Abstract
Chronic intermittent hypoxia is considered to play an important role in cardiovascular pathogenesis during the development of obstructive sleep apnea (OSA). We used a well-described OSA rat model induced with simultaneous intermittent hypoxia. Male Sprague Dawley rats were individually placed into plexiglass chambers with air pressure and components were electronically controlled. The rats were exposed to intermittent hypoxia 8 hours daily for 5 weeks. The changes of cardiac structure and function were examined by ultrasound. The cardiac pathology, apoptosis, and fibrosis were analyzed by H&E staining, TUNNEL assay, and picosirius staining, respectively. The expression of inflammation and fibrosis marker genes was analyzed by quantitative real-time PCR and Western blot. Chronic intermittent hypoxia/low pressure resulted in significant increase of left ventricular internal diameters (LVIDs), end-systolic volume (ESV), end-diastolic volume (EDV), and blood lactate level and marked reduction in ejection fraction and fractional shortening. Chronic intermittent hypoxia increased TUNNEL-positive myocytes, disrupted normal arrangement of cardiac fibers, and increased Sirius stained collagen fibers. The expression levels of hypoxia induced factor (HIF)-1α, NF-kB, IL-6, and matrix metallopeptidase 2 (MMP-2) were significantly increased in the heart of rats exposed to chronic intermittent hypoxia. In conclusion, the left ventricular function was adversely affected by chronic intermittent hypoxia, which is associated with increased expression of HIF-1α and NF-kB signaling molecules and development of cardiac inflammation, apoptosis and fibrosis.
Keywords: obstructive sleep apnea, model chronic intermittent hypoxia, cardiac dysfunction, inflammation
Introduction
Obstructive sleep apnea (OSA), which is a common sleep-related breathing disorder with repetitive upper airway collapse during sleep[1], is a global health problem affecting at least 10% of the general population[2-5]. Recurrent episodes of upper airway collapse and obstruction lead to chronic intermittent hypoxia (CIH), oxygen desaturation, sleep fragmentation, arousal and daytime sleepiness. All of these changes can further cause significant endocrine and metabolic disturbance, increasing the risk of metabolic and cardiovascular diseases[6-7]. Moreover, OSA activates the sympathetic nervous system and inflammatory pathways[8].
The relevant factors of OSA to cardiovascular consequences include chronic intermittent hypoxia, exaggerated swings of intrathoracic pressure, and post-apneic arousal[9]. CIH is considered to play an important role in cardiovascular pathogenesis during the development of OSA. CIH animal models of chronic intermittent hypoxia have been widely used in the research of OSA[10]. There are few data available on cellular and molecular mechanism of cardiac injury in the CIH model. How exactly CIH triggers pathophysiological changes in the cardiovascular system has not been completely elucidated. In the present study, we use a rat model of CIH to observe OSA-induced changes in the heart and delineate the underlying mechanism for such changes.
Materials and methods
Rat OSA model
Ten weeks old male Sprague Dawley (SD) rats (body weight about 250 g) were purchased from the Laboratory Animal Center of Southeast University. All animal protocols were reviewed and approved by the Institutional Animal Care and Usage Committee of Southeast University. The rats were kept in a temperature-controlled (22-24°C) room with 12-hour light and 12-hour dark cycle and allowed to acclimate to new environment for a week. Sixteen rats were randomly divided into the control and OSA groups. Each rat was placed in a 40 cm × 30 cm × 18 cm (length × width × height) plexiglass container with a control device from Puhe Biotechnology (Jiangyin, Jiangsu, China). The rats in the control group received air while OSA rats were given intermittent low oxygen (O2) for 5 weeks with a cycling program 8 hours a day (9:00 am to 5:00 pm) with N2 injection for 20 seconds to lower O2 to 7.4% to 7.8% and pressure was lowered to 600 mmHg for 12 seconds before injecting air for 28 seconds to recover O2 to 21% and ambient pressure. At the end of 5-week treatment, the rats were scanned on a VisualsonicsVevo 2100 ultrasound system (Toronto, Canada) before the rat heart tissues were harvested and processed for histology, protein, and RNA works. Blood gas analysis was performed by the central laboratory of the Affiliate Hospital of South-eastern University.
Haematoxylin and eosin (H&E) staining
Frozen sections (5 µm thick) were fixed in 4% paraformaldehyde for 10 to 30 seconds, rinsed, stained in hematoxylin for 3 to 5 minutes, rinsed, differentiated in 75% ethanol in 1% HCl for 5 to 10 seconds and rinsed, stained in eosin for 30 to 60 seconds, rinsed, dehydrated, cleared and mounted and then checked under a Olympus ix71 inverted microscope (Olympus, Shanghai, China)
TUNNEL assay
The apoptotic cells in rat hearts were detected using a TUNEL Apoptosis Detection Kit (KGA7032, KeyGENBioTECH, Nanjing, Jiangsu, China) following the manufacturer's instructions. Briefly, sections were dewaxed, hydrated, permeabilized, blocked using conventional methods and then incubated in the dark with 45 µL equilibration buffer, 1 µL biotin-11-dUTP, and 4 µL TdT for 60 minutes at 37°C, washed 5 minutes in PBS for 3 times, incubated in 0.5 µL streptavidin-HRP in 49.5 µL 1x PBS in the dark at 37°C for 30 minutes, washed 5 minutes in PBS for 3 times, incubated in 2.5 µL DAB-A solution mixed with 50 µL distilled H2O first and then mixed in 2.5 µL of DAB-B and DAB-C solutions at room temperature for 30 seconds to 5 minutes, and washed 3 times in PBS for 5 minutes each and then observed and photographed under a Olympus ix71 microscope.
Sirius staining
Rat heart tissue sections were stained with a Picric acid-Sirius staining kit (Senbeijia Biotech, Nanjing, China). The sections were washed 3 times in 1xPBS for 2 minutes each, incubated with Sirius staining solution at room temperature for 30 minutes, and washed as before, counterstained with hematoxylin for 5 minutes, washed 3 times in PBS for 1-2 minutes each and then checked and photographed under an Olympus ix71 microscope.
Western blotting
The rat heart total proteins (50 µg) were separated on a 12% SDS-polyacrylamide gel, and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). The membranes were blocked with 5% nonfat milk for 30 minutes at room temperature and then incubated with anti-MMP-2 (ab80737, Abcam, Cambridge, MA), HIF-1α (MAB1536, R&D Systems, Shanghai, China), NF-kB (ab16502, Abcam), IL-6 (ab6672, Abcam), or β-actin (ab8227, Abcam) antibodies overnight at 4°C. After wash, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratory, West Grove, PA, USA) for 1 hour at room temperature. The blots were then visualized by using the enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). Densitometry was performed with a Hewlett-Packard scanner and NIH Image software (Image J).
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted from rat heart tissues using RNeasy mini kits (Qiagen, Venlo, the Netherlands). Reverse transcription was performed with SuperScript® III First-Strand Synthesis System (Life Tech, Shanghai, China) according to the supplier's instruction using 1 µg total RNA. Quantitative real-time PCR was performed using the SYBR® Green PCR Master Mix (Life Tech) on a ABI 7300 (Applied Biosystems, Foster City, CA) with the following program: 95°C for 3 minutes followed by 40 cycles of 95°C 30 seconds, 58°C 15 seconds, and 68°C 30 seconds. The primers used were 5′-ATCTGTCCTCAAACCAGTTG-3′ and 5′-GTTGTTGGTCTTCAGTTTCC-3′ for HIF-1α, 5′-TATACCACTTCACAAGTCGG-3′ and 5′-CAGAGCAGATTTTCAATAGGC-3′ for IL-6, 5′-ACAACAGCTGTACCACCGA-3′ and 5′-TGGTGCAGCTCTCATACTTG-3′ for MMP-2, 5′-CGGAGGCATGTTCGGTAGT-3′ and 5′-TTGGCACAATCTCTAGGCTC-3′ for NF-kB, and 5′-AGGGAAATCGTGCGTGACAT-3′ and 5′-GAGCCACCAATCCACACAGA-3′ for β-actin. The relative transcription levels were calculated with 2-DDCt method using β-actin as the internal control.
Statistical analysis
The data were expressed as mean±standard deviation. Difference between groups were analyzed by one way analysis of variance using Graphpad Prism 5. A P value less than 0.05 was considered statistically significant.
Results
CIH caused cardiac dysfunction
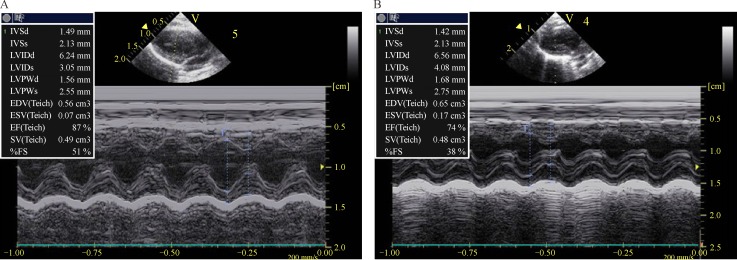
After 5-week intermittent hypoxia treatment, the rat left ventricles were dilated (Fig. 1) with expanded left ventricular internal diameter in systole (LVIDs) (4.094 mm±1.131 (CIH) versus 3.060±0.923 mm (control), P < 0.01) (Table 1). The end-systolic volume (ESV) was more than doubled in CIH rats (from 0.070±0.024 cm3 in control to 0.171±0.054 cm3 in CIH rats, P < 0.01). The end-diastolic volume (EDV) was markedly increased whereas ejection fraction (EF) and fractional shortening (FS) were both significantly reduced in CIH rats (Table 1).
Fig. 1. Chronic intermittent hypoxia (CIH) caused left ventricle hypertrophy.
Ultrasonography shows the cardiac images of the control rats (A) and CIH rats (B).
Table 1. Echocardiographic parameters of the control and CIH rats.
| Parameter | Control | CIH | P |
|---|---|---|---|
| IVSd (mm) | 1.495±0.422 | 1.425±0.386 | 0.1037 |
| IVSs (mm) | 2.137±0.562 | 2.151±0.498 | 0.7951 |
| LVIDd (mm) | 6.261±1.875 | 6.582±2.015 | 0.0876 |
| LVIDs (mm) | 3.060±0.923 | 4.094±1.131 | 0.0002 |
| LVPWd (mm) | 1.565±0.181 | 1.686±0.365 | 0.0319 |
| LVPWs (mm) | 2.559±0.423 | 2.759±0.598 | 0.0301 |
| EDV (cm3) | 0.562±0.109 | 0.652±0.102 | 0.0029 |
| ESV (cm3) | 0.070±0.024 | 0.171±0.054 | < 0.0001 |
| EF (%) | 87.290±9.436 | 74.247±10.345 | 0.0022 |
| SV (cm3) | 0.492±0.112 | 0.482±0.010 | 0.4190 |
| FS (%) | 51.170±12.425 | 38.127±11.564 | 0.0002 |
CIH: chronic intermittent hypoxia; IVSd: the intervernicular septum in diastole; IVSs: the intervernicular septum in systole; LVIDd: left ventricular internal diameter in diastole; LVIDs: left ventricular internal diameter in systole; LVPWd: the left ventricle posterior wall in diastole; LVPWs: the left ventricle posterior wall in systole; EDV: the volume of blood within a ventricle immediately before a contraction is known as the end-diastolic volume; ESV: the volume of blood left in a ventricle at the end of contraction is end-systolic volume; EF: ejection fraction is the fraction of blood in the left and center ventricles pumped out with each heartbeat; SV = EDV-ESV; FS: fractional shortening.
CIH induced cardiac injuries and fibrosis
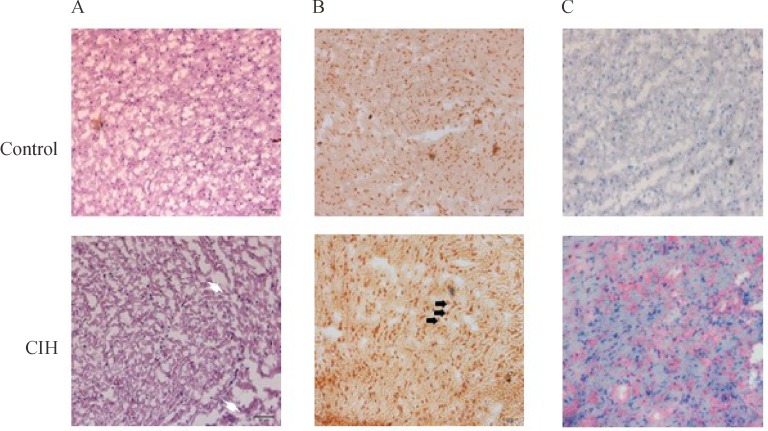
In the control group, myocardial cells were orderly arranged with structural integrity while the myocytes were disarrayed with some edema and necrosis, and cardiac structure integrity was compromised with bending cardiac fibers in CIH rats (Fig. 2A). The number of TUNNEL-positive apoptotic cells were markedly increased in the cardiac tissues of CIH-treated rats compared to the control rats (Fig. 2B). Sirius staining showed a significant amount of collagen fibers in the heart of CIH rats, especially in the left ventricle, whereas collagen fiber was rarely detected in the control rat hearts (Fig. 2C).
Fig. 2. CIH caused cellular death and structural changes in rat hearts.
A: H&E staining showed increased cardiomyocyte size, disrupted cardiac structure (arrow head) in CIH rat hearts. B: Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay detected significantly increased apoptotic myocytes (arrows) in CIH rats. C: CIH rats had significant fibrosis in Sirius-stained slices.
CIH activated cardiac hypoxia and inflammatory responses
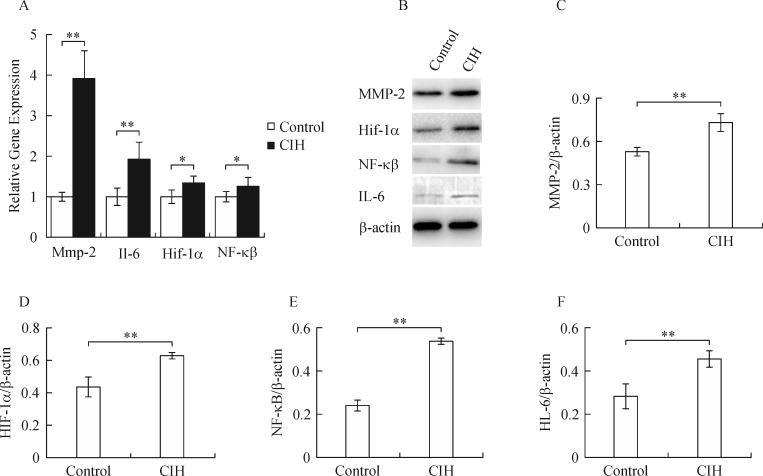
Chronic intermittent hypoxia significantly upregulated hypoxia induced factor 1α (Hif-1α) mRNA (Fig. 3A) and protein (Fig. 3 B & D) levels. Meanwhile, the expression levels of nuclear factor kappa B (NF-kB) (Fig. 3 A, B, & E) and interleukin 6 (IL-6) (Fig. 3 A, B, & F) were also markedly increased in the cardiac tissue of CIH rats compared to the control rats. The matrix mRNA (Fig. 3A) and protein (Fig. 3 B & C) levels of matrix metallopeptidase 2 (MMP-2) were also significantly increased in rat cardiac tissue in the CIH group.
Fig. 3. CIH promoted cardiac expression of inflammation and fibrosis marker genes.
The mRNA (A) and protein (B-F) levels of Mmp-2 (A-C), Hif-1α (A, B, D), NF-kB (A, B, E), and IL-6 (A, B, F) were significantly higher in CIH rats. *P < 0.05; **P < 0.01.
Discussion
CIH has been shown to be associated with hypertension and sympathetic activation in animal models[10], both of which could influence cardiac function[11]. However, there are little data on cardiac function in CIH model[12].
In this study, we used a well-known model to study the mechanisms of myocardial dysfunction in OSA. We found that the LV function was adversely affected by CIH, as indicated by increased LV cavitary volumes, decreased FS and EF by echocardiographic measurements. We demonstrated that the development of myocardial dysfunction is associated with increased expression of molecules in the HIF-1α and NF-kB signaling pathways and enhanced cardiac inflammation, apoptosis and fibrosis.
Several cellular and molecular changes have been suggested to be involved in the development of ventricular remodeling and dysfunction, including myocardial inflammation and fibrosis[13]. Loss of cardiomyocytes due to apoptosis directly impacts cardiac dysfunction[14]. We observed significant apoptosis in the myocardium in the CIH group, as indicated by increased TUNEL-positive myocytes. Consistently, upregulation of proapoptotic proteins and downregulation of antiapoptotic proteins in chronic sustained hypoxia have been observed[15]. Myocardial apoptosis contributes to myocardial damage in cultured myocytes exposed to hypoxia, postinfarct remodeling in rodents, and patients with heart failure. So we inferred that apoptosis may also play an important role in cardiac dysfunction in CIH; however, its exact contribution as well as the initial mechanisms is still to be determined.
Oxidative stress caused by CIH in OSA is considered to be one of the most important mechanisms of cardiac dysfunction[16]. Hypoxia can lead to oxidative stress and overproduction of reactive oxygen species. Reactive oxygen species can also activate nuclear transcriptional factors, including NF-kB and HIF-1α[17], which stimulates production of inflammatory mediators such as interleukin (IL)-6[18], as we observed in this model.
Myocardial inflammation and fibrosis have been observed in animal models and patients with heart disease[17]. In the present study, we observed histological evidence of myocardial inflammation and collagen fiber increased on H&E- and Sirius-stained heart slices from CIH animals, suggesting that their role in cardiac dysfunction is essential. Myocardial collagen remodeling process was regulated by matrix metalloproteinases (MMPs). The NF-kB signaling pathway plays an important role in the regulation of MMPs transcription, and the inhibition of NF-kB activation may improve the interstitial collagen remodeling[19]. In the present study, we found that the expression of MMP-2 increased significantly in the CIH group, consistent with the increased expression of NF-kB. NF-kB also has other important roles, including stimulating production of inflammatory mediators such as tumor necrosis factor-α and IL-6, which was also observed in the present study.
In conclusion, we successfully established an OSA rat model and found inflammation and cardiac apoptosis, which in turn resulted in ventricle fibrosis and hypertrophy and weakening of cardiac ejection power.
Acknowledgements
This study was supported by Medical Key Talents Foundation of Jiangsu Province, China (No: 904-KJXW18) and by National Natural Science Youth Foundation of China (No. 81300227 and No. 81300159).
References
- [1].Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea[J]. Lancet, 2014, 383(9918): 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sforza E, Chouchou F, Collet P, et al. Sex differences in obstructive sleep apnoea in an elderly French population[J]. EurRespir J, 2011, 37(5): 1137–1143. [DOI] [PubMed] [Google Scholar]
- [3].Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults[J]. Am J Epidemiol, 2013, 177(9): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharma SK, Ahluwalia G. Epidemiology of adult obstructive sleep apnoea syndrome in India[J]. Indian J Med Res, 2010, 131: 171–175. [PubMed] [Google Scholar]
- [5].Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong[J]. Chest, 2001, 119(1): 62–69. [DOI] [PubMed] [Google Scholar]
- [6].Vrints H, Shivalkar B, Hilde H, et al. Cardiovascular mechanisms and consequences of obstructive sleep apnoea[J]. ActaClinBelg, 2013, 68(3): 169–178. [DOI] [PubMed] [Google Scholar]
- [7].Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea[J]. N Engl J Med, 2014, 370(24): 2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Loredo JS, Clausen JL, Nelesen RA, et al. Obstructive sleep apnea and hypertension: are peripheral chemoreceptors involved[J]? Med Hypotheses, 2001, 56(1): 17–19. [DOI] [PubMed] [Google Scholar]
- [9].Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease[J]. J Am CollCardiol, 2003, 41(9): 1429–1437. [DOI] [PubMed] [Google Scholar]
- [10].Chen L, Einbinder E, Zhang Q, et al. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats[J]. Am J RespirCrit Care Med, 2005, 172(7): 915–920. [DOI] [PubMed] [Google Scholar]
- [11].Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications[J]. J Am CollCardiol, 2011, 57(2): 119–127. [DOI] [PubMed] [Google Scholar]
- [12].Kasai T. Sleep apnea and heart failure[J]. J Cardiol, 2012, 60(2): 78–85. [DOI] [PubMed] [Google Scholar]
- [13].Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea[J]. Nat Rev Cardiol, 2012, 9(12): 679–688. [DOI] [PubMed] [Google Scholar]
- [14].Gao YH, Chen L, Ma YL, et al. Chronic intermittent hypoxia aggravates cardiomyocyte apoptosis in rat ovariectomized model[J], 2012, 125(17):3087–3092. [PubMed] [Google Scholar]
- [15].Lee SD, Kuo WW, Lin JA, et al. Effects of long-term intermittent hypoxia on mitochondrial and Fas death receptor dependent apoptotic pathways in rat hearts[J]. Int J Cardiol, 2007, 116(3): 348–356. [DOI] [PubMed] [Google Scholar]
- [16].Badran M, Ayas N, Laher I. Cardiovascular Complications of Sleep Apnea: Role of Oxidative Stress[J]. Oxid Med Cell Longev, 2014, 2014:985258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Javaheri S, Javaheri S, Javaheri A. Sleep apnea, heart failure, and pulmonary hypertension[J]. Curr Heart Fail Rep, 2013, 10(4): 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li S, Feng J, Wei S, et al. Delayed neutrophil apoptosis mediates intermittent hypoxia-induced progressive heart failure in pressure-overloaded rats[J]. Sleep Breath, 2016, 20(1): 95–102. [DOI] [PubMed] [Google Scholar]
- [19].Kumar S, Seqqat R, Chigurupati S, et al. Inhibition of nuclear factor kB regresses cardiac hypertrophy by modulating the expression of extracellular matrix and adhesion molecules[J]. Free RadicBiol Med, 2011, 50(1): 206–215. [DOI] [PubMed] [Google Scholar]