Abstract
Thyroid stimulating hormone receptor (TSHR) is thought to be a significant candidate for genetic susceptibility to Graves' disease (GD). However, the association between TSHR gene polymorphism and the risk of GD remains controversial. In this study, we investigated the relationship between the two conditions by meta-analysis. We searched all relevant case-control studies in PubMed, Web of Science, CNKI and Wanfang for literature available until May 2015, and chose studies on two single nucleotide polymorphisms (SNPs): rs179247 and rs12101255, within TSHR intron-1. Bias of heterogeneity test among studies was determined by the fixed or random effect pooled measure, and publication bias was examined by modified Begg's and Egger's test. Eight eligible studies with 15 outcomes were involved in this meta-analysis, including 6,976 GD cases and 7,089 controls from China, Japan, Poland, UK and Brazil. Pooled odds ratios (ORs) for allelic comparisons showed that both TSHR rs179247A/G and rs12101255T/C polymorphism had significant association with GD (OR=1.422, 95%CI=1.353–1.495, P<0.001, Pheterogeneity=0.448; OR=1.502, 95%CI: 1.410–1.600, P<0.001, Pheterogeneity=0.642), and the associations were the same under dominant, recessive and co-dominant models. In subgroup analyses, the conclusions are also consistent with all those in Asian, European and South America subgroups (P<0.001). Our meta-analysis revealed a significant association between TSHR rs179247A/G and rs12101255T/C polymorphism with GD in five different populations from Asia, Europe and South America. Further studies are needed in other ethnic backgrounds to independently confirm our findings.
Keywords: Graves' disease, thyroid stimulating hormone receptor, polymorphism, meta-analysis
Introduction
Graves' disease (GD) is one of the most common organ-specific endocrine diseases, and also a multifactorial disease with genetic susceptibilities and environmental factors. It affects up to approximately 1% of the general population[1], and the studies in twins suggest that genetic factors contribute 80% to the etiology of GD[2]. Previous works have identified several gene loci that are associated with the risk to develop GD, which include human leukocyte antigen (HLA)[3-4], cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4)[5], thyroid stimulating hormone receptor (TSHR)[6-9], thyroglobulin (TG)[10-11], protein tyrosine phosphatase (PTPN) gene[12-13], CD40 gene[14-16], Fc receptor-like protein 3 (FCRL3) gene[16], vitamin D receptor (VDR) gene[17], and interleukin-1 gene[18]. In recent years, a number of new genes have been reported which may be associated with the etiology of GD, including the methylenetetrahydrofolate reductase (MTHFR) gene[19], CD24 gene[20], mannose-binding lectin2 (MBL2) gene[21], NACHT leucinerich repeat protein 1 (NLRP1) gene[22], and signal transducer and activator of transcription 3 (STAT3) gene[23]. Among these genes, the TSHR gene is a significant candidate, and failure of tolerance to TSHR is central to the pathogenesis of GD.
Many studies have investigated the association between TSHR gene polymorphism and the risk of GD, but yielded conflicting results. Early case-control studies mainly concentrated on three polymorphic sites, including D36H, P52T and D727E[24-26], but gave mixed results. Later studies focused on SNPs in intron-1, -7 and -8, and significant associations were found in multi-ethnic origins. In 2003, Ho et al.[27] firstly found the association of C/G+63IVS1 (in intron-1) with GD in a cohort of Singaporean patients of multi-ethnic origins. Later, association screening by two independent studies identified strong association within TSHR intron-7 and -8 in Japanese[28] and TSHR intron-1 in UK Caucasian cohorts[29], which firstly provided convincing evidence for TSHR association with GD. Recent studies focus on two SNPs, including rs179247 and rs12101255[30-37], within TSHR intron-1, in Asian, European and South America descents, but still generated conflicting results. To further examine their potential role in influencing the risk of GD, we performed a meta-analysis on case-control studies to investigate their effects in populations.
Materials and methods
Search strategy
We performed an exhaustive search on studies that examined the association of TSHR gene polymorphism with GD. Data were collected from PubMed, Web of Science, CNKI and Wanfang databases, and completed on May 2015. Search strategy was based on combinations of “TSHR”, “thyroid stimulating hormone receptor”, “gene”, “polymorphism”, “thyroid”, “Graves”, “Hashimoto”, and “autoimmune thyroid disease” limited to humans without language restriction. All the searched studies were retrieved, and their references were checked as well for other relevant publications. All searches were conducted independently by two investigators.
Study selection
The following criteria were used for report selection for the meta-analysis: (a) studies evaluating the associations between either one or both of the two TSHR SNPs (rs179247 and rs12101255) with GD risk; (b) in an case-control design; (c) with sufficient data available to estimate an odds ratio (OR) with its 95% confidence interval (95%CI); (d) genotype distributions in the control group should be consistent with Hardy-Weinberg equilibrium (HWE).
Studies were excluded if one of the following was present: (a) case reports, review articles, abstracts and editorials; (b) reports with incomplete data; (c) studies with overlapping samples; (d) articles based on pedigree data; (e) genotype distribution of the controls deviated from HWE.
Data extraction
The following information was extracted from each study by two investigators independently and reached a consensus on all items: first author's name, year of publication, ethnicity, genotyping methods, number of cases and controls, available allele, and genotype frequencies. Not all the necessary statistics were reported directly in the papers; therefore, we had to estimate OR from the reported data as necessary. When studies included two or more populations, the data were extracted separately in all analyses. We defined no minimum number of patients to include a study in our meta-analysis.
Statistical analysis
We firstly assessed HWE in the controls for each study using a goodness-of-fit χ2 analysis, and P<0.05 was considered as significant disequilibrium. Heterogeneity between the studies was evaluated with χ2-based Q statistic and I 2 metric. P>0.1 indicated a lack of heterogeneity across studies, allowing for the use of a fixed-effects model. Otherwise, a random--effects model was used. Association between GD and rs179247 and rs12101255 polymorphisms was estimated using OR, with the corresponding 95%CI. The significance of pooled OR was determined by Z-test, and P<0.05 was considered statistically significant. We performed primary analyses using the per-allele model for each polymorphism, and addressed a dominant model, a recessive model, and a co-dominant model as secondary analyses. We also carried out stratified analyses by ethnicity in rs179247. Sensitivity analysis was conducted by sequentially excluding a single study each time to identify the potential influence of individual data set to pooled ORs. Potential publication bias was estimated using modified Begg's and Egger's tests. The significance of intercept was determined by the t-test suggested by Egger's test (P<0.05 was considered representative of statistically significant publication bias). All of the statistical analyses were performed using Stata version 11.0 (Stata, College Station, TX, USA).
Results
Study characteristics
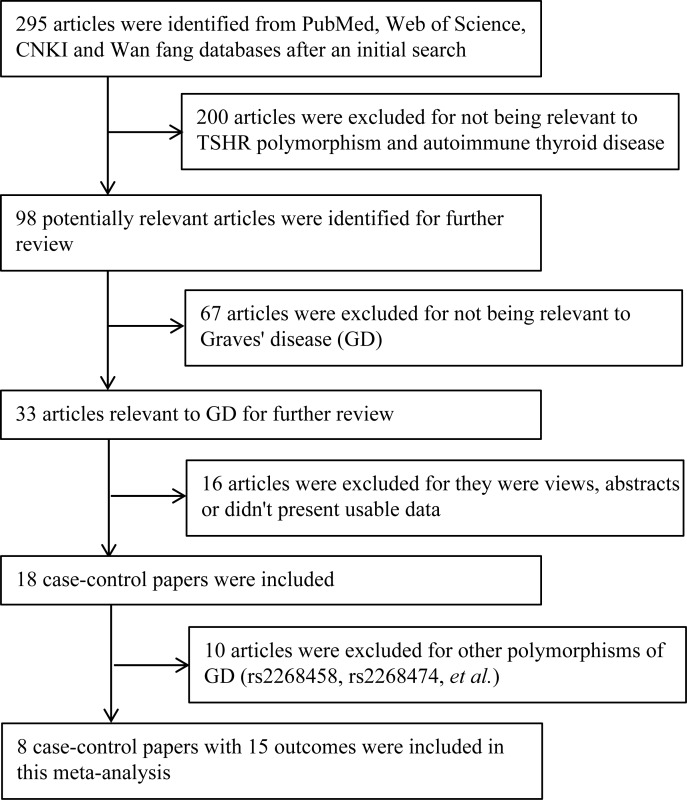
The detailed steps of our literature search are shown in Fig. 1. According to the study inclusion criteria, a total of eight relevant case-control studies with different samples were selected in the meta-analysis, including 6,976 GD cases and 7,089 controls of five different populations. Studies had been carried out in China, Japan, Poland, UK and Brazil. One of these reports included three populations, and we included this report as three separate studies. Therefore, a total of 10 association studies were considered for association between the rs179247 and rs12101255 polymorphism and GD. Characteristics of each study are shown in Table 1 and Table 2. These studies included 15 association outcomes; in which 10 examined association between rs179247 polymorphism (6,976 cases and 7,089 controls) and GD, and five for the rs12101255 polymorphism (4,430 cases and 4,512 controls). Among 10 studies, four were from Caucasians [30-31], five were from Asians[32-36], and one was from South America[37]. The allele and genotype distributions in the included studies are summarized in Table 1 and Table 2 for TSHR rs179247 and rs12101255 polymorphisms, respectively.
Fig. 1. Selection of articles for inclusion and excluding in the meta-analysis.
Table 1. Characteristics of the rs179247A/G polymorphism allelic and genotype distribution for GD risk in studies included in the meta-analysis.
| Authors[ref.] | Year | Ethnicity | Methods | HWE | Total/Genotypes(AA/AG/GG) | A allele frequency(%) | OR(95% CI) | ||
| Cases | Controls | Cases | Controls | ||||||
| Oliver J. Brand et al | 2009 | European | Taq man | 0.454 | 768 (279 /359 /100) | 768 (182/ 322 / 160) | 46.0 | 38.0 | 1.54 (1.32–1.78) |
| Rafał Płoski et al | 2010 | European Set 1 | Taq man | 0.443 | 558 (139 / 270 /149) | 520 (81 / 259 / 180) | 49.1 | 40.5 | 1.42 (1.20–1.68) |
| Rafał Płoski et al | 2010 | European Set 2 | Taq man | 0.857 | 196 (58 / 84 / 54) | 198 (34 / 98 / 67) | 51.0 | 41.7 | 1.46 (1.10–1.93) |
| Rafał Płoski et al | 2010 | European Set 3 | Taq man | 0.393 | 2504 (879 /1110 / 351) | 2784 (737 /1243 / 561) | 61.3 | 53.5 | 1.38 (1.27–1.49) |
| Shaoying Yang et al | 2011 | Asian | Taq man | 0.471 | 1066 (564/427/75) | 1107 (486/503/118) | 72.9 | 66.6 | 1.35 (1.19-1.54) |
| Lin Liu et al | 2012 | Asian | MALDI-TOFMS | 0.504 | 404 (230/140/24) | 242 (120/88/20) | 76.14 | 71.93 | 1.25 (0.96-1.62) |
| Ran Wang et al | 2012 | Asian | Taq man | 0.859 | 618 (353/220/45) | 646 (260/298/88) | 74.92 | 63.31 | 1.73 (1.46-2.05) |
| Haili Wang et al | 2013 | Asian | Taq man | 0.713 | 471 (258/174/38) | 472 ( 216/204/52) | 73.4 | 67.4 | 1.34 (1.10-1.63) |
| N. Inoue1 et al | 2013 | Asian | PCR-RFLP | 0.066 | 112 (57/44/11) | 56 (18/33/5) | 70.5 | 61.6 | 1.49 (0.93-1.50) |
| N. E. Bufalo et al | 2015 | South America | Taq man | 0.213 | 279 (117/138/24) | 296 (92/154/50) | 66.7 | 57.1 | 2.82 (1.56–4.99) |
Table 2. Characteristics of the rs12101255T/C polymorphism allelic and genotype distribution for GD risk in studies included in the meta-analysis.
| Authors[ref.] | Year | Ethnicity | Methods | HWE | Total/Genotypes(TT /TC/ CC) | T allele frequency(%) | OR(95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||
| Oliver J. Brand et al | 2009 | European | Taq man | 0.964 | 768 (150/345 /197) | 768 (89/ 295 / 268) | 35.0 | 28.0 | 1.55( 1.33–1.81) |
| Rafał Płoski et al | 2010(1) | European Set 1 | Taq man | 0.473 | 558 (75 /245 /238) | 520 (35 / 212 / 273) | 35.4 | 27.1 | 1.47( 1.23–1.77) |
| Rafał Płoski et al | 2010(2) | European Set 2 | Taq man | 0.261 | 196 (32 /94 /70) | 198 (17 /71 /110) | 40.3 | 26.5 | 1.87( 1.39–2.53) |
| Rafał Płoski et al | 2010(3) | European Set 3 | Taq man | 0.412 | 2504 (482/1136 / 687) | 2784 (338/1148 / 1046) | 45.6 | 36.0 | 1.49( 1.37–1.61) |
| Lin Liu et al | 2012 | Asian | MALDI-TOFMS | 0.550 | 404 (187/179/38) | 242 (86/119/35) | 68.44 | 60.63 | 1.41(1.11-1.78) |
Meta-analysis
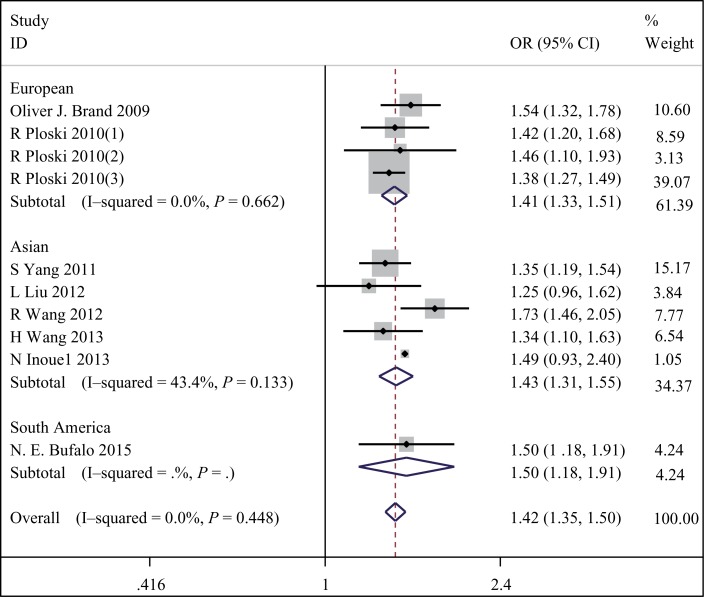
To summarize the published data, we performed a comprehensive meta-analysis. Table 3 shows the pooled ORs and heterogeneity tests from different analysis models. We firstly pooled overall effects for rs179247A/G polymorphism and GD risk in 10 studies with a total of 6,976 cases and 7,089 controls. A significant association between the TSHR rs179247 A/G polymorphism and GD was found, and the pooled OR for allelic frequency comparison showed that A allele significantly increased the risk of GD in people from China, Japan, Poland, UK and Brazil, with OR=1.422, 95%CI=1.353–1.495, P<0.001, Pheterogeneity=0.448. Moreover, this significant association was found in all genetic models (dominant model: OR=1.632, 95%CI=1.484–1.796, P<0.001, Pheterogeneity=0.537; recessive model: OR=1.552, 95%CI=1.445–1.667, P<0.001, Pheterogeneity=0.264; and co-dominant model: OR=2.022, 95%CI=1.816–2.252, P<0.001, Pheterogeneity=0.653). Then, subgroups analysis for ethnicityt was performed, and the results were similar that rs179247 A/G polymorphism was also significantly associated with increased GD risk both in two Asian populations (OR=1.426, 95%CI: 1.310–1.553, P<0.001, Pheterogeneity=0.133), two European populations (OR=1.415, 95%CI: 1.327–1.508, P<0.001, Pheterogeneity=0.662) and one South America population (OR=1.503, 95%CI: 1.183–1.910, P<0.001, Pheterogeneity=0), and the association was the same under dominant, recessive and co-dominant models. Furthermore, whatever we calculated the pooled OR for rs179247 A/G polymorphism and GD risk under random or fixed effects model risk, the results were similar. It indicated that there was no significant heterogeneity among these studies (Table 1, 3 and Fig. 2).
Table 3. Pooled measures for the association between the rs179247A/G and rs12101255T/C polymorphisms and susceptibility to GD risk.
| SNPs | Inherited model | Ethnicity | Studies(cases/controls) | Model | Effects | |||
| p | I2(%) | OR(95%CI) | p | |||||
| rs179247 | Per allele (A vs. G) | overall | 10(6976/7089) | 0.448 | 0 | FEM | 1.422(1.353-1.495) | <0.0001 |
| European | 4(4026/4270) | 0.662 | 0 | FEM | 1.415(1.327-1.508) | <0.0001 | ||
| Asian | 5(2671/2523) | 0.133 | 43.4 | FEM | 1.426(1.310-1.553) | <0.0001 | ||
| South America | 1(279/296) | 0 | 0 | FEM | 1.503(1.183-1.910) | 0.001 | ||
| Dominant (AA +AG vs. GG) | overall | 10(6976/7089) | 0.537 | 0 | FEM | 1.632(1.484-1.796) | <0.0001 | |
| European | 4(4026/4270) | 0.263 | 24.7 | FEM | 1.618(1.447-1.810) | <0.0001 | ||
| Asian | 5(2671/2523) | 0.589 | 0 | FEM | 1.609(1.327-1.952) | <0.0001 | ||
| South America | 1(279/296) | 0 | 0 | FEM | 2.160(1.287-3.623) | 0.004 | ||
| Recessive (AA vs. AG+GG) | overall | 10(6976/7089) | 0.264 | 19.4 | FEM | 1.552(1.445-1.667) | <0.0001 | |
| European | 4(4026/4270) | 0.395 | 0 | FEM | 1.550(1.406-1.710) | <0.0001 | ||
| Asian | 5(2671/2523) | 0.086 | 51.0 | REM | 1.561(1.311-1.858) | <0.0001 | ||
| South America | 1(279/296) | 0 | 0 | FEM | 1.601(1.137-2.255) | 0.007 | ||
| Co-dominant (AA vs. GG) | overall | 10(6976/7089) | 0.653 | 0 | FEM | 2.022(1.816-2.252) | <0.0001 | |
| European | 4(4026/4270) | 0.572 | 0 | FEM | 2.028(1.779-2.312) | <0.0001 | ||
| Asian | 5(2671/2523) | 0.443 | 0 | FEM | 1.937(1.585-2.368) | <0.0001 | ||
| South America | 1(279/296) | 0 | 0 | FEM | 2.649(1.516-4.630) | 0.001 | ||
| rs12101255 | Per allele (T vs. C) | overall | 5(4430/4512) | 0.642 | 0 | FEM | 1.502(1.410-1.600) | <0.0001 |
| Dominant (TT + TC vs. CC) | overall | 5(4430/4512) | 0.528 | 0 | FEM | 1.672(1.525-1.834) | <0.0001 | |
| Recessive (TT vs. TC + CC) | overall | 5(4430/4512) | 0.766 | 0 | FEM | 1.741(1.548-1.957) | <0.0001 | |
| Co-dominant (TT vs. CC) | overall | 5(4430/4512) | 0.882 | 0 | FEM | 2.236(1.956-2.556) | <0.0001 | |
Fig. 2. Stratified analysis pooled odds ratios for the association between the rs179247A/G polymorphism and susceptibility to GD risk.
The association between rs12101255 T/C polymorphism and GD was investigated in five studies with a total of 4,430 cases and 4,512 controls. For rs12101255 T/C polymorphism, T allele displayed an increased frequency in GD cases from these four different counties. There was a significant association between rs12101255 T/C polymorphism and GD risk in additive (OR=1.502, 95%CI: 1.410–1.600, P<0.001, Pheterogeneity=0.642), dominant (OR=1.672, 95%CI: 1.525–1.834, P<0.001, Pheterogeneity=0.528), recessive (OR=1.741,95%, CI:1.548–1.957, P<0.001, Pheterogeneity=0.766) and co-dominant (FEM OR=2.236, 95%CI: 1.956–2.556, P<0.001, Pheterogeneity=0.882) models (Table 2, 3 and Fig. 3).
Fig. 3. Stratified analysis pooled odds ratios for the association between the rs12101255T/C polymorphism and susceptibility to GD risk.
In conclusion, our meta-analysis revealed significant association between TSHR rs179247A/G and rs12101255T/C polymorphisms with GD in four different populations from Asia and Europea.
Heterogeneity and sensitivity analyses
As shown in Table 3, no significant heterogeneity was observed among studies for rs179247A/G and rs12101255 T/C polymorphisms in all models in overall populations. However, when the data were stratified by ethnicity, the heterogeneity among studies of rs179247A/G polymorphism was eliminated in Asian descents in allele and recessive models. Therefore, in these two models, we chose a fixed-effects model, and in the other models, a random-effects model was used.
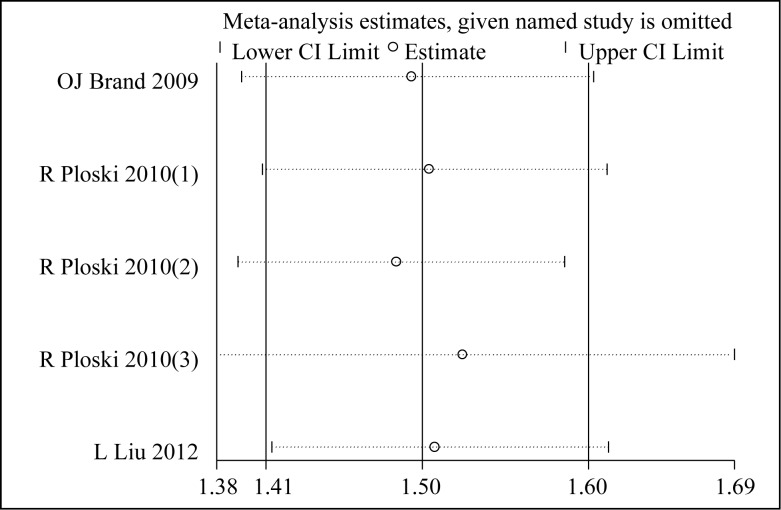
Sensitivity analyses were conducted to assess whether each individual study influenced the final results (Fig. 4 and Fig. 5). In the sensitivity analysis, the influence of each study on the pooled OR was examined by repeating the meta-analysis while omitting each study, one at a time. The results showed no significant changes in the pooled OR, indicating that the results of our meta-analysis were reliable and stable.
Fig. 4. Sensitivity analyses for rs179247A/G polymorphisms in GD.
Fig. 5. Sensitivity analyses for rs12101255T/C polymorphisms in GD.
Evaluation of publication bias
Publication bias was performed using Egger's test (Table 4) and Begg's funnel plots. The funnel plots were asymmetrical and the statistical results showed no publication biases for any of the polymorphisms examined (rs179247A/G: P=0.898; rs12101255 T/C: P=0.688), indicating that our results were statistically reliable.
Table 4. Egger's publication bias test for rs179247A/G and rs12101255T/C polymorphisms in GD.
| Coef. (a) | Std. Err. (b) | t | P>t | 95% CI of intercept | ||
| rs179247A/G | –.2301901 | 1.744951 | -0.13 | 0.898 | -4.254053 | 3.793673 |
| rs12101255T/C | –3.959451 | 8.958683 | -0.44 | 0.688 | -32.46998 | 24.55108 |
a Coefficient; b Standard error.
Discussion
TSHR is primarily expressed on thyroid follicular cell surface membrane, and via its ligand, TSH, regulates thyroid growth and hormone production. However, in autoimmune thyroid disease (AITD) patients, the body produces auto-antibodies including thyroid-stimulating antibody (TSAb) and TSH-stimulation blocking antibody (TSBAb) against TSHR, and TSAb have a predominant effect in GD affecting thyroid cell growth and differentiation and finally leading to thyroid dysfunction[38]. Genetic variation within TSHR region may influence TSHR structure, expression and/or post-translational processing, which in turn could initiate or exacerbate autoimmune response against TSHR in GD.
TSHR gene is located on chromosome 14q31 and contained 10 exons and 9 introns. Early case-control studies on TSHR gene single nucleotide polymorphisms mainly concentrated on three polymorphic sites, including D36H, P52T and D727E[24-26], and no associations of GD were found with all the three polymorphisms. Later studies turned to focus on SNPs in intron-1, -7 and -8, and significant associations were found in multi-ethnic origins. In 2003, Ho et al.[27] found the association of C/G+63IVS1 (in intron-1) with GD in a cohort of mixed Chinese, Indian and Malay ancestry. In 2005, Hiratani et al.[28] found that the strong association within TSHR intron-7 and -8 in Japanese (especially rs2268475, rs1990595 and rs3783938), and the study also investigated three variants within intron-1 and identified some evidence of association with TSHR intron-1 SNP, rs2268474 (P=0.026) in the Japanese, and TSHR gene became the first GD’s specific locus. Later, Dechairo et al.[29] found that the strongest association for SNP was rs2268458 in intron-1 in a large Caucasian cohort, and it is the first time that the association of TSHR rs2268458 with GD was demonstrated in a large sample cohort. More recently, several case-control studies provided a comprehensive analysis of TSHR association, with the strongest SNP associations at rs179247 and rs12101255.
rs179247 and rs12101255 were both in intron-1, and although early genetic studies provided inconclusive evidence of TSHR association with GD, our results indicated that rs179247 and rs12101255 polymorphisms were GD susceptibility loci in five populations (Chinese, Japanese, Polish, British and Brazil). The associations were the same under all genetic models. In subgroup analyses, the conclusion is also consistent with those in Asian, European and South America subgroups. However, according to another case-control cohort study in China[39], which involved 199 GD patients and 208 control subjects, three SNPs (rs179247, rs12101255 and rs2268458) were not involved in the pathogenesis of GD in a northern Chinese population. This difference may be attributed to its small sample size. In one of the included studies, Liu et al.[33] detected five SNPs (rs179247, rs12101255, rs2268475, rs1990595, and rs3783938) in TSHR gene to evaluate the association between SNP of TSHR and AITD in a Chinese Han population. However, none of the three SNPs in intron-7 and -8 (rs2268475, rs1990595, and rs3783938) was associated with GD pathogenesis, and these were different from those on the Japanese population[28], which investigated 22 intronic SNPs across the TSHR region and identified single SNP association with GD primarily located within intron-7 of TSHR. However, in Liu’ study, the result suggested rs3783938 within intron-8 was significantly increased in HT patients. In another study, Brand et al.[30] screened an extended region of TSHR (800Kb) and investigated a combined panel of 98 SNPs in a UK Caucasian cohort. In this study, 28 SNPs revealed evidence of association with GD (P<0.05), all of which were located within a 340 kb window spanning TSHR intron-1 and neighbouring C14ORF145. The strongest SNP associations were all located within intron-1 of TSHR and were separated by just 18.5 kb; it ruled out any association within intron-7 and -8 in Caucasians. This once again serves to highlight the presence of geographical genetic variation and the need for detailed analysis in different populations; therefore, further studies are now required in Japanese and other ethnic backgrounds to independently confirm previous reports of association within TSHR intron-7 and -8.
Furthermore, recently a new study drew an conclusion that allele A of rs179247 polymorphism in TSHR gene was associated with lower risk of Graves’ orbitopathy (GO) in GD patients, but only in young patients, not in either elderly patients or the group analyzed as a whole[40]. However, our article showed that allele A of rs179247 polymorphism in TSHR gene can increase the risk of GD. This should also be focused as a new research subject for us to study next step and find out association between TSHR gene polymorphism and the risk of GO.
Since rs179247 and rs12101255 are located 18.5Kb apart within TSHR intron-1, it is necessary to resolve if the two SNPs had independent effects or if the effect of one SNP was secondary to linkage disequilibrium (LD) with the other. Logistic regression was unable to split association between rs179247 and rs12101255, and different studies suggested different conclusions. The case-control studies in three European Caucasian cohorts[31] and in a Chinese Han population from seaboard in Shandong[35] suggested that association at rs179247 may be driven by rs12101255 or that rs12101255 was in stronger LD with the aetiological variants within the region, while another study on Chinese cohort[32] suggested that rs179247 may be the major susceptibility locus. Diversity of races or other different elements were possible problems, and interactions with other SNPs that have not been reported cannot be ignored. One of the included studies[31] revealed that a number of SNPs were in strong LD (r²>.0.80) with rs17927, locating within 5 kb of rs179247 itself within TSHR intron-1. At least four additional SNPs were in strong LD (r²>0.80) with rs12101255, and all located within the proposed 40 kb region, separated by just 18.5 kb from rs12101255.
Although the studies have identified two highly associated-SNPs with GD, the pathogenesis of AITD has not been fully elucidated. It has been suggested that TSHR sometimes undergoes post-translational intra-molecular cleavage into distinct A and B subunits[41], and that TSHR A-subunit is the critical autoantigen and shedding of the A subunit may result in the production of TSAb[42]. ST4 and ST5, two of the truncated TSHR mRNA transcripts, encode the majority of soluble A-subunit directly. Thus, increased ST4 and ST5 transcription may enhance the generation of a shed A subunit and trigger autoimmune response and the production of TSHR autoantibodies[40]. Furthermore, it has been found that genotypes of GD-associated SNPs were associated with expression of ST4 and ST5[30]. In rs179247 polymorphism of TSHR gene, the frequency of AA genotype and A allele was higher among GD patients compared with controls, and ST4 expression was higher in AA genotype compared with GG genotype as well. A similar trend of expression was found between ST5 and rs179247 genotypes, although not significant. The other SNP highly associated with GD, rs12101255, also revealed higher ST4 and ST5 mRNA expression with genotype, and GD-associated TT genotype revealed a higher expression of ST4 and ST5. These may support the hypothesis for GD pathogenesis.
In conclusion, our meta-analysis confirmed strong association of TSHR intron-1 rs179247 and rs12101255 polymorphisms with GD in five different populations from Asian, European and South America. Further studies are required in other ethnic backgrounds to independently confirm previous reports of association within TSHR intron, which may lead to advances towards developing TSHR targeted therapeutics.
Acknowledgements
Author contribution statement
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81102032).
References
- [1].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III)[J]. J Clin Endocrinol Metab, 2002, 87(2): 489-499. [DOI] [PubMed] [Google Scholar]
- [2].Brix TH, Kyvik KO, Christensen K, et al. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts[J]. J Clin Endocrinol Metab, 2001, 86(2): 930-934. [DOI] [PubMed] [Google Scholar]
- [3].Li Y, Yao Y, Yang M, et al. Association between HLA-B*46 allele and Graves’ disease in Asian populations: a meta-analysis[J]. Int J Med Sci, 2013, 10(2): 164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hodge SE, Ban Y, Strug LJ, et al. Possible interaction between HLA-DRbeta1 and thyroglobulin variants in Graves’ disease[J]. Thyroid, 2006, 16(4): 351-355. [DOI] [PubMed] [Google Scholar]
- [5].Du L, Yang J, Huang J, et al. The associations between the polymorphisms in the CTLA-4 gene and the risk of Graves’ disease in the Chinese population[J]. BMC Med Genet, 2013, 14(46): 2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shao L, Jiang H, Liang J, et al. Study on the relationship between TSHR gene and thyroid diseases[J]. Cell Biochem Biophys, 2011, 61(2): 377-382. [DOI] [PubMed] [Google Scholar]
- [7].Drexhage HA. Are there more than antibodies to the thyroid-stimulating hormone receptor that meet the eye in Graves’ disease[J]. Endocrinology, 2006, 147(1): 9-12. [DOI] [PubMed] [Google Scholar]
- [8].Chistiakov DA. Thyroid-stimulating hormone receptor and its role in Graves’ disease[J]. Mol Genet Metab, 2003, 80(4): 377-388. [DOI] [PubMed] [Google Scholar]
- [9].Yin X, Latif R, Bahn R, et al. Influence of the TSH receptor gene on susceptibility to Graves’ disease and Graves’ ophthalmopathy[J]. Thyroid, 2008, 18(11): 1201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ban Y, Tozaki T, Taniyama M, et al. Multiple SNPs in intron 41 of thyroglobulin gene are associated with autoimmune thyroid disease in the Japanese population[J]. PLoS One, 2012, 7(5): e37501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caputo M, Rivolta CM, Mories T, et al. Analysis of thyroglobulin gene polymorphisms in patients with autoimmune thyroiditis[J]. Endocrine, 2010, 37(3): 389-395. [DOI] [PubMed] [Google Scholar]
- [12].Zeitlin AA, Heward JM, Brand OJ, et al. Use of Tag single nucleotide polymorphisms (SNPs) to screen PTPN21: no association with Graves’ disease[J]. Clin Endocrinol (Oxf), 2006, 65(3): 380-384. [DOI] [PubMed] [Google Scholar]
- [13].Chabchoub G, Teixiera EP, Maalej A, et al. The R620W polymorphism of the protein tyrosine phosphatase 22 gene in autoimmune thyroid diseases and rheumatoid arthritis in the Tunisian population[J]. Ann Hum Biol, 2009, 36(3): 342-349. [DOI] [PubMed] [Google Scholar]
- [14].Hsiao JY, Hsieh MC, Hsiao CT, et al. Association of CD40 and thyroglobulin genes with later-onset Graves’ disease in Taiwanese patients[J]. Eur J Endocrinol, 2008, 159(5): 617-621. [DOI] [PubMed] [Google Scholar]
- [15].Tomer Y, Concepcion E, Greenberg DA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves’ disease[J]. Thyroid, 2002, 12(12): 1129-1135. [DOI] [PubMed] [Google Scholar]
- [16].Inoue N, Watanabe M, Yamada H, et al. Associations between autoimmune thyroid disease prognosis and functional polymorphisms of susceptibility genes, CTLA4, PTPN22, CD40, FCRL3, and ZFAT, previously revealed in genome-wide association studies[J]. J Clin Immunol, 2012, 32(6): 1243-1252. [DOI] [PubMed] [Google Scholar]
- [17].Chen RH, Chang CT, Chen HY, et al. Association between vitamin-D receptor gene FokI polymorphism and Graves’ disease among Taiwanese Chinese[J]. J Clin Lab Anal, 2007, 21(3): 173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu N, Li X, Liu C, et al. The association of interleukin-1alpha and interleukin-1beta polymorphisms with the risk of Graves’ disease in a case-control study and meta-analysis[J]. Hum Immunol, 2010, 71(4): 397-401. [DOI] [PubMed] [Google Scholar]
- [19].Mao R, Fan Y, Zuo L, et al. Association study between methylenetetrahydrofolate reductase gene polymorphisms and Graves’ disease[J]. Cell Biochem Funct, 2010, 28(7): 585-590. [DOI] [PubMed] [Google Scholar]
- [20].Inoue N, Watanabe M, Hayashi F, et al. The association between a functional polymorphism in the CD24 gene and the development of autoimmune thyroid diseases[J]. Tissue Antigens, 2013, 81(3): 161-163. [DOI] [PubMed] [Google Scholar]
- [21].Filho CB, Rodrigues FF, Segat L, et al. Association of MBL2 gene exon 1 variants with autoimmune thyroid disease in Brazilian patients[J]. Int J Immunogenet, 2012, 39(4): 357-361. [DOI] [PubMed] [Google Scholar]
- [22].Alkhateeb A, Jarun Y, Tashtoush R. Polymorphisms in NLRP1 gene and susceptibility to autoimmune thyroid disease[J]. Autoimmunity, 2013, 46(3): 215-221. [DOI] [PubMed] [Google Scholar]
- [23].Xiao L, Muhali FS, Cai TT, et al. Association of single-nucleotide polymorphisms in the STAT3 gene with autoimmune thyroid disease in Chinese individuals[J]. Funct Integr Genomics, 2013, 13(4): 455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ban Y, Greenberg DA, Concepcion ES, et al. A germline single nucleotide polymorphism at the intracellular domain of the human thyrotropin receptor does not have a major effect on the development of Graves’ disease[J]. Thyroid, 2012, 12(12): 1079-1083. [DOI] [PubMed] [Google Scholar]
- [25].Muhlberg T, Herrmann K, Joba W, et al. Lack of association of nonautoimmune hyperfunctioning thyroid disorders and a germline polymorphism of codon 727 of the human thyrotropin receptor in a European Caucasian population[J]. J Clin Endocrinol Metab, 2000, 85(8): 2640-2643. [DOI] [PubMed] [Google Scholar]
- [26].Chistiakov DA, Savost’anov KV, Turakulov RI, et al. Further studies of genetic susceptibility to Graves’ disease in a Russian population[J]. Med Sci Monit, 2002, 8(3): 180-184. [PubMed] [Google Scholar]
- [27].Ho SC, Goh SS, Khoo DH. Association of Graves’ disease with intragenic polymorphism of the thyrotropin receptor gene in a cohort of Singapore patients of multi-ethnic origins[J]. Thyroid, 2003, 13(6): 523-528. [DOI] [PubMed] [Google Scholar]
- [28].Hiratani H, Bowden DW, Ikegami S, et al. Multiple SNPs in intron 7 of thyrotropin receptor are associated with Graves’ disease[J]. J Clin Endocrinol Metab, 2005, 90(5): 2898-2903. [DOI] [PubMed] [Google Scholar]
- [29].Dechairo BM, Zabaneh D, Collins J, et al. Association of the TSHR gene with Graves’ disease: the first disease specific locus[J]. Eur J Hum Genet, 2005, 13(11): 1223-1230. [DOI] [PubMed] [Google Scholar]
- [30].Brand OJ, Barrett JC, Simmonds MJ, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves’ disease[J]. Hum Mol Genet, 2009, 18(9): 1704-1713. [DOI] [PubMed] [Google Scholar]
- [31].Ploski R, Brand OJ, Jurecka-Lubieniecka B, et al. Thyroid stimulating hormone receptor (TSHR) intron 1 variants are major risk factors for Graves’ disease in three European Caucasian cohorts[J]. PLoS One, 2010(11), 5e15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang SY, Liu W, Xue LQ. Association between TSHR gene intron1 polymorphisms and Graves’ disease[J]. Chin J Endocrinol Metab (in Chinese), 2011, 27(3): 478-481. [Google Scholar]
- [33].Liu L, Wu HQ, Wang Q, et al. Association between thyroid stimulating hormone receptor gene intron polymorphisms and autoimmune thyroid disease in a Chinese Han population[J]. Endocr J, 2012, 59(8): 717-723. [DOI] [PubMed] [Google Scholar]
- [34].Wang R, Zhang XM, Liu BL, et al. Association between thyroid stimulating hormone receptor gene1 intron polymorphisms and Graves’ disease[J]. Chin J Endocrinol Metab (in Chinese), 2012, 28(4): 306-310. [Google Scholar]
- [35].Wang HL, Yang LH, Jia ZT. Association between thyroid stimulating hormone receptor gene polymorphisms and autoimmune thyroid disease in a Chinese Han population from seaboard in Shangdong provience[J]. Chin J Endocrinol Metab (in Chinese), 2013, 29(4): 108-111. [Google Scholar]
- [36].Inoue N, Watanabe M, Katsumata Y, et al. Different genotypes of a functional polymorphism of the TSHR gene are associated with the development and severity of Graves’ and Hashimoto’s diseases[J]. Tissue Antigens, 2013, 82(4): 288-290. [DOI] [PubMed] [Google Scholar]
- [37].Bufalo NE, Dos Santos RB, Marcello MA, et al. TSHR intronic polymorphisms (rs179247 and rs12885526) and their role in the susceptibility of the Brazilian population to Graves’ disease and Graves’ ophthalmopathy[J]. J Endocrinol Invest, 2015, 38(5): 555-561 [DOI] [PubMed] [Google Scholar]
- [38].Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies[J]. Thyroid, 2007, 17(10): 923-938. [DOI] [PubMed] [Google Scholar]
- [39].Xu LD, Zhang XL, Sun HM, et al. Lack of association between thyroid-stimulating hormone receptor haplotypes and Graves’ disease in a northern Chinese population. [J] Tissue Antigens, 2011(1), 77: 2. [DOI] [PubMed] [Google Scholar]
- [40].Beata JL, Rafal P, Dorota K. Association between Polymorphisms in the TSHR Gene and Graves’ Orbitopathy[J]. PLOS ONE, 2014, 9(7): e102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brand OJ, Gough SC. Genetics of thyroid autoimmunity and the role of the TSHR[J]. Mol Cell Endocrinol, 2010, 322(1-2): 135-143. [DOI] [PubMed] [Google Scholar]
- [42].Chen CR, Pichurin P, Nagayama Y, et al. The thyrotropin receptor autoantigen in Graves’ disease is the culprit as well as the victim[J]. J Clin Invest, 2003, 111(12): 1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]