2016;16:2765. doi: 10.1111/ajt.13873. [DOI] [PubMed] [Google Scholar]
- 34.Wadei H.M., Gonwa T.A., Taner C.B. Simultaneous liver kidney transplant (SLK) allocation policy change proposal: Is it really a smart move? Am J Transplant. 2016;16:2763–2764. doi: 10.1111/ajt.13844. [DOI] [PubMed] [Google Scholar]
- 35.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan Menon K.V., Nyberg S.L., Harmsen W.S. MELD and other factors associated with survival after liver transplantation. Am J Transplant. 2004;4:819–825. doi: 10.1111/j.1600-6143.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P., Schaubel D.E., Sima C.S. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575–1581. doi: 10.1053/j.gastro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Merion R.M., Schaubel D.E., Dykstra D.M. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P., Schaubel D.E., Guidinger M.K., Merion R.M. Effect of pretransplant serum creatinine on the survival benefit of liver transplantation. Liver Transpl. 2009;15:1808–1813. doi: 10.1002/lt.21951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P., Welch K., Eikstadt R. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142–1148. doi: 10.1002/lt.21821. [DOI] [PubMed] [Google Scholar]
- 41.Ruebner R., Goldberg D., Abt P.L. Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction. Am J Transplant. 2012;12:2958–2965. doi: 10.1111/j.1600-6143.2012.04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected patient characteristics before and after propensity score matching according to renal function and dialysis status by transplant type (LK1–LK4).