Abstract
Purpose
Surface wipe sampling for various hazardous agents has been employed in many occupational settings over the years for various reasons such as evaluation of potential dermal exposure and health risk, source determination, quality or cleanliness, compliance, and others. Wipe sampling for surface residue of antineoplastic and other hazardous drugs in healthcare settings is currently the method of choice to determine surface contamination of the workplace with these drugs. The purpose of this article is to review published studies of wipe sampling for antineoplastic and other hazardous drugs, to summarize the methods in use by various organizations and researchers, and to provide some basic guidance for conducting surface wipe sampling for these drugs in healthcare settings.
Methods
Recommendations on wipe sampling methodology from several government agencies and organizations were reviewed. Published reports on wipe sampling for hazardous drugs in numerous studies were also examined. The critical elements of a wipe sampling program and related limitations were reviewed and summarized.
Results
Recommendations and guidance are presented concerning the purposes of wipe sampling for antineoplastic and other hazardous drugs in the healthcare setting, technical factors and variables, sampling strategy, materials required, and limitations. The reporting and interpretation of wipe sample results is also discussed.
Conclusions
It is recommended that all healthcare settings where antineoplastic and other hazardous drugs are handled consider wipe sampling as part of a comprehensive hazardous drug ‘safe handling’ program. Although no standards exist for acceptable or allowable surface concentrations for these drugs in the healthcare setting, wipe sampling may be used as a method to characterize potential occupational dermal exposure risk and to evaluate the effectiveness of implemented controls and the overall the safety program. A comprehensive safe-handling program for antineoplastic drugs may utilize wipe sampling as a screening tool to evaluate environmental contamination and strive to reduce contamination levels as much as possible, using the industrial hygiene hierarchy of controls.
Keywords: Antineoplastic drugs, hazardous drugs, surface wipe sampling healthcare settings, exposure assessment
INTRODUCTION
Surface wipe sampling has been used extensively in healthcare settings over the past two decades to determine: workplace contamination with antineoplastic drugs, the need and effectiveness for engineering controls, the effect of improved work practice controls, and what type of personal protective equipment (PPE) is required.(1–7) Similar methodology has been successfully employed in other occupational settings for toxic agents such as lead, chromium VI, beryllium, arsenic, cadmium and nickel,(8) asbestos, polychlorinated biphenyls, pesticides and antibiotics.(9–12) Ashley et al.(13) identify the three primary reasons for conducting surface wipe sampling: (1) evaluation of the potential health risk; (2) hazard management or evaluation of the source; and (3) hazard compliance. These authors also list a number of reasons for wipe sampling (Table I), most of which can be applied to healthcare settings.
It has been postulated that dermal uptake is the most likely route of occupational exposure to most hazardous drugs in healthcare settings, especially low-molecular-weight antineoplastic drugs.(14–17) Inhalation of aerosolized droplets or vapors and accidental hand-to-mouth ingestion following contact with contaminated surfaces are also possible routes of exposure.(18,19) OSHA states that in some cases skin absorption may be a more important route of exposure than inhalation and may not be noticed by the employee, especially for non-volatile hazardous chemicals which remain on work surfaces for long periods of time. (20) It is generally assumed that dermal absorption is more likely for drugs with a molecular weight of <500 Daltons and less likely for those >1000 Daltons.(21,22) In addition, lipid soluble compounds more readily penetrate the skin than water-soluble ones and uptake may be enhanced by the use of carrier solvents. (22) Many of the first-generation antineoplastic drugs are relatively small molecules with molecular weights <500 Daltons. Some newer antineoplastic drugs, such as monoclonal antibodies, can have a molecular weight >40,000 Daltons, which presumably limit their potential for dermal uptake from contaminated surfaces.(23–25) Although 500 Daltons is considered the maximum molecular weight for effective uptake of transdermal drugs by pharmaceutical companies, it is not an absolute cut-off point and some drugs with molecular weights of >1000 Daltons can penetrate the skin, but they do so at a lower efficiency. (22) In addition, solvents in some pharmaceuticals, such as N,N-Dimethylacetamide may pose an additional health risk (26) or may enhance drug penetration of the skin. (27) It should be noted that healthcare workers, especially nursing personnel, often have damage to their skin from repeated hand washing that could facilitate uptake of drugs with higher molecular weights. (20.28.29)
The first published U.S. study of surface contamination with antineoplastic drugs was by McDevitt et al.(30) at Johns Hopkins Hospital. It involved one cancer drug, cyclophosphamide, and contamination was demonstrated in both the pharmacy and patient treatment areas. Around the same time, several articles were published by Sessink and colleagues, (31–34) based on studies conducted in the Netherlands. These studies measured surface concentrations of several cancer drugs by wipe sampling, including cyclophosphamide, ifosfamide, methotrexate, and 5-fluorouracil. As in the U.S. study, surface contamination with these drugs was demonstrated in areas where they were prepared and administered to patients. These studies also documented occupational exposure (uptake of the drugs) based on their measurement in the urine of healthcare workers.(32–34) In 1999, Connor et al.(1) published a large-scale study that included three cancer hospitals in the United States and three in Canada. This study documented surface contamination with cyclophosphamide, ifosfamide, and 5-fluorouracil in the pharmacy and patient treatment areas, and also in areas adjacent to the pharmacy. Since publication of these initial studies, several studies have been published in the United States and more than 100 have been published worldwide, demonstrating widespread workplace surface contamination with antineoplastic drugs (a complete listing of these studies can be found at http://www.cdc.gov/niosh/topics/antineoplastic/). While it was not possible to summarize the information to this large body of work, key elements have been selected from them in order to describe a framework for wipe sampling for hazardous drugs in healthcare settings.
Currently, surface wipe sampling is the method of choice to evaluate surface contamination within healthcare settings for antineoplastic drugs. With such a large number of studies published from many countries around the world, the methodology used for surface wipe sampling and analytical laboratory analysis for antineoplastic drugs has varied considerably. In the United States, several guidance documents published by government and other organizations on wipe sampling for chemicals other than antineoplastic drugs provide background information on methodology.(8,10,11,35–37) This article reviews published studies of surface sampling for antineoplastic drugs and summarizes the methods in use by various organizations and researchers. It provides minimal (basic) requirements for surface sampling and general guidance for conducting wipe sampling for these drugs in healthcare and other settings where they are handled.
METHODS
The following is an example of a sample wipe sampling strategy for antineoplastic and other hazardous drugs in healthcare settings. It can be modified to meet specific needs, but it should include all the basic steps. The information included herein has been abstracted from a number of sources, and most of it is common to all protocols.
Reasons for wipe sampling
Prior to performing wipe sampling, it is important to have a clear purpose and intent concerning the approach to be taken and how the results will be used. Because many antineoplastic drugs are classified as hazardous,(19) employers may use wipe sampling when assessing potential workplace risks. For instance, 29 CFR 1910.132 requires employers to “assess the workplace to determine if hazards are present, or are likely to be present, which necessitate the use of personal protective equipment (PPE).” In addition, OSHA(35) recommends that surface wipe sampling is helpful in performing a risk assessment for dermal uptake of chemicals when the most likely route of exposure is not inhalation. In support of a risk assessment, specific reasons for conducting wipe sampling for antineoplastic drugs in the healthcare setting include the following:
evaluating the efficacy of engineering or administrative controls for eliminating or minimizing potential drug release during the handling lifecycle
preemptive screening for potential exposure of healthcare workers, before any concerns are raised
compliance with workplace health standards or recommended guidelines
support for a comprehensive safe-handling program
Assessment of contamination before moving or performing maintenance on equipment
determination of baseline surface contamination levels [e.g. at a new or refurbished facility]
assessment of contamination on the outside of drug vials as received from manufacturers
evaluating the efficiency of deactivation, decontamination, and cleaning procedures in the facility
verification of cleanliness [e.g. following a spill or decommissioning of equipment].
Data obtained from surface wipe sampling are indicative not of worker exposure but of workplace environmental contamination as a potential source of exposure.
Factors and considerations
When developing a wipe sampling strategy for antineoplastic drugs, one must consider certain factors and variables (summarized in Table II). The types, frequency of use, and general quantities of antineoplastic drugs used at the study site should first be reviewed, to aid in selecting drug to use as surrogates for overall contamination. It is also prudent to determine what types of questions the facility is trying to answer when conducting the wipe sampling. Once these are selected, the availability of sampling and analytical methodologies should be determined. This may require selecting and partnering with a contract or research laboratory. Currently there are no standards or proficiency programs for surface sampling and analysis for antineoplastic drugs in an occupational exposure setting. Thus, it is desirable to use a laboratory that is both experienced in analyzing wipe samples for antineoplastic drugs and that holds appropriate, quality certification and accreditation, such as from the American Industrial Hygiene Association Laboratory Accreditation Program (AIHA-LAP).(38) As an alternative, a laboratory could follow Good Laboratory Practices (GLPs) which are US Food and Drug Administration Regulations for Bioresearch Monitoring (http://www.fda.gov/downloads/.../ucm070107). The laboratory’s method validation process should be carefully evaluated for the specific drug or drug combinations that will be targeted, as this information may help determine some of the factors and considerations for wipe sampling. For instance, the drug recovery rate is influenced by both the recovery from the wipe media as well as the recovery from the specific type of surface: smooth (stainless steel, Formica®, glass, plastic); soft (fabric, leather); rough (carpet, wood); porous (vinyl, rubber); and contaminated (dirty).(13) In general, smooth, nonporous surfaces demonstrate a better rate of recovery, often greater than 90%. It is critical that contract laboratories predetermine the extraction efficiencies for each drug from each surface type and the wipe media, using a standardized, measured surface sampling area of the same material (39–40) within a range of measured surface area sizes. An extraction efficiency of >75% is acceptable, but >90% is preferred.(36) Some contract laboratories provide sampling kits for a number of antineoplastic drugs. The kits should provide the user with the same wipe-sample medium (swabs, wipes, etc.), wetting solution, and sample containers that were used for validation. Other important laboratory method validation factors include sample storage stability and preservation techniques, wipe-sample medium desorption efficiency, method selectivity and sensitivity (including the linearity of calibration curves, limits of detection [LODs], and limits of quantitation [LOQs]), and quality control measures.
Most guidelines on surface sampling (8,10,11,37) suggest sampling an area of 100 cm2 because this is assumed to be the average surface area of the palm of the hand.(22) However, surface area and the surface type (smooth/coarse), along with the location and number of samples collected, can greatly affect the final surface concentrations and all factors should be included in the sampling strategy. For example, one 100-cm2 sample taken in a standard 4-foot biological safety cabinet represents <4% of the total surface area. Therefore, some researchers utilize larger sampling areas, such as 500 or 600 cm2, or multiple samples from the same location when sampling for hazardous drugs.(31,41 If a sampling area of 100 cm2 is employed, it would be advisable to samples many locations (such as several areas of the BSC), but this could be cost-prohibitive since most testing laboratories charge by the sample. A better approach would be to use a sampling size of 400 cm2, thus sampling a larger surface area while keeping costs down. If larger surface areas are sampled, it is important that the surface area size selected for wipe sampling is validated as discussed above or studied such that the reported concentrations may be corrected for recovery efficiencies to acceptable levels of statistical confidence.
After reviewing the sampling plan and procedures and a laboratory has been identified a number of other factors should be considered. It should be determined why the sampling is being done and what locations and surface area sizes are to be sampled. Next, the necessary equipment should be assembled, and it should be ensured that personnel are adequately trained and supplies are available at the site. In order to minimize variability, one person (such as an Environmental Health and Safety [EHS] staff member, pharmacist, or consultant) should be identified to collect all of the wipe samples.(10,11) This list summarizes the preparation and sampling steps:
Since the wipe sampling process may result in worker exposure, precautions should be taken to avoid exposure such as when removing PPE.
Determine that the drugs of interest are currently in use and their frequency of use.
Determine if fully validated sampling and analytical methods are available for these drugs, and evaluate the laboratory’s quality standards and accreditations.
Identify the locations that will be sampled by observing the handling process for the selected drugs, from the point of introduction into the work environment to the point of use and/or disposal. For general hazard assessment, focus on the top one to three most frequently touched locations for each unique job task, such as receiving and storage, preparation, transport, patient administration, and disposal. (See Table III for typical locations.)
Obtain all necessary materials for the sampling (Table IV).
Obtain and read the laboratory method–specific wipe sampling protocol.
Record all of the sampling information including: the location/surface material/sample area size; sample identification number; time/date collected; sampler’s name; and comments.
Don appropriate protective equipment as required: gloves, gowns, shoe covers, etc.
When applicable, secure a template in position with tape; disposable, nonporous templates are preferable.
For irregularly shaped objects, measure surface area to be sampled and delineate with tape.
Take digital photos or video of sampling location and/or sketch the location so that it can be identified later.
Carefully remove gloves and don a clean pair of gloves.
Add required amount of solvent or wetting agent to the surface or to the wiping material per laboratory guidance.
Wipe surface according to a predetermined pattern (see Appendix for some examples of wipe sampling schemes).(8,42) Note that excess wiping of the surface can reduce recovery of the chemical being sampled.(11)
Place wipe in a proper, pre-labeled storage container and seal its top.
If the protocol calls for an additional wipe of the same sample location, repeat previous three steps.
Don a new pair of gloves for each subsequent wipe sampling to reduce the potential for cross-contamination from the previous wipe sample.
Add quality control samples (field blanks or spike samples) based on the requirements of the laboratory method. The recommended practice for the number of field blanks varies from one protocol to another, but is two for each 10 samples with a maximum of 10 is a workable number.(43) Also, if included in the protocol for the laboratory, spike several wipes with a known amount of the drug being analyzed for use as a control sample.
Pack samples in a shipping container per laboratory guidance, using the preservation methods (such as ice packs or dry ice) specified by the method validation, if applicable.
Ship or transport samples to selected laboratory for analysis.
Follow standard chain of custody procedures.
Although the sampling procedure may effectively remove some drugs, the area should be cleaned according to institution guidelines to remove any remaining drug residue.
All material used for wipe sampling should be disposed of according to state and federal requirements.
Analysis of wipe samples
Details of the analysis of wipe samples for the presence of antineoplastic drugs are beyond the scope of this document. A 2003 review by Turci et al.(44) describes some of the analytical methods that have been employed. These have included gas chromatography (GC), liquid chromatography (LC), high-performance liquid chromatography (HPLC), and ultra-performance liquid chromatography (UPLC); all in combination with mass spectrometry (MS) or tandem mass spectrometry (MS/MS). Inductively coupled plasma mass spectrometry (ICP-MS) has been used for the analysis of total platinum in platinum-based drugs. Liquid chromatography with mass spectrometry LC-MS/MS is the current method of choice for analysis of most antineoplastic drugs and does not require derivatization of the drug, as GC methods do.(31) Currently, several contract laboratories offer services using a variety of these analytical techniques. Since there is no certification for laboratories that analyze antineoplastic and other hazardous drugs, it is critical to obtain proper validation of the methodology from the laboratory.
Reporting of results
The sensitivity of current analytical methods allows for the determination of values in the picogram (pg) to nanogram (ng) range. Surface-wipe sample values are typically reported as ng/cm2 in order to standardize results across locations and studies. Analytical laboratory reports should also include basic information regarding the analytical method: the LOD and LOQ; the absolute value of drug analyte detected in the samples; the calculated surface concentration, if applicable; the serial dilutions performed to determine values within the working range of the validated method; and the results of the analysis of quality control samples.
Interpretation of results
The concentration ranges reported in many studies cover several orders of magnitude, from picograms to micrograms, and data interpretation can be challenging. Some authors have suggested the use of actionable guidance levels using surface concentration percentiles derived from historical published data. In one such example, Merger et al (41) sampled for cyclophosphamide, ifosfamide, and methotrexate in 33 Canadian hospitals. They recommended that corrective measures should be taken for hospitals with values above the 75th percentile. Schierl and coauthors (45) have recommended using “threshold guidance values” (TGVs) based on the results of sampling data from more than 100 German hospitals. Based on their observation, contamination values above the 75th percentile were considered high and that handling procedures needed to be optimized. In another German report, Kiffmeyer et al (46) recommended the use of the 90th percentile and suggested a guidance value of 0.1 ng/cm2 based on their findings for 5-fluorouracil contamination. However, such an approach requires a large amount of wipe sampling data which is not available in the U.S and suggested values would not be based on a health outcome. An approach from the Netherlands took into account a cancer risk assessment and levels of cyclophosphamide in the urine of healthcare workers in that country. (47) The recommendation was to strive for a surface contamination level for cyclophosphamide of < 0.1 ng.cm2. The pharmaceutical industry has used a method to calculate an allowable surface concentration using the drug’s maximum allowable dose, the 8-hour time-weighted average (TWA) air concentration, and the approximate surface area of a worker’s hand.(22) But for some antineoplastic agents, the pharmaceutical industry–derived TWA air concentration limits cannot be found on safety data sheets and have not been validated by an authoritative organization in the U.S.
No regulations or standards exist for allowable or acceptable antineoplastic-drug surface concentrations in the healthcare setting, and currently little is known about the potential health risks associated with low-level multidrug environmental surface contamination. Because of the mutagenic, teratogenic, and carcinogenic nature of many antineoplastic drugs, it has been suggested that occupational exposures be reduced with use of a principle from radiation safety: as low as reasonably achievable (ALARA) (48–50) (10 CFR 20.1003). A comprehensive safe-handling program for antineoplastic drugs that adopts the ALARA principle may utilize wipe sampling as a screening tool to evaluate environmental contamination as a potential exposure source and strive to reduce contamination levels as much as possible, using the industrial hygiene hierarchy of controls: Elimination; Substitution; Engineering Controls; Administrative Controls, PPE.(51) Elimination and Substitution are typically not compatible with hazardous drug handling. However, with increasing analytical knowledge and technology and lower LODs/LOQs, it can be difficult to determine reasonable achievability. Although there have been several attempts to correlate surface contamination with uptake of the drugs in healthcare workers and/or adverse health effects, such correlations have not provided sufficient information that can be used quantitatively to set a value for surface contamination. (31,47, 52–55) Given the large number of drugs in use at any given time by a healthcare facility, it would not be possible to conduct risk assessments on all the drugs and the possibility of interactions (e.g. additivity or synergism) between drugs would not be possible to evaluate. Using urinary excretion data, one recommendation for cyclophosphamide is to keep values as low as 0.1 ng/cm2.(56) Since cyclophosphamide and 5-fluorouracil continue to be used in most cancer treatment facilities, this value may serve as a benchmark for other drugs until a better understanding of adverse health effects can be determined.
Limitations
It is important to caution EHS professionals, who are accustomed to interpretation of air sampling data, that interpretation of surface sampling results is quite different. Surface sampling data are best used as a screening tool but, as noted above, can be used to evaluate the effectiveness of engineering and administrative controls and the spread of contamination to other areas. Healthcare workers need to be aware that the sampling only captures a moment in time and that many variables such as the technique of the last person to work in the area, recent spills, and improper cleaning can greatly affect the sampling results. There are many additional variables with surface sampling and additional difficulties with reproducibility of sampling techniques in comparison with air sampling. Some EHS professionals consider surface sampling data as “semi-quantitative” or rank-ordered data, as compared to traditional quantitative air sampling data.(22) Comparison of data sets from different surveys should be limited to the same work environment (baseline, trending, post clean-up, etc.) and as collected by the same sampler when possible. EHS professionals should proceed with caution when comparing data sets across different work environments.
There are a number of other limitations to performing wipe sampling for antineoplastic drugs, as summarized below:
Some drugs may not be stable in the environment or on the sampling materials and will be under-reported or may not be detected during analysis.
Other drugs such as cyclophosphamide are very stable in the environment and may represent spills or other sources of contamination that have taken place at an earlier date (e.g., days or weeks).
In some instances, interferences may be caused by agents used to clean surfaces and other contaminates.
If a robust sampling strategy is not developed with the sampling locations carefully selected or are selected at random, contamination may be missed and/or inappropriate conclusions may result.
If the incorrect solvent or wetting agent is used for a specific drug, the sampling efficiency may be very low. The solvent or wetting agent should be pre-determined by the testing laboratory.
If the wipe sampling is not performed correctly, the amount of drug recovered may be low.
Collecting too few samples may lead to problems with characterizing potential sources of surface contamination.
No accepted standards are available for these drugs for surface contamination in healthcare settings, but, wipe sampling can be utilized to determine if a drug is present, and the effectiveness of engineering and administrative controls.
The data obtained from surface wipe sampling is not an indication of workplace exposure but an indication of workplace environmental contamination as a potential source of exposure.
CONCLUSIONS
Dermal absorption has been suggested as the most likely route of exposure to antineoplastic drugs in the healthcare setting, especially low-molecular-weight drugs. Surface wipe samples for antineoplastic and other hazardous drugs from potentially contaminated surfaces in healthcare facilities and other areas where they are used may be evaluated by occupational health professionals to determine the effectiveness of existing controls and potential health risks from exposure to these drugs. However, if sampling is done incorrectly or without proper knowledge of the potential pitfalls, then the results may be misleading.
In U.S. healthcare facilities, there are no dermal exposure limits for allowable levels of surface contamination with antineoplastic. The ALARA principle has been suggested as a strategy to employ as part of a comprehensive safe handling program, but with increasing analytical capabilities it can be difficult to assign reasonable achievability. On the basis of the numerous published reports in healthcare settings, it is possible to ascertain if contamination levels are relatively low or high, compared with values reported for other institutions. USP Chapter <797> recommends an ‘action level’ of 1.0 ng/cm2 for cyclophosphamide, but a recommendation to keep values as low as 0.1 ng/cm2 would seem to be a reasonably achievable goal.(46,56)
Surface sampling for antineoplastic/hazardous drugs should be a part of a comprehensive hazardous drug program. Each organization should carefully consider how surface sampling fits into its comprehensive hazardous drug program. It may help characterize potential dermal exposure risk in different areas in the facility and it may help evaluate the effectiveness of the overall program.
Acknowledgments
The authors would like to thank Shelley Rae Carry, Kaiser Permanente for her valuable assistance in developing this manuscript and Seleen S. Collins, technical writer Education and Information Division, NIOSH. The authors would also like to thank Kevin Ashley and Teresa Lane for their review of the manuscript.
Appendix. Examples of Schemes used for Surface Wipe Sampling
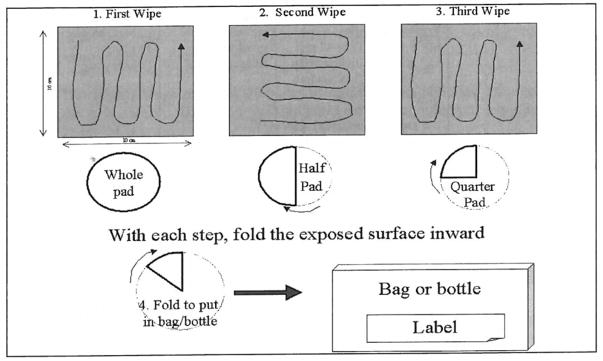
FIGURE 1.
An Example for a Scheme for Wipe Sampling with Filter Paper (8)
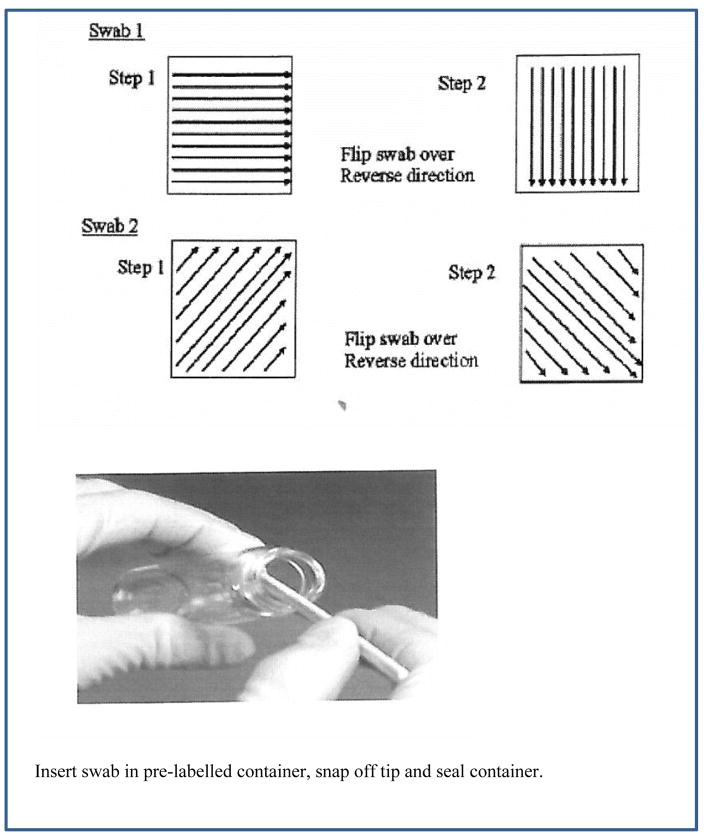
FIGURE 2.
An Example of a Scheme for Wipe sampling with a swab. (Modified from Hollands et al. (38)
TABLE I.
Reasons for Conducting Surface Wipe Sampling (12)
| Hazard identification and evaluation |
| Exposure assessment |
| Facility characterization |
| Housekeeping |
| Selection of engineering controls |
| Evaluation of engineering and administrative/work practice controls |
| Evaluation of exposure pathways |
| Selection of personal protective equipment |
| Compliance with regulations and standards |
| Source identification |
| Education and training |
| Investigation of complaints |
TABLE II.
Technical Factors and Variables to Consider when Doing Surface Wipe Sampling
| Validated sampling and analytical methods |
| Solubility of drug in extraction solvent |
| Type of wipe sampling material |
| Extraction efficiency of drug from surface material |
| Recovery of drug from sampling material |
| Extraction solvent |
| Limit of detection (LOD) |
| Limit of quantification (LOQ) |
| Compatibility of extraction solvent with solvent system used for analysis |
| Drugs currently in use at the study site |
| Design and layout of area to be sampled |
| Number of samples to be taken |
| Locations to be sampled |
| Size of samples to be taken |
TABLE III.
Locations Typically Sampled for Hazardous Drugs in Healthcare Settings
| Pharmacy areas |
|---|
| Surface of BSC |
| Airfoil of BSC |
| Surface of CACI |
| Floor in front of BSC or CACI |
| Floor in pharmacy |
| Pass-through (inside and outside, both for a CACI and from inside the pharmacy) |
| Countertops |
| Equipment |
| Storage trays |
| Drug vials |
| Door handles, door knobs, other high-touch areas |
| Computer keyboard/mouse |
|
Nursing and patient areas
|
| Nurses’ station |
| Storage area for IV bags |
| Countertops |
| Furniture in patient room |
| Infusion pump |
| Door handles, door knobs, other high-touch areas |
| Computer keyboard/mouse |
| Floor in patient room |
| Floor in restroom |
Notes: BSC, biological safety cabinet; CACI, compounding aseptic containment isolator; IV, intravenous.
TABLE IV.
Materials Required for Collecting Surface Wipe Samples
| Disposable templates |
| Marker/tape |
| Ruler |
| Solvent or wetting agent |
| Device for applying solvent (pipette) |
| Wipes or swabs |
| Sample container for wipes |
| Disposable gloves |
| Container to store samples |
| Container for waste materials |
| Notebook/laptop/tablet with chart |
| Digital camera |
Footnotes
DISCLAIMERS
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH. In addition, citations to Web sites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not responsible for the content of these Web sites. All Web addresses referenced in this document were accessible as of the publication date.
The findings and conclusions in this report are those of the author and do not necessarily represent the views of FedEx Ground. Mention of any company or product does not constitute endorsement by FedEx Ground. In addition, citations to Web sites external to FedEx Ground do not constitute FedEx Ground endorsement of the sponsoring organizations or their programs or products. Furthermore, FedEx Ground is not responsible for the content of these Web sites. All Web addresses referenced in this document were accessible as of the publication date.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the N.C. Department of Labor (NCDOL). Mention of any company or product does not constitute endorsement by NCDOL. In addition, citations to Web sites external to NCDOL do not constitute NCDOL endorsement of the sponsoring organizations or their programs or products. Furthermore, NCDOL is not responsible for the content of these Web sites. All Web addresses referenced in this document were accessible as of the publication date.
Contributor Information
Thomas H. Connor, Email: tmc6@cdc.gov, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, Cincinnati, OH.
Matthew D. Zock, Email: matthew.zock@fedex.com, FedEx Ground, Moon Township, PA.
Amy H. Snow, Email: Amy.snow@labor.nc.gov, Occupational Safety and Health Division, North Carolina Department of Labor, Raleigh, NC*.
References
- 1.Connor TH, Anderson RW, Sessink PJM, Broadfield L, Power LA. Surface contamination with antineoplastic agents in six cancer treatment centers in the United States and Canada. Am J Health-Syst Pharm. 1999;56:1427–1432. doi: 10.1093/ajhp/56.14.1427. [DOI] [PubMed] [Google Scholar]
- 2.Connor TH, DeBord G, Pretty JR, et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J Occup Environ Med. 2010;52:1019–1027. doi: 10.1097/JOM.0b013e3181f72b63. [DOI] [PubMed] [Google Scholar]
- 3.Wick C, Slawson MH, Jorgenson JA, Tyler LS. Using a closed-system protective device to reduce personnel exposure to antineoplastic agents. Am J Health-Syst Pharm. 2003;60:2314–2320. doi: 10.1093/ajhp/60.22.2314. [DOI] [PubMed] [Google Scholar]
- 4.Harrison BR, Peters BG, Bing MR. Comparison of surface contamination with cyclophosphamide and fluorouracil using a closed-system drug transfer device versus standard preparation techniques. Am J Health-Syst Pharm. 2006;63:1736–1744. doi: 10.2146/ajhp050258. [DOI] [PubMed] [Google Scholar]
- 5.Sessink PJM, Connor TH, Jorgenson JA, Tyler TG. Reduction in surface contamination with antineoplastic drugs in 22 hospital pharmacies in the US following implementation of a closed-system drug transfer device. J Oncol Pharm Pract. 2011;17:39–48. doi: 10.1177/1078155210361431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sessink PJM, Trahan J, Coyne JW. Reduction in surface contamination with cyclophosphamide in 30 US hospital pharmacies following implementation of a closed-system drug transfer device. Hosp Pharm. 2013;48:204–212. doi: 10.1310/hpj4803-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sessink PJM, Leclercq GM, Wouters DM, Halbardier L, Hammad C, Kassoul N. Environmental contamination, product contamination and workers exposure using a robotic system for antineoplastic drug preparation. J Oncol Pharm Pract. 2014 doi: 10.1177/1078155214522840. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Brookhaven National Laboratory. IH75190: Surface Wipe Sampling Procedure. Upton, NY: Brookhaven National Laboratory, Safety & Health Services Division, Industrial Hygiene Group; 2014. [Google Scholar]
- 9.Ness SA. Surface and Dermal Monitoring for Toxic Substances. New York: Van Nostrand Reinhold; 1994. [Google Scholar]
- 10.EPA. A Literature Review of Wipe Sampling Methods for Chemical Warfare Agents and Toxic Industrial Chemicals. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development; 2007. EPA/600/R-07/004. [Google Scholar]
- 11.ASTM. D6661-10: Standard Practice for Field Collection of Organic Compounds from Surfaces Using Wipe Sampling. West Conshohocken, Penn: ASTM International; 2010. [Google Scholar]
- 12.Nygren O, Lindahl R. Development of a method for screening spill and leakage of antibiotics on surfaces based on wipe sampling and HPLC-MS/MS analysis. J ASTM Intl. 2011;8(6) doi: 10.1520/JAI103678. [DOI] [Google Scholar]
- 13.Ashley K, Brisson MJ, White KT. Review of standards for surface and dermal sampling. J ASTM Intl. 2011;8(6) doi: 10.1520/JAI103678. [DOI] [Google Scholar]
- 14.Kromhout H, Hoek F, Uitterhoeve R, et al. Postulating a dermal pathway for exposure to antineoplastic drugs among hospital workers: applying a conceptual model to the results of three workplace surveys. Ann Occup Hyg. 2000;44:551–560. doi: 10.1016/s0003-4878(00)00050-8. [DOI] [PubMed] [Google Scholar]
- 15.Fransman W, Vermeulen R, Kromhout H. Occupational dermal exposure to cyclophosphamide in Dutch hospitals: A pilot study. Ann Occup Hyg. 2004;48:237–244. doi: 10.1093/annhyg/meh017. [DOI] [PubMed] [Google Scholar]
- 16.Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Health. 2005;78:403–412. doi: 10.1007/s00420-004-0595-1. [DOI] [PubMed] [Google Scholar]
- 17.Hon CY, Teschke K, Demers PA, Venners S. Antineoplastic drug contamination on the hands of employees working throughout the hospital medication system. Ann Occup Hyg. 2014;58:1–10. doi: 10.1093/annhyg/meu019. [DOI] [PubMed] [Google Scholar]
- 18.NIOSH. [accessed May 27, 2015];Personal Protective Equipment for Health Care Workers Who Work with Hazardous Drugs. 2009 DHHS (NIOSH) Publication No. 2009-106. Available at http://www.cdc.gov/niosh/docs/wp-solutions/2009-106/
- 19.NIOSH. [accessed May 27, 2015];NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings. 2014 Available at http://www.cdc.gov/niosh/docs/2014-138/
- 20.Surface Contaminants, Skin Exposure, Biological Monitoring and Other Analyses. Section II, Chapter 2. [accessed November 2, 2015]; https://www.osha.gov/dts/chemicalsampling/data/CH_235600.html.
- 21.Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of compounds and drugs. Exp Dermatol. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimmel TA, Sussman RG, Ku RH, Adar AW. Developing acceptable surface limits for occupational exposure to pharmaceutical substances. [accessed November 6, 2015];J ASTM Intl. 2011 8(8) doi:10.1520JAI103480. http://www.usp.org/pdf/EN/referenceStandards/msds/1601485.pdf. [Google Scholar]
- 23.Halsen G, Krämer I. Assessing the risk to health care staff from long-term exposure to anticancer drugs: the case of monoclonal antibodies. J Oncol Pharm Pract. 2011;17:68–80. doi: 10.1177/1078155210376847. [DOI] [PubMed] [Google Scholar]
- 24.Connor TH, MacKenzie BM. Should monoclonal antibodies and their conjugates be considered occupational hazards? [accessed May 27, 2015];Saf Consid Oncol Pharm Special edition. 2011 Fall;:13–16. Available at www.ppme.eu.
- 25.Alexander M, King J, Bajel A, et al. Australian consensus guidelines for the safe handling of monoclonal antibodies for cancer treatment by healthcare personnel. Intern Med J. 2014;44:1018–1026. doi: 10.1111/imj.12564. [DOI] [PubMed] [Google Scholar]
- 26.USP. Safety Data Sheet. [accessed November 23, 2015];N,N-Dimethylacetamide. http://static.usp.org/pdf/EN/referenceStandards/msds/1601500.pdf.
- 27.Munro DD, Stoughton RB. Dimehtylacetamide (DMAC) and dimethylformamide (DMFA) effect on percutaneous absorption. Arch Dermatol. 1965;92:585–586. [PubMed] [Google Scholar]
- 28.Health and Safety Executive. Work-Related Contact Dermatitis in the Health Services. London, UK: Health and Safety Executive; no date. [accessed July 20, 2015]. Available at http://www.hse.gov.uk/skin/employ/highrisk/healthcare.htm. [Google Scholar]
- 29.Kedrowski DA, Waeshaw EM. Hand dermatitis: a review of clinical features, diagnosis, and management. Dermatol Nurs. 2008;20:17–25. [PubMed] [Google Scholar]
- 30.McDevitt JJ, Lees PSJ, McDiarmid MA. Exposure of hospital pharmacists and nurses to antineoplastic agents. J Occup Med. 1993;35:57–60. [PubMed] [Google Scholar]
- 31.Sessink PJM, Anzion RB, Van den Broek PHH, Bos RP. Detection of contamination with antineoplastic agents in a hospital pharmacy department. Pharm Wkly (Sci) 1992;14:16–22. doi: 10.1007/BF01989220. [DOI] [PubMed] [Google Scholar]
- 32.Sessink PJM, Van de Kerkhof MCA, Anzion RB, Noordhoek J, Bos RP. Environmental contamination and assessment of exposure to antineoplastic agents by determination of cyclophosphamide in urine of exposed pharmacy technicians: Is skin absorption an important exposure route? Arch Environ Health. 1994;49:165–169. doi: 10.1080/00039896.1994.9940377. [DOI] [PubMed] [Google Scholar]
- 33.Sessink PJM, Friemèl NSS, Anzion RBM, Bos RP. Biological and environmental monitoring of occupational exposure of pharmaceutical plant workers to methotrexate. Int Arch Occup Environ Health. 1994;65:401–403. doi: 10.1007/BF00383251. [DOI] [PubMed] [Google Scholar]
- 34.Sessink PJM, Wittenhorst BCJ, Anzion RBM, Bos RP. Exposure of pharmacy technicians to antineoplastic agents: reevaluation after additional protective measures. Arch Environ Health. 1997;52:240–244. doi: 10.1080/00039899709602893. [DOI] [PubMed] [Google Scholar]
- 35.OSHA. OSHA Technical Manual. Washington, DC: OSHA; 2002. Sampling for surface contamination. Section II, Chapter 2. [Google Scholar]
- 36.OSHA. Evaluation Guidelines for Surface Sampling Methods. Salt Lake City, Utah: OSHA Salt Lake Technical Center; 2001. [Google Scholar]
- 37.NIOSH. NIOSH Manual of Analytical Methods (NMAM), Fourth Edition, 8/15/94. Cincinnati, OH: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 1994. Lead in Surface Wipe Samples: Method 9100. [Google Scholar]
- 38.American Industrial Hygiene Association. AIHA’s Laboratory Accreditation Programs, LLC. Falls Church, Va: AIHA; 2015. [accessed May 27, 2015]. Available at http://www.aihaaccreditedlabs.org/ [Google Scholar]
- 39.Pretty JR, Connor TH, Spasojevic I, et al. Sampling and mass spectrometric analytical methods for five antineoplastic drugs in the healthcare environment. J Oncol Pharm Pract. 2010;18:23–36. doi: 10.1177/1078155210389215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.B’Hymer C, Connor TH, Stinson D, Pretty J. Validation of an HPLC-MS/MS and wipe procedure for mitomycin C contamination. J Chromatogr Sci. 2015;53:619–624. doi: 10.1093/chromsci/bmu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merger D, Tanguay C, Langlois E, Lefebvre M, Bussieres JF. Multicenter study of environmental contamination with antineoplastic drugs in 33 Canadian hospitals. Int Arch Occup Environ Health. 2014;87:307–313. doi: 10.1007/s00420-013-0862-0. [DOI] [PubMed] [Google Scholar]
- 42.Hollands W, Siegerman H, Strauss M. How to succeed in the search for nothing: effective swabbing techniques for cleaning validation. [accessed May 27, 2015];Controlled Environments. 2007 Available at http://www.cemag.us/articles/2007/02/how-succeed-search-nothing-effective-swabbing-techniques-cleaning-validation.
- 43.NIOSH. [accessed May 27, 2015];NIOSH Manual of Analytical Methods. 2003 Available at http://www.cdc.gov/niosh/docs/2003-154/
- 44.Turci R, Sottani C, Spagnoli G, Minoia C. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agent: a review of analytical methods. J Chromatog B. 2003;789:169–209. doi: 10.1016/s1570-0232(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 45.Schierl R, Bohlandt A, Nowak D. Guidance values for surface monitoring of antineoplastic drugs in German pharmacies. Ann Occup Hyg. 2009;53:1–9. doi: 10.1093/annhyg/mep050. [DOI] [PubMed] [Google Scholar]
- 46.Kiffmeyer T, Tuerk J, Hahn M, et al. Application and assessment of a regular environmental monitoring of the antineoplastic drug contamination level in pharmacies: the MEWIP project. Ann Occup Hyg. 2013;57:444–455. doi: 10.1093/annhyg/mes081. [DOI] [PubMed] [Google Scholar]
- 47.Sessink PJM, Kroese ED, van Kranen HJ, Bos RP. Cancer risk assessment for health care workers occupationally exposed to cyclophosphamide. Inter Arch Occup Environ Health. 1993;67:317–323. doi: 10.1007/BF00385647. [DOI] [PubMed] [Google Scholar]
- 48.Baker ES, Connor TH. Monitoring occupational exposure to cancer chemotherapy drugs. Am J Health-Syst Pharm. 1996;53:2713–2723. doi: 10.1093/ajhp/53.22.2713. [DOI] [PubMed] [Google Scholar]
- 49.Zeedijk M, Greijdanus B, Steenstra FB, Uges DRA. Monitoring exposure to cytotoxics on the hospital ward: measuring surface contamination of four different cytotoxic drugs from one wipe sample. Eur J Hosp Pharm Sci. 2005;11:18–22. [Google Scholar]
- 50.Polovich M, Bolton DL, Eisenberg S, et al. Safe handling of hazardous drugs. 2. Pittsburgh: Oncology Nursing Society; 2011. [Google Scholar]
- 51.NIOSH. [accessed May 27, 2015];Hierarchy of Controls. 2015 Available at http://www.cdc.gov/niosh/topics/hierarchy/
- 52.Villarini M, Dominici L, Piccinini RR, et al. Assessment of primary, oxidative and excision repaired DNA damage in hospital personnel handling antineoplastic drugs. Mutagenesis. 2011;26:359–369. doi: 10.1093/mutage/geq102. [DOI] [PubMed] [Google Scholar]
- 53.Sottani C, Porro B, Cornelli M, Imbriani M, Minoia CC. An analysis to study trends in occupational exposure to antineoplastic drugs among health care workers. J Chromatog B. 2010;878:2593–2605. doi: 10.1016/j.jchromb.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida J, Koda S, Nishida S, Yoshida T, Miyajima K, Kumagai S. Association between occupational exposure levels of antineoplastic drugs and work environment in five hospitals in Japan. J Oncol Pharm Practice. 2011;17:29–38. doi: 10.1177/1078155210380485. [DOI] [PubMed] [Google Scholar]
- 55.Sabatini L, Barbieri A, Lodi V, Violante FS. Biological monitoring of occupational exposure to antineoplastic drugs in hospital settings. Med Lav. 2012;103:394–401. [PubMed] [Google Scholar]
- 56.Sessink PJM. Environmental contamination with cytostatic drugs: past, present, future. [accessed May 27, 2015];Saf Consid Oncol Pharm. 2011 Fall; Special edition. Available at www.ppme.eu.