Abstract
Fluorine-18 (18F) is one of the most common positron-emitting radionuclides used in the synthesis of positron emission tomography radiotracers due to its ready availability, convenient half-life and outstanding imaging properties. In Part 1 of this review, we presented the first analysis of patents issued for novel radiotracers labeled with fluorine-18. In Part 2, we follow-up with a focus on patents issued for new radiochemistry methodology using fluorine-18 issued between January 2009 and December 2015.
Keywords: : 18F, fluorine-18, PET radiotracers, positron emission tomography, radiochemistry
Positron emission tomography (PET) imaging is a functional nuclear medicine imaging technique in which patients receive an intravenous injection of a radiotracer (a bioactive molecule tagged with a positron-emitting radionuclide). A positron is emitted from the radiotracer, which annihilates with an electron producing two 511-keV gamma rays emitted simultaneously in opposite directions. The photons are detected by the PET scanner for all annihilation events that occur throughout the entire duration of the PET scan, and then mapped to provide an image with high spatial resolution that can provide functional information on, for example, biochemical and metabolic processes occurring in the patient. Due to the important role that PET plays in both disease diagnosis and biomedical research, there are over 1.5 million PET scans conducted every year [1].
Fluorine-18 (18F) is one of the most commonly used PET radionuclides because of its excellent imaging properties (a clean decay process involving 97% β+ emission and limited positron migration of approximately 1 mm, leading to high-resolution images) and the ready accessibility of Curie amounts of no-carrier-added [18F]fluoride from small medical cyclotrons. Moreover, the half-life of fluorine-18 (109.77 min) is long enough to allow manufacturing of PET drugs at centralized nuclear pharmacies and shipping (by ground or air) to satellite imaging centers and/or mobile PET scanners that do not have their own PET drug-manufacturing facilities. As popularity of the technique continues to grow, both in academic medical centers and the pharmaceutical industry (where labeling with fluorine-18 is highly compatible with the growing trend of incorporating fluorine into pharmaceutical scaffolds [2,3]), significant effort in the PET radiochemistry community is being devoted to the design and synthesis of new PET tracers labeled with fluorine-18. Fluorine-18 labeled radiotracers disclosed in the patent literature from 2009 to 2015 were the focus of Part 1 of this review series [4]. Many ‘classical’ fluorine-18 labeling strategies are available to the radiochemist (for a comprehensive review, see [5]), and continue to be used extensively to synthesize new PET radiotracers. However, as demand for new and more complex PET radiotracers continues to grow, there is a concomitant demand for new methods that allow for the incorporation of fluorine-18 into more diverse bioactive molecules, frequently at sites that cannot be labeled using classical fluorination methods. Reflecting this, late-stage radiofluorination has become an extremely active research area in its own right, with the last few years witnessing the genesis of many powerful [18F]fluorination reactions, either adapted for use from existing fluorine-19 methods or developed entirely de novo (for reviews highlighting new developments, see [6–8]). In Part 2 of this series of review articles on Intellectual Property concerning fluorine-18, patents disclosing new radiolabeling methods using fluorine-18 are surveyed.
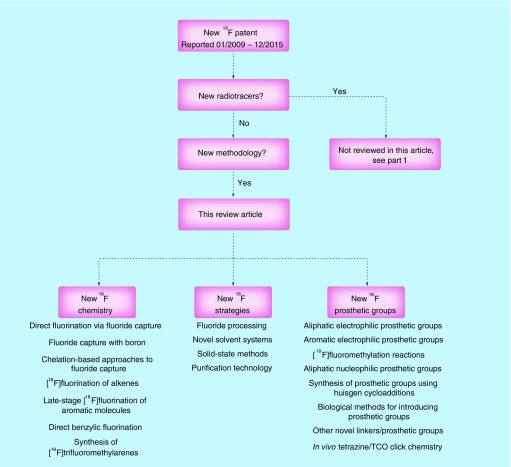
Fluorine-18 patent search (January 2009–December 2015)
Patent searches were performed in February 2016 using SciFinder®. More than 1000 patents containing ‘fluorine-18’ and ‘18F’ were issued between January 2009 and December 2015, and these patents were the starting point for the vetting process that went into selecting the patents for this review. The number of patents containing fluorine-18 has risen steadily since the 1990s, and currently ≥100 fluorine-18-containing patents are issued per year. Abstracts from the patent search were individually evaluated to determine the topic of the material that was covered in the claims. Patents involving the synthesis of new fluorine-18-labeled radiotracers were separated and reviewed in Part 1 of this series [4], while in Part 2 we focus on patents describing novel fluorine-18 radiolabeling strategies. Patents that generally cited the use of fluorine-18 among other radionuclides were excluded, as were those that focused on kit formulations or dealt with cyclotron design/targetry and synthesis module/microfluidic systems/shielding design. Methodology patents covered in this review fall into three broad categories: first, new strategies for ‘working with fluorine-18’ (includes novel fluorine-18 processing techniques, solvent systems, reaction media (e.g., solid-state synthesis) and purification strategies (solid-phase extraction [SPE])); second, new radiolabeling strategies and third, development of new prosthetic groups or novel strategies to synthesize existing prosthetic groups. The organization of this review follows this grouping (Figure 1) and summarizes technical improvements in the ability to label molecules with fluorine-18 that have occurred over the last 5 years.
Figure 1. . Roadmap of this article.
New strategies for working with fluorine-18
Synthesis of 18F-labeled radioligands for PET imaging is a multistep process that involves production of fluorine-18 in a cyclotron followed by [18F]fluoride processing. For the purposes of this review, [18F]fluoride processing involves the transfer of fluorine-18 from the cyclotron to the synthesis module, trapping/elution of [18F]fluoride from a quaternary methylammonium (QMA) or other SPE cartridge, and drying of the [18F]fluoride. The several patents highlighted in this section focus on alternative [18F]fluoride processing methods, including alternative eluents, phase transfer agents, solid-phase materials and (surprisingly) reaction solvents containing water. The primary purpose of these new approaches is shortening (or even elimination) of the [18F]fluoride-drying step, which is done primarily by altering the constitution of the aforementioned processing steps and/or allowable reaction conditions.
Fluoride processing
Inventors at the Technical University of Munich claimed the use of alkali and alkaline earth metal hydroxide solutions as superior eluents that enable dry-free [18F]fluoride reprocessing. This was claimed to have an impact on the development of ‘lab-on-a-chip’ [18F]fluorination assemblies [9]. The [18F]fluoride is first trapped on the QMA cartridge, then eluted with an organic (MeCN, DMF, etc.) solution containing a cryptate paired with an alkali or alkaline earth metal hydroxide. The novelty lies in the fact that the organic-soluble hydroxide salt does not require water during the elution process and therefore makes the [18F]fluoride-drying step unnecessary. PSA-HCO3 anion exchange cartridges were compared with the more commonly used Waters QMA-light cartridges for the [18F]fluoride capture process, as was the effect of different preconditioning counter ions (HCO3 - and CO3 2-), with QMA cartridges yielding better results with both counterions.
The Asan Foundation [10] patented their reported [11] method of eluting [18F]fluoride from an anion exchange cartridge with a pH-buffered eluent (6.0–8.0). The advantages of this novel eluent include the exclusion of base from the eluent, which is useful for base-sensitive precursors, such as those of a biogenic origin. This novel eluent system was also claimed to decrease the amount of organic impurities produced during synthesis of radiopharmaceuticals.
Different phase transfer agents have also been explored within the patent literature. Naguib et al. described the complexation of [18F]fluoride with a variety of different cryptands for use in radiolabeling of PET tracers for clinical use [12]. The cryptands are variably substituted in order to ensure stability of the resulting complex. Inventors at Bayer also explored alternative methods of capturing and eluting 18F- using various quaternary ammonium polymers and a range of solvents, including water and aqueous alcohols [13]. They utilized these methods to label multiple clinically relevant PET tracers [14].
Similarly, researchers at GE Healthcare Ltd. explored the use of a polymer solid support connected to various cryptands (including Kryptofix 2.2.2) via an alkyl/alkenyl linker for trapping and elution of [18F]fluoride [15]. The cartridge is kept at a pH of ca. 5 to ensure that anionic [18F]fluoride will complex with the cryptand, which contains fluorophilic-protonated amine residues. [18F]Fluoride is then eluted with a somewhat more basic solution (pH 9), to presumably deprotonate the amine residues. In the methods presented, the cryptand is attached to the solid supports, in other words, polystyrene, etc. via a C1–50 linker that may include aromatic rings.
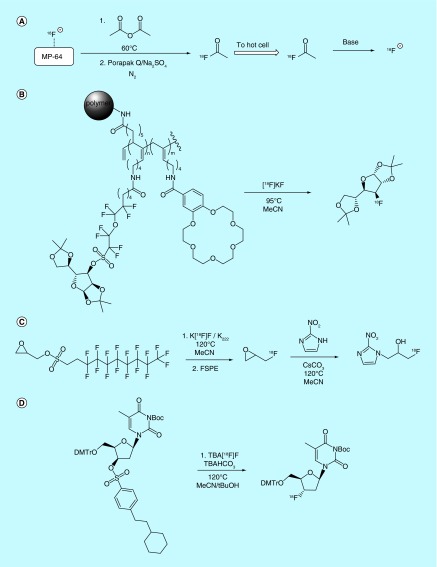
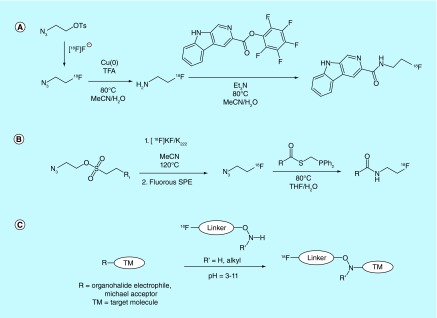
In a very different example of [18F]fluoride-processing methodology, researchers at the Mayo Foundation for Medical Education and Research patented a novel method of producing gaseous fluorine-18 synthons [16] using a method similar to their published report [17]. In this method, gaseous [18F]acetyl fluoride or other [18F]acyl fluorides are synthesized directly in the vault of the cyclotron, or in a small module adjacent to it, via treatment of cyclotron-produced [18F]fluoride with the corresponding acid anhydride. The gaseous synthons produced in this way are then routed to the synthesis module and converted back to reactive [18F]fluoride in a basic solution for subsequent radiolabeling reactions (Figure 2A). This technique is claimed to reduce the amount of activity lost during transfer from the cyclotron to the synthesis module. The inventors report the synthesis of the acetyl [18F]fluoride carrier with a specific activity of more than 0.035 Ci/µmol (>1.3 GBq/µmol) in the patent, while specific activities of more than 32 Ci/µmol (>1200 GBq/µmol) are reported in the accompanying publication.
Figure 2. . New methods for working with fluorine-18 and new solid-state methods.
Novel solvent systems
The design of novel solvent systems for [18F]fluorination reactions are also the subject of several patents, again primarily with the goal of eliminating the time-consuming [18F]fluoride-drying step during processing. Inventors at Trasis S.A. and the University of Liège patented a [18F]fluorination method using a solvent system containing up to 3% water and a tertiary alcohol additive [18]. A similar patent from inventors at Siemens also describes a ‘drydown-free’ [18F]fluoride-processing method where a balance is achieved between the minimal amount of water required for elution and the maximal amount of water allowable for [18F]fluorination [19]. This method claims that [18F]fluoride elution from a QMA cartridge can be accomplished with as little as approximately 5% water, and radiolabeling with as much as approximately 1% water. To reduce the toxicity of the solvent systems used for [18F]fluorination, inventors at the University of Michigan [20] patented a ‘green approach’ to fluorine-18 radiochemistry that employs wet ethanol as a solvent system for [18F]fluorination of radiopharmaceuticals such as [18F]FDG. An optimal ratio of 15:85 water:ethanol produced the best radiochemical conversions (RCCs) that were (surprisingly) almost on par with conventional methods. This method precludes the need for residual solvent analysis after radiolabeling due to the absence of problematic solvents (e.g., MeCN and DMF) as water and ethanol are the only solvents used throughout the entire radiopharmaceutical manufacturing process. A report detailing this method is also published in the academic literature [21].
Solid-state methods
Methods where all steps of the radiosynthesis are conducted on a polymer-filled cartridge have been patented by several inventors. This approach to [18F]fluorination is desirable as it does not require the use of complicated synthesis module setup and is potentially more automatable. For example, a column design method patented [22] and reported [23] by inventors at the Technical University of Denmark details a reusable phosphazene-PEG appended polystyrene column design for solid-phase [18F]fluorination reactions. Target water is first passed over the resin, thereby trapping the [18F]HF present in target water via reaction with immobilized phosphazene. Fluorine-18 trapping is followed by a wash of the material with a water-miscible solvent and pressurized gas to remove residual water. Precursor compounds in organic media are then loaded onto the column. The fluorination reaction (SN2, SNAr) takes place directly on the column (a heating system is described) without the need for additional reaction vessels. Elution with additional organic media then releases the fluorinated compound and excess precursor, but not any unreacted [18F]fluoride, thereby simplifying purification, and the column can be washed and reused. Inventors discuss resin synthesis as well as appropriate selection of phosphazenes and support agents, as only several phosphazene/linker/support combinations that were tested produced appreciable yields of radiotracer. The inventors do not report the specific activities of products produced using this method in the patent. However, in the publication describing this method, they report the synthesis of [18F]FDG using solid-supported phosphazine base PS-P2-t-Bu with a specific activity of greater than 0.3 Ci/µmol (>11 GBq/µmol). A similar column design that utilizes anion exchange appended packing material to trap [18F]fluoride and a luer lock system to prevent solvent leaks was separately patented by Chi et al. [10].
Another solid-state [18F]fluorination technique involves the use of column-packing materials that are chemically preloaded with the substrate. This approach was taken by researchers at Tokyo Institute of Technology and Niigata University, who patented a ‘react and release’ approach for the radiosynthesis of [18F]fluorosugar derivatives [24]. The claimed method relies on the synthesis of a suitably protected sugar bound to a cryptand-bearing polymeric support through a sulfonate ester linkage. Upon reaction of the polymer with [18F]fluoride, the labeled fluorosugar is released and can be separated from the unreacted precursor, cryptand and by-products via elution with appropriate solvent (Figure 2B). The same inventors also patented a similar method for the synthesis of [18F]FMISO, again from a polymeric precursor as a means of facilitating purification [25]. Other examples of this approach include the use of bifunctional materials reported by investigators at Sogang University, where triazole-appended linkers assist in [18F]fluoride adsorption and phase transfer [26], only releasing the [18F]fluorinated product on elution [27].
Purification technology
Several patents also describe novel uses of SPE processes for the purification of [18F]fluorinated products. For example, Isis Innovation Limited patented a strategy [28] that was also later reported in the academic literature [29] for improving the speed and ease of purification of [18F]-labeled radiotracers and radioligands by making use of precursors bearing perfluorinated sulfonyl-leaving groups (Figure 2C). The described isolation/purification strategy relies on ‘fluorous detagging’, whereby reaction of perfluorinated precursor with [18F]fluoride results in the production of the desired (monofluorinated) radiolabeled product, along with a perfluorinated sulfonate anion. Most of the precursor remains unreacted and consequently still bears the perfluorinated tag as well. The radiolabeled product is consequently the only species in the mixture not bearing the perfluorinated tag, so the reaction mixture can be loaded onto a fluorous SPE (FSPE) resin, and the product eluted with an appropriate fluorophobic solvent (such as a water/MeCN mixture). An additional advantage of this method is that unreacted precursor can be purified and recovered after the reaction by subsequent elution of the FPSE resin with a fluorophillic solvent, such as toluene. Considering the use of fluorous precursors in this method, the specific activity of the resulting [18F]-labeled products is of interest. While a discussion of this concept is provided in the patent, the measured specific activities of [18F]-4-phenylbutyl fluoride produced from the tosyl-precursor (>3 Ci/µmol, >100 GBq/µmol) is higher than the corresponding fluorous precursor (0.03–0.3 Ci/µmol, 1–10 GBq/µmol), suggesting some leaching of fluoride from the precursor occurs during the synthesis. A similar method was also patented by Bayer Schering Pharma AG, and describes an approach whereby perfluorinated sulfonate esters were employed as leaving groups on biorelevant scaffolds for SN2 reactions with [18F]fluoride, followed by FSPE to purify the resultant products [30].
Solid-phase separation of precursors and by-products from the desired radiolabeled target molecule has also been explored in the context of vastly different logP values between the product, precursor and by-products. Bayer Schering Pharma patented a number of highly lipophilic sulfonate esters as electrophiles for reaction with [18F]fluoride, including 4-(2-cyclohexylethyl)benzenesulfonyl-, 4-(2,2-dicyclohexylethyl)benzenesulfonyl- and a cholesterylmethylenebenzenesulfonyl derivatives (Figure 2D) [31]. The inventors claim that the difference in logP between the precursors and the products (up to 9.5 units for the cholecystyl derivatives) is sufficient to allow for facile purification using reverse-phase SPE methods, and a number of methods for the separation of the precursor and product are claimed.
New radiochemical reactions
Direct fluorination via [18F]fluoride ‘capture’
An alternate approach to covalent [18F]fluorination is the introduction of a fluorine-18 label via dative (or coordinative) bond formation, sometimes described as [18F]fluoride ‘capture’. There are two approaches to this, and both take advantage of the affinity of fluoride, which is a ‘hard’ Lewis base, for ‘hard’ Lewis acids. The processes work as follows: first, [18F]fluoride is ligated to a cationic metal center toward which fluoride has a high affinity (e.g., aluminium). The metal is then conjugated to the target molecule with a bifunctional chelate [32,33] and second, [18F]fluoride is ligated to a Lewis acidic center that is not traditionally thought of as metallic (i.e., metalloids such as boron, silicon, etc.), with the Lewis acid conjugated to the rest of the target molecule via conventional covalent bonds, with or without a linker/prosthetic group [34]. The benefit of such exchange-based methods is that they are typically very simple and mild, allowing for rapid labeling in an aqueous environment given the correct choice of pH with/without heating. However, this simplification of radiolabeling comes at a cost due to the possible difficulty in separating labeled/unlabeled molecule, which can theoretically reduce specific activity significantly. In addition, introduction of a chelate and/or Lewis acid can have deleterious effect on the PK properties of the target molecule. Many of the patents in this section are accompanied by a wealth of academic literature that will not be discussed here, and some of these strategies are quite well developed, albeit infrequently utilized for clinical radiolabeling.
Fluoride capture with boron
Boronates are capable of forming reversible, yet kinetically inert covalent complexes with the [18F]fluoride ion. This can occur via isotopic exchange of [18F]fluoride for existing fluorides or alternately, exchange of [18F]fluoride for various leaving groups, most notably boronic acid hydroxyls. Boronic acid esters can also capture fluoride without concomitant loss of the ester, thereby forming singly [18F]fluorinated boronate salts. This section focuses on patents employing various boronate functional groups as [18F]fluoride capture agents, an area that has very recently been reviewed by one of the inventors below [35]. The patented method describes the use of a carrier, and while no specific activities of the radiolabeled peptides are reported, specific activities are expected to be low because of the presence of carrier fluoride.
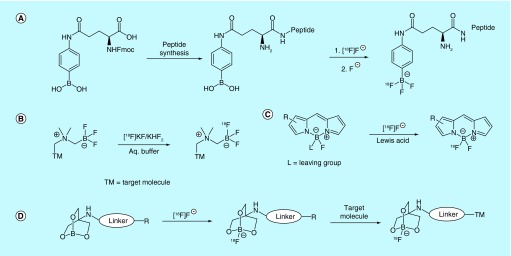
A method for the preparation of aryl-[18F]BF3-substituted peptides is described by the University of Kent, UK [36]. In this method, artificial amino acids appended with either aryl boronic acids or their pinacol esters are introduced into peptides during solid-phase peptide synthesis. The peptide is then treated with [18F]fluoride and with naturally occurring fluoride in the form of KHF2 to produce the aryl-[18F]BF3-substituted peptides in a manner similar to that described by the Perrin laboratory (Figure 3A) [37,38]. The methods for synthesis of amino acids and their conjugation to peptides comprise most of the patent examples and claims, including methods such as click chemistry and methods akin to those used for succinimidyl-4-[18F]fluorobenzoate where the 4-position is instead [18F]BF3. This method eliminates the need for lysine-reactive fluorine-18-containing prosthetic groups in situations where lysine and/or other amino acid targets of bioorthogonal labeling are important to the structure/function of the peptide.
Figure 3. . [18F]Fluoride capture with boron.
Another patent filed during the same year (2009) by inventors at the University of British Columbia also described [18F]fluoride exchange into boronic acids as a simple means of fluorine-18 labeling, but with a focus on the effect that aryl ring substituents have on the fluorophilicity of the boronate [39]. Specifically, the inventors linked the presence of electron-withdrawing substituents on the aryl ring to increased [18F]fluoride stability (i.e., slower [18F]defluorination from the fluoroborate complex). The authors found that favorable kinetics for [18F]fluoride capture and retention occur when the overall σ is at least 0.06 with an electron-withdrawing group (EWG) positioned ortho to the boronate and at least 0.2 without an ortho-positioned EWG, with both situations encompassing the primary claim of the patent. Polyaromatic and heteroaromatic systems are also included in this claim in addition to simpler aryl systems. This carrier-added method for the synthesis of [18F]BF3-labeled agents has a reported decay-corrected specific activity of 0.12 Ci/µmol (4.4 GBq/µmol).
A second patent filed in 2014 by the same University of British Columbia group expanded this methodology to nonaryl boronates as fluorine capture agents [40]. It similarly takes advantage of EWGs such as carbonyl, carboxylic acid and quaternary ammonium salts positioned alpha to the boronate as the driving force for fluoride capture and retention. As in previous patents, fluoride capture is accomplished in aqueous buffer, but the starting reagent is a preformed trifluoroborate salt with [18F]fluoride capture occurring solely via isotopic exchange (Figure 3B). The resultant radiolabeled complex is highly impervious to [18F]defluorination, with several examples described listing half-lives in the tens or hundreds of thousands of minutes. Aside from enhanced half-life, the benefit of this moiety when compared with arylboronates is that the resultant reactive group has a much smaller footprint and careful positioning of potentially reactive EWGs is no longer required.
Another patent from the University of Southern California focused on the use of 18F-fluorinated boron-dipyrromethene (BODIPY) groups as dual imaging prosthetic groups for labeling of pharmaceutical imaging agents (Figure 3C) [41]. BODIPY dyes have high quantum yields, with emission wavelength easily tunable by appropriate functionalization, which allows for facile tissue imaging, specifically at longer emission wavelengths. The boron atom at the core of the dye can also be [18F]fluorinated under relatively mild conditions with carrier-added or no-carrier-added [18F]fluoride in the presence of a Lewis acid catalyst. These properties lead to a single prosthetic group that is both fluorescent and positron-emitting, and an example BODIPY derivative with a specific activity of higher than 1.4 Ci/µmol (52 GBq/µmol) is described. The inventors note that proper choice of ring substituents is an important consideration when forming fluoroborate complexes. As in the prior [18F]fluoride exchange patents, electron-donating ring substituents lead to rapid [18F]defluorination of the fluoroborate complex, thus rendering the molecule unsuitable for use as a tracer. Ring substituents also affect the spectral properties of the dye, so substituent optimization is required in these types of systems to maximize both [18F]defluorination half-lives and the desired emission wavelength of the dye. As such, the inventors also claim variable functionalization on the boron atom, as this can also influence fluoride binding and release kinetics, as well as several BODIPY-like dye scaffolds. Several examples of BODIPY prosthetic groups are also included in the patent, as well as methods for conjugation to biomolecules, and a report on the synthesis of BODIPY PET/fluorescent probes is presented in the academic literature [42].
In contrast to the heterosubstituted boronates described in the former patents, inventors from the University of South Florida and the Moffitt Cancer Center patented a tris-aliphatic boronate group that forms charged fluoroborate complexes with fluorine-18 [43]. The group consists of a tridentate boronic ester that puts significant strain on the bridgehead boron atom, thereby increasing fluorophilicity and significantly disfavoring [18F]defluorination (Figure 3D). The authors claim a [18F]defluorination half-life of 52 h for this complex, more than sufficient for most PET imaging studies. The neutral bicyclic group can be formed from commercially available reagents, and is attached to a linker that terminates with an electrophilic reactive group, a nucleophile or an amino acid residue for conjugation or subsequent solid-state peptide synthesis. The authors alternately claim that the linker between the boronate and reactive group can be shortened or removed altogether if appropriate. Although synthetic details are not explicitly stated in the patent, it can be assumed that mild aqueous methods used to fluorinate aryl boronates will likewise work for this bicyclic group as well.
Chelation-based approaches to fluoride capture
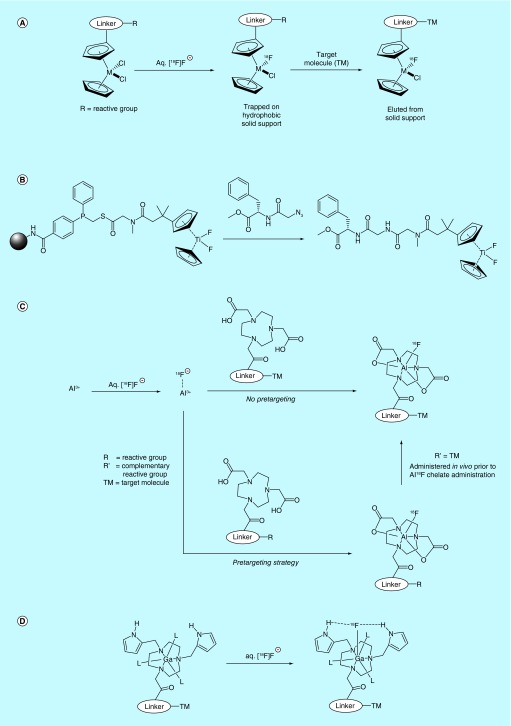
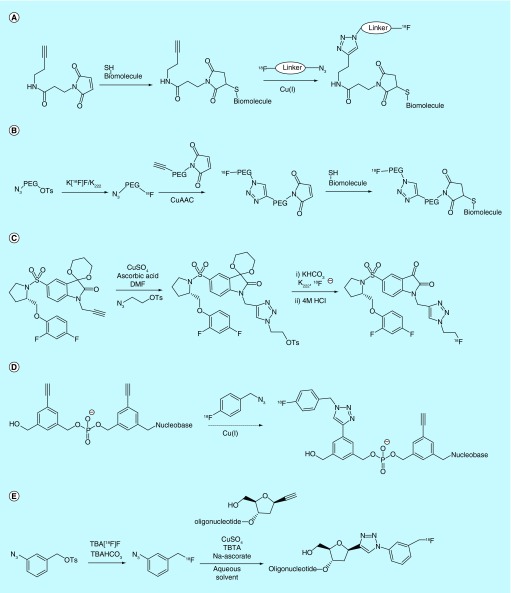
Researchers affiliated with Aptenia S.R.L patented a method wherein titanium, hafnium and zirconium metallocenes are utilized as substrates for [18/19F]fluoride [44]. Due to the fluorophilicity of these metals, [18F]fluoride can displace one of two ligands originally coordinated to the metallocene metal centers. The inventors take advantage of this affinity by using these metallocenes to functionalize biomolecules, thereby allowing them to be easily labeled with [18F]fluoride in biologically relevant media such as saline (Figure 4A). The core of the invention outlines the method by which metallocenes are labeled with [18F]fluoride and then conjugated to biomolecules in a more or less expedient manner. A metallocene appended with a linker containing a reactive moiety is first immobilized on a hydrophobic solid support, then labeled with [18F]fluoride and washed with water. An appropriately functionalized biomolecule that will react with the reactive moiety on the metallocene linker is then passed through the solid support, thereby generating biomolecule-metallocene-[18F]fluoride conjugates, which are washed off the support and purified further prior to use. A similar method for radiolabeling of azide-bearing bioactive compounds with polymer-supported difluorotitanocene derivatives by a modified Staudinger ligation has also been reported by Scabini (Figure 4B) [45].
Figure 4. . [18F]Fluoride chelation.
Inventors affiliated with Immunomedics Inc. have filed several patents on the use of group 13 metal cations (notably Al3+) as high-affinity substrates for rapid fluoride labeling (Figure 4C) [46–52]. Boron complexes are also claimed in one of the patents, but the methodology required with boron is not described [47]. Strongly Lewis acidic metal cations such as Al3+ and Ga3+ are quite fluorophilic, forming a coordinative bond that is stable in vivo. Thus, the patented strategy relies on the use of a bifunctional chelate approach, wherein a biomolecule conjugated to a 5- or 6-coordinate chelate such as NODA (other examples are also provided) is lf with M3+ followed by [18F]fluoride, or instead reacted with a preprepared aqueous M18F2+ complex, which is then incarcerated within the chelate– biomolecule conjugate (with or without heat, microwave irradiation). Either route [18F]fluorinates the biomolecule for use in imaging, purportedly without the need to separate the unlabeled starting material from the labeled complex. In Asome examples, the inventors report that the Al complex is inseparable from the [18F]AlF complex. In both cases, the specific activity is determined by the amount of aluminium and precursor utilized in the complex-formation step, along with the activity of isolated [18F]-labeled product. In cases where they did isolate the radiofluorinated species, they reported specific activities ranging from 0.46 Ci/µmol (17 GBq/µmol) to 4.1 Ci/µmol (153 GBq/µmol), depending on the chelator, buffer and specific peptide used in the labeling reaction. The inventors claim that an in vivo click chemistry ‘pretargeting’ approach can also be used, which combats issues such as slow target engagement that are seen with some radiolabeled biomolecules, for example, antibodies. This approach is described in greater detail in a later section. The inventors discuss the need to balance the hydrophobic and hydrophilic properties of the chelator, linker and peptide to enhance targeting, as well as chelator choice to increase RCY, and provide several examples of properly balanced systems as well as kit formulations. The patents also provide examples of biomolecule labeling, autoradiography biodistribution data of Al18F bioconjugates, including a comparison of results when pretargeting and non-pretargeting strategies are utilized, as well as a variety of reactive groups capable of either bio-orthogonal or conventional click chemistry such as azide, nitrone, cyclooctyne and active ester [51]. Al–F [18F]fluoride capture toward the radiofluorination of biomolecules was recently reviewed by the inventors [32].
A similar process was also claimed in a series of patents from a collaboration between GE Healthcare Limited and the University of Southampton. The inventors describe a set of conditions for M3+-chelate [18F]fluorination that are purportedly more suitable for work with biomolecules (Figure 4D) [53–55]. Specifically, the inventors focus on Ga3+ and In3+ as the metals of choice due to the lower temperatures required to synthesize GaF2+ and InF2+ complexes relative to AlF2+, as well as a broader pH range during labeling (AlF2+ is formed under a more or less narrow range around pH 4). The inventors also claim the use of tridentate triazacyclononane chelators, as opposed to the penta- or hexa-dentate NODA-type chelators described by Immunomedics Inc., as well as the use of additional pendant ligands for M3+ that are outfitted with hydrogen-bonding donors, presumably as a means to ‘attract’ the fluoride to the chelate and thereby improve radiolabeling kinetics at lower temperatures.
[18F]Fluorination of alkenes
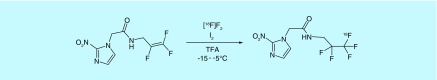
In an example of formally oxidative [18F]fluorination, researchers from the University of Pennsylvania and the University of Florida disclosed a new method for the fluorination of alkenes with [18F]F2 (Figure 5) [56]. The method described is general, but was developed specifically for the preparation of PET hypoxia imaging agent [18F]EF5, an analog of [18F]fluoromisonidazole. The reaction utilized I2 and trifluoroacetic acid to catalyze the reaction and the method was described in greater detail in a related publication [57]. Interestingly, in a later paper on the dosimetry of [18F]EF5, an earlier synthesis was used instead of the patented method [58].
Figure 5. . [18F]Fluorinatin of alkenes.
Late-stage [18F]fluorination of aromatic molecules
In the past 5 years, the [18F]fluorination of arenes has seen a significant amount of innovation, which has made electron neutral and election-donating arene [18F]fluorination more accessible and increased the scope of precursors available for such reactions [6]. Many of these innovations, which have been published in field-specific literature, have also been patented.
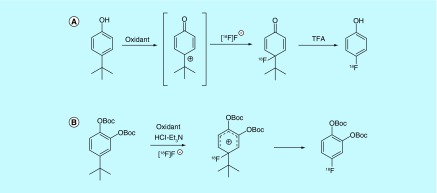
Isis Innovation Limited patented a method utilizing a phenol umpolung strategy to activate a phenol for [18F]fluorination with [18F]KF para to the phenol [59], which was based on work developed in the Gouverneur laboratory and described in greater detail in a journal article (Figure 6A) [60]. While there is no discussion of the specific activity of the products produced by this oxidative method in the patent, the accompanying journal article reports the specific activity of 2-bromo-4-[18F]fluorophenol produced by this method as 11.3 Ci/µmol (420 GBq/µmol).
Figure 6. . Synthesis of [18F]phenol derivatives.
In similar work, the use of a tert-butyl group para to a modified phenol (protected with tert-butyloxycarbonyl) as a site of fluoride incorporation was reported (Figure 6B) [61]. Unlike the prior method, which utilized chemical oxidation to facilitate an umpoloung strategy, the method reported by the University of California utilized electrochemical anodic oxidation where Et3N-HCl was utilized for elution of the [18F]fluoride from the quaternary ammonium resin and later used as a ‘Et3N-[18F]HF’-like reagent to carry out [18F]fluorination. The electrochemical method was used for the synthesis of di-O-Boc-protected 4-[18F]fluorocatechol where the product was found to have a specific activity of 0.001 Ci/µmol (0.043 GBq/µmol), similar to that obtained during carrier added fluorination with [18F]F2. Examples provided in the patent are the same as those described in a later publication [62].
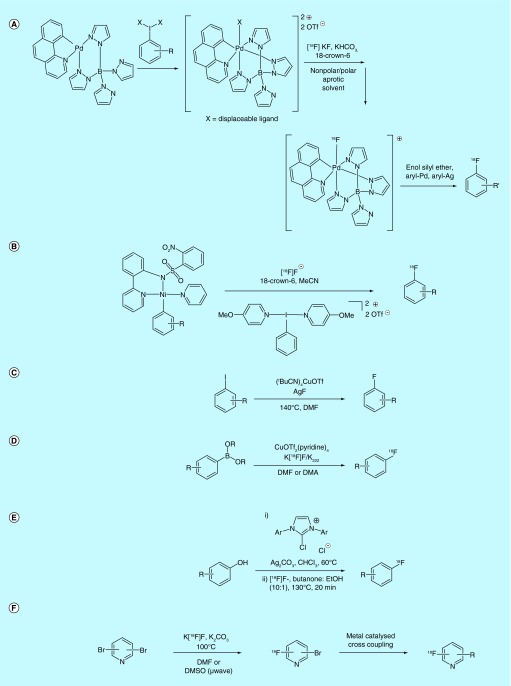
An exciting emerging approach for fluorinating aromatic rings involves the development of transition-metal-mediated nucleophilic radiofluorinations (for reviews, see [6–8]). For example, a method for the synthesis of [18F]arylfluorides via Pd(IV)-18F complexes was patented by Harvard University [63] and published separately in the academic literature by the Ritter laboratory [64]. This body of work describes methods used for the production of a ‘high-valent’ Pd(IV) complex which, when reacted with [18F]KF, generates an electrophilic (‘F2-like’) source of [18F]fluorine from nucleophilic [18F]fluoride. The Pd(IV)-18F complex formed in this way can then be used to carry out [18F]fluorination reactions on a range of substrates (Figure 7A), including silver arenes, palladium(II) arenes and silyl enol ethers, which are typically fluorinated with electrophilic-fluorinating reagents such as Selectfluor. The specific activity of [18F]fluoroarenes formed using this methodology is quite high (1.03 Ci/µmol [38.1 Gbq/µmol]) for the single example given in the patent) in comparison to products formed from [18F]F2-derived electrophilic fluorination reagents. The synthesis of a palladium(II) arene substrate (which is similar to the Ni species in the patent that follows) is also described, as is the synthesis and spectral properties of multiple Pd(IV) catalyst variants.
Figure 7. . Late-stage [18F]fluorination of aromatics 1.
It quickly became apparent that this transformation was not compatible with the strenuous demands of routine clinical PET radiotracer production, a fact recently addressed by the authors in the literature [65]. Thus, subsequent work from the Ritter laboratory has concentrated on the radiofluorination of Ni(II) aryl complexes that are silica and water stable. This work was also patented by Harvard [66] and has been reported in the literature (Figure 7B) [67]. This method is the first of several to utilize metal species to conduct ‘electrophilic-like’ [18F]fluorination using formally nucleophilic [18F]fluoride. The patent describing the nickel-mediated oxidative fluorination of arenes does not note the specific activity of the products, though an example is found in the publication describing this work, where fluorination of an estrone derivative was found to produce a product with a specific activity of 1.1 Ci/µmol (40.7 GBq/µmol). The methodology has been used to synthesize radiotracers for preclinical use [68] and has recently also been validated for clinical production of [18F]5-fluorouracil [69,70].
Toward the same goal, a method developed at the University of California for the fluorination of aryl iodides by copper mediation and silver fluoride treatment was also patented (Figure 7C) [71]. The method for [18F]fluorination with excess AgF was reported by Hartwig's laboratory in a later publication [72]. However, while application toward [18F]fluorination is claimed, no example is given in the patent or accompanying literature. Later work by Sanford, Scott and colleagues showed that the reaction could be used for noncarrier added [18F]fluorination but required a newly developed Ag[18F]F preparation [73]. The silver reactions as presently described provide only modest yields and are challenging to purify due to the silver(I/0) present in the reaction medium.
Motivated by Ritter's initial work on [18F]fluorinating Pd- and Ni-complexes, the Sanford group developed copper(III)-mediated fluorination reactions due to concerns regarding the cost and toxicity of palladium [74]. This line of thought has generated several patents among various groups, including methods for the [18F]fluorination of diaryliodonium salts [75] and organoborons [76]. The former is discussed in a later section that focuses on new methods for fluorinating hypervalent I(III) compounds. The latter patent describes copper(II)-catalyzed methods for the [18F]fluorination of arylboronates (and their esters) and was patented by Isis Innovation Limited [76] and reported in the academic literature [77] by the Gouverneur laboratory. This method utilizes CuOTf2(pyridine)4 as a catalyst for late-stage [18F]fluorination of diverse arenes and arylenes using nucleophilic [18F]fluoride under relatively mild conditions (Figure 7D). The inventors describe this method as an improvement over other [18F]fluorodeboronation reactions that can be found in the literature, which have traditionally relied on electrophilic sources of fluorine (e.g., [18F]Selectfluor bis-triflate), and/or the use of metals (Pd, Ni) that pose safety concerns. Specific emphasis is placed on reagent ratios/concentrations, temperature and solvent as key factors. Atmospheric oxygen was also found to significantly improve yields, which points to a Sanford fluorodeborylation-like mechanism involving hypervalent Cu(III) species [74]. While the literature report by Tredwell et al. is quite extensive [77], several key findings, such as improvement of yield in dimethylacetamide solvent, are only described in the patent. Conversely, while the patent does not report the specific activity of the products, the article describing the same work reports that 4-[18F]fluoro-1,1′-biphenyl synthesized using this method was found to have a specific activity of 3.0 Ci/µmol (112 GBq/µmol). Further developments in the [18F]fluorination of organoborons have also been reported by Gouverneur and colleagues [78], Neumaier and colleagues [79] as well as Sanford, Scott and colleagues [80], and this continues to be an active area of research.
The recently reported concerted nucleophilic SNAr fluorination from the Ritter laboratory [81] was also patented by Harvard [82]. In this work, a phenol is treated with a chloroimidazolium chloride in the presence of [18F]fluoride. This generates a uronium, which can react with [18F]fluoride and subsequently undergo a deoxyfluorination to generate an [18F]arylfluoride (Figure 7E). The method works well for arenes bearing EWGs, as well as a range of heteroarenes. The method was found to produce products with high specific activity as noted in a recent report, where a [18F]fluorinated benzofuran derivative was synthesized with a specific activity of 3.0 Ci/µmol (111 GBq/µmol). Proof-of-concept was also demonstrated for electron-rich aromatics although yields were lower for these substrates. The inventors suggest this is due to issues with the solubility of [18F]fluoride and efforts continue to improve the process for electron-rich arenes.
GE Healthcare Ltd. patented a method for the synthesis of 2-[18F]fluoro-6-bromopyridine and 3-[18F]fluoro-5-bromopyridine from dibromopyridine precursors [83]. Their method describes both a simple SNAr reaction in DMF for the [18F]fluorination of the 2,6-substituted precursors and a microwave-assisted reaction in DMSO for the more challenging [18F]fluorination of 3,5-substituted precursors (Figure 7F). The authors also claim the use of the [18F]fluoropyridine products as prosthetic groups useful in a variety of metal-catalyzed cross couplings, including Heck, Suzuki, and Stille reactions, among others.
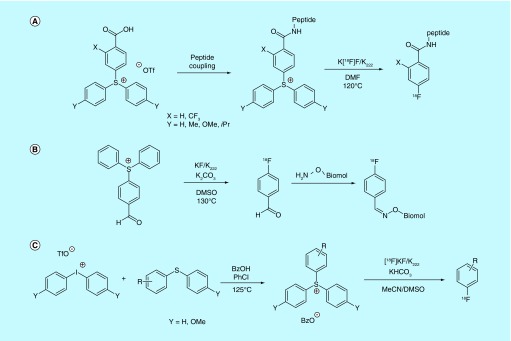
Hypervalent I(III) compounds have been known for over 100 years and have been used as substrates for nucleophilic [18F]fluorination since Pike's report in 1995 of [18F]fluorination via thermal decomposition of aryliodonium salts [84]. Since then, the scope of I(III) species utilized for [18F]fluorination has grown to include various aryliodonium salts, aryliodyl compounds and aryliodonium ylides, as well as substrates based on other hypervalent centers, such as sulfonium precursors [85]. Patent literature has followed suit, and several novel methods detailing the [18F]fluorination of these species were patented within the timeframe of this review. Most utilize thermal methods, wherein coordination of [18F]fluoride to the iodonium is followed by high-temperature thermal decomposition to the [18F] aryl fluoride, and also include a patent utilizing milder copper-mediated [18F]fluorination conditions.
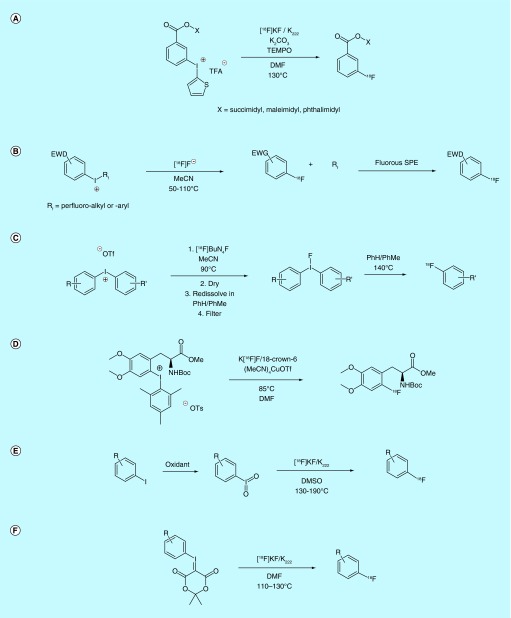
A patent by the inventors at the University of Newcastle-upon-Tyne claimed the synthesis and thermal [18F]fluorination of iodonium salt derivatives that contained caboxysuccininmidyl-, carboxymaleimidyl- and carboxyphthalimidyl-substituted aryliodoniums (Figure 8A) [86]. These compounds were synthesized with an iodonium thiophene salt present in the meta-position of the benzene ring. [18F]Fluorination at an ideal temperature of 130°C (8–23% yield) provides access to meta-[18F]fluorinated benzoic-acid derivatives for the use in biomolecular-coupling reactions or small-molecule arylcarboxylate radioligands and at least one additional report for the [18F]fluorination of these species can be found in the academic literature [87]. The inventors also claim that solid-state methods can be used for this chemistry.
Figure 8. . Late-stage [18F]fluorination of aromatics 2.
Inventors from GE Healthcare and Medi-Physics Inc. outlined the use of perfluoro iodonium salts as leaving groups for thermal [18F]fluorination (Figure 8B) [88]. The perfluorinated iodonium salt moiety was claimed to be a suitable leaving group for [18F]fluorination of electron-withdrawing arenes under standard nucleophilic [18F]fluorination conditions. As in previously discussed patents, the use of a perfluorinated leaving group also simplifies product purification via FSPE. While the precursors in the former patent were claimed to be shelf-stable for at least 4–6 months, another patent by inventors at GE Healthcare details the formulation, [18F]fluorination and development of a kit for less shelf-stable iodonium salts [89], and discusses the need for these precursors to be stored in the presence of a free radical trap (10–400 mol% TEMPO), which is claimed to also increase radiochemical yields during thermal [18F]fluorination.
Another attempt at improving labeling yields comes from a pair of patents by inventors at NUtech Ventures. These patents outline the methods involved in the [18F]fluorination iodonium salts and the process by which excess salt is removed after ligation of [18F]fluoride to the precursor but prior to thermal decomposition to the [18F]aryl fluoride (Figure 8C) [90,91]. The inventors claim that this extra purification step leads to a yield increase of greater than 10%. Multiple methods were utilized to remove the undesirable salts, including size exclusion chromatography, which could be implemented into production by way of kit formulation.
Spurred by the transition-metal-mediated fluorination chemistries discussed earlier, Sanford, Scott and colleagues looked to improve the synthesis of [18F]fluoroarenes by combining the benefits of transition metal catalysis with the [18F]fluorination of diaryliodonium salts. They developed a general Cu-catalyzed [18F]fluorination of mesityl(aryl)iodonium salts using (MeCN)4CuOTf with [18F]fluoride in DMF (Figure 8D), which was patented by the University of Michigan [75] and subsequently reported in the literature [92]. This method performs well at much lower temperatures than typical of most thermal iodonium [18F]fluorinations (85°C) and is suitable for fluorinating both electron-poor and electron-rich arenes. The utility of the method was demonstrated by the synthesis of a protected [18F]FDOPA derivative, which is traditionally accessed through an operationally challenging electrophilic fluorination method using [18F]F2 gas. Out of concern for the possibility fluoride exchange between the tetrafluoroborate counterion of the iodonium salt and [18F]fluoride, the inventors investigated the specific activity of 4-[18F]fluoroanisole produced using their copper-catalyzed method with tosylate and tetrafluoroborate iodonoum salts. They found that the specific activity of the resultant product to be 1.8 ± 0.8 Ci/µmol (66 ± 30 GBq/µmol) and 3.0 ± 1 Ci/µmol (111 ± 37 GBq/µmol) for the tetrafluoroborate and tosylate salts, respectively, demonstrating that fluoride exchange under these conditions was negligible.
Two patents by the inventors at the University of California pertain to the use of hypervalent aryliodine species as substrates for thermal [18F]fluorination. The first of these patents pertains to [18F]fluorination of aryliodyl (IO2) substrates that possess electron-donating or -withdrawing substituents (Figure 8E) [93]. Production of the precursors is relatively straightforward, involving the oxidation of the respective aryliodide with either sodium hypochlorite or dimethyldioxirane. [18F]Fluorination is then carried out with [18F]KF, [18F]CsF or [18F]Alk4N+F- using standard SNAr methods. The second patent outlines the use of aryliodonium ylides for the [18F]fluorination of substituted arenes [94]. Synthesis proceeds via oxidation of the corresponding aryliodide to furnish an unstable diacetoxyaryliodonium species, which is then treated with meldrum's acid to furnish the stable ylide (Figure 8F). [18F]Fluorination from these precursors is claimed to significantly reduce the occurrence of side reactions over other hypervalent iodine species, and this chemistry is compatible with both electron-withdrawing and -donating arenes.
Inventors affiliated with the General Hospital Corporation also patented [95], and separately reported [96], an alternate method for the thermal [18F]fluorination of iodonium ylides. This patent extends the scope of ylide ‘auxiliary groups’ from Meldrum's acid to a number of different groups, notably including acid diester and barbiturate-based spirocycles. Precursors with auxiliaries appended with cyclobutyl and cyclopentyl groups (instead of the dimethyl moiety in Meldrum's acid) showed an increase in RCC to greater than 70 versus 43% with Meldrum's acid. The patent provides synthetic methodology both to carry out precursor synthesis as well as [18F]fluorination. The inventors also demonstrated that their method produced products with high specific activity. They describe the synthesis of 4-[18F]fluorobenzyl azide with a specific activity of 0.3 Ci/µmol (11 GBq/µmol), while two radiotracers, 5-[18F]fluorouracil and [18F]FPEB, were synthesized from the spirocyclic iodonium ylide precursors with specific activities of 0.4 Ci/µmol (15 GBq/µmol) and 18 ± 1.4 Ci/µmol (666 ± 52 GBq/µmol), respectively.
Several patents, albeit less diverse in precursor scope, also focus on the use of arylsulfonium salts as [18F]fluorination substrates. A patent from the inventors at Bayer Schering Pharma utilized varying para-substituted-triaryl-sulfonium salt derivatives to [18F]fluorinate biomolecules (Figure 9A) [97]. The sulfonium salt is first immobilized on solid support, coupled to the desired biomolecule, and the bioconjugate is finally eluted and [18F]fluorinated using standard SNAr methods. The inventors highlight that the resultant [18F]-fluorinated biomolecules were produced with low specific activity, and attribute this to the [18F]fluorination conditions being nonoptimal. A similar patent from GE Healthcare Ltd. also utilized sulfonium salts for [18F]fluorination of peptides, however, their method was geared toward the simplification of [18F]fluorobenzaldehyde synthesis (Figure 9B) [98]. The standard method for [18F]fluorobenzaldehyde synthesis entails nucleophilic radiofluorination of 4-trimethylammoniumbenzaldehyde, but this method suffers from several drawbacks, most notably that the precursor undergoes demethylation to the uncharged dimethyl aniline, which is not fully separable from the product when SPE is utilized. Purification of reaction mixtures using the patented method is simplified, as the two major contaminants are removed by charge difference (precursor) and difference in lipophilicity (diarylsufide by-product), both of which can be fully removed by standard SPE methods.
Figure 9. . Late-stage [18F]fluorination of aromatics 3.
A patent [99] and report [100] by inventors affiliated with UCL Business PLC claimed that the substrate scope of arylsulfonium precursors can be broadened by utilizing electron-donating substituents on the sulfonium center (Figure 9C). Also described was the effect of temperature on reaction yield, as there was significant reduction in side product formation when the [18F]fluorination was conducted at ambient temperature versus reaction heating/UV irradiation. These findings were implemented in the successful [18F]fluorination of radiotracers with multiple functional groups including unprotected amines, alcohols and esters. While not discussing specific activity in the patent, the method was used for the synthesis of a candidate radiotracer used for a small-animal PET imaging study, which was determined to have a specific activity of 0.1 Ci/µmol (4 GBq/µmol).
Direct benzylic fluorination
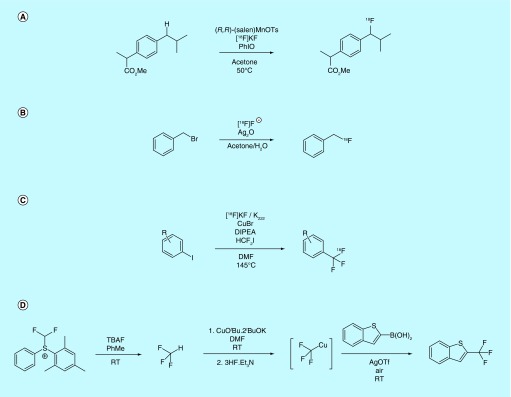
Benzylic fluorination has also seen renewed interest as methods to compliment the traditional SN2 methods used in radiochemistry have been described over the last 5 years. A method from the laboratories of Groves and Hooker for benzylic C–H fluorination utilizing Mn(salen)OTs, an [18F]fluoride phase transfer reagent (Figure 10A), was reported in the literature [101] and patented by Princeton University [102]. Using this method, the authors of the accompanying publication report the benzylic [18F]fluorination of celestolide, thereby producing a product with a specific activity of 2.7 Ci/µmol (100 GBq/µmol). An additional method from Caires and Guccione was patented by Stanford University wherein a benzyl bromide is converted to the corresponding [18F]benzyl fluoride (Figure 10B) [103]. This method utilizes AgO to promote the reaction and [18F]HF as the source of fluoride. One fluorine-18 example is given: the conversion of benzyl bromide to benzyl [18F]fluoride with a radiochemical conversion of 46% when 1 mCi of [18F]HF is reacted with 600 mM benzyl bromide and 181 mM Ag2O for 45 min in a 3:2 mixture of acetone and water.
Figure 10. . New approaches for C–H fluorination and synthesis of [18F]trifluoromethyl groups.
Synthesis of [18F]trifluoromethylarenes
Several new trifluoromethyl-radiolabeling methods have also been developed in the course of time covered by this review, and have been patented [104,105] and/or reported in the academic literature [106–109]. While limitations still exist, particularly low specific activities due to exchange with natural fluoride from the excess precursor, the new methods offer a variety of new strategies for labeling bioactive molecules through a trifluoromethyl group. For example, a method from the Riss laboratory at the University of Oslo converts aryliodides to [18F]trifluoromethylarenes via a copper-mediated process from HCF2I precursor (Figure 10C). The precursor is first converted to [18F]CuCF3, then reacted with the aryliodide substrate. This method was found to produce products with lower specific activities. For example, [18F]-4-(trifluoromethyl)benzonitrile was produced with a specific activity of 0.004 Ci/µmol (0.14 GBq/µmol). The method was patented by the University of Oslo [104] and published as a communication [106].
The synthesis and application of fluoroform (CF3H) and its fluorine-18-labeled analog as a synthon for the trifluoromethylation of boronic acids and boronates under copper and silver catalysis was claimed in a patent by Institut National des Sciences Appliquees de Rouen and Centre National de la Recherche Scientifique [105]. The inventors claim that CF3H can be generated via reaction of a fluoride source (such as TBAF) with difluoromethyl-substituted sulfonium and sulfoximinium salts, or difluoromethyl triflate. The CF3H is then distilled into a solution containing a copper(I) or silver(I) salt, with intermediary ‘CuCF3’ and ‘AgCF3’ species reacting efficiently with various B(II) and B(III) species to furnish the corresponding [18F]fluorides (Figure 10D). While the analogous work with [18F]fluoride is claimed in the patent, no examples of such radiochemistry are described.
Synthesis of prosthetic groups
Synthesis of aliphatic electrophilic prosthetic groups
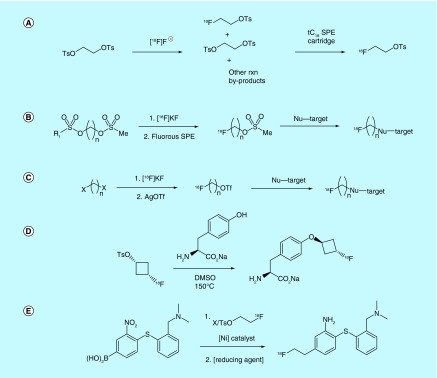
Researchers from GE Healthcare Ltd. and Med-Physics Inc. patented a method for the automated synthesis and SPE-based purification of [18F]fluoroaklyl tosylates, specifically [18F]fluoroethyl tosylate from the ditosylate precursor (Figure 11A) [110]. This patent discloses the use of a tC18 SPE system that, when eluted with 35% MeCN in H2O, is an ideal method for the rapid purification of [18F]fluoroethyl tosylate using widely available FASTLab and TRACERLab systems.
Figure 11. . Aliphatic electrophilic prosthetic groups.
A novel SPE approach geared toward the synthesis of fluorine-18-labeled mesylates was also patented by the inventors affiliated with GE Healthcare Ltd. and Medi-Physics, Inc. [111]. FSPE is used to separate the fluorine-18-containing prosthetic group product from the perfluorinated bis-sulfonic ester precursor (Figure 11B). Reactivity of [18F]fluoride toward the perfluorinated end of the bis-sulfonic ester in polar aprotic solvents is claimed to be several orders of magnitude greater than that of the mesylate end due to the former's greater electrophilicity (similar to triflate in terms of reactivity). After initial radiolabeling, the reaction mixture is passed through a perfluorinated alkyl matrix, which adsorbs the perfluorinated components while permitting the [18F]fluoroalkylmesylate pass through. This greatly simplifies and shortens purification of this prosthetic group.
To improve reactivity of alkylhalide prosthetic groups toward [18F]fluoride, inventors at Gachon University of Medicine and Science filed a patent on the trifluoromethanesulfonylation (triflylation) of [18F]fluoroalkylhalide prosthetic groups, as the greater electrophilicity of the triflyl leaving group enables milder and more rapid reaction with nucleophilic [18F]fluoride than the corresponding alkylhalides (Figure 11C) [112]. In their method, a dihaloalkane and nucleophilic [18F]fluoride are heated at sufficient temperature to evaporate the [18F]fluoroalkylhalide product, but not the dihaloalkane. The gaseous [18F]fluoroalkylhalide passes through a packed column of sand/AgOTf (other metal triflate salts are also claimed), thereby replacing the halide moiety with a more reactive triflate. The [18F]fluoroalkyltriflate is then condensed in a cooled solution of the target molecule to be labeled.
Although most electrophilic prosthetic groups link the [18F]fluoride to the electrophilic group via a straight aliphatic chain, presumably to lower the ‘footprint’ that the prosthetic group has on the resultant conjugate, this is not necessarily beneficial, and in some cases the resultant conjugate is metabolically unstable. In order to combat this issue, the inventors at Bayer Schering Pharma patented a method of synthesizing prosthetic groups containing [18F]fluorinated cycloalkyl moieties as metabolically stable alternatives to [18F]alkylfluorides (Figure 11D) [113]. Emphasis is placed on [18F]fluorocyclobutyl-appended molecules, which (in the aldehyde and carboxylic acid analogs) have a cLogP of ca. 0.4 and thus do not contribute much lipophilicity to the target molecule, as do many otherwise stable [18F]fluoroaromatic prosthetic groups.
An alternate and novel method for conjugating electrophilic prosthetic groups to other molecules was patented by Hammersmith Imanet Limited and Medi-Physics, Inc. [114]. [18F]Fluoroalkyl halides or sulfonates are conjugated with boronic acids and esters via Ni(II)-catalyzed Suzuki reaction to give [18F]fluoroalkylated products. However, the inventors offer only hypothetical examples of the utilization of this method for the production of [18F]-radiolabeled molecules, such as the synthesis of serotonin transporter ligand [18F]AFE (Figure 11E).
Synthesis of aromatic electrophilic prosthetic groups
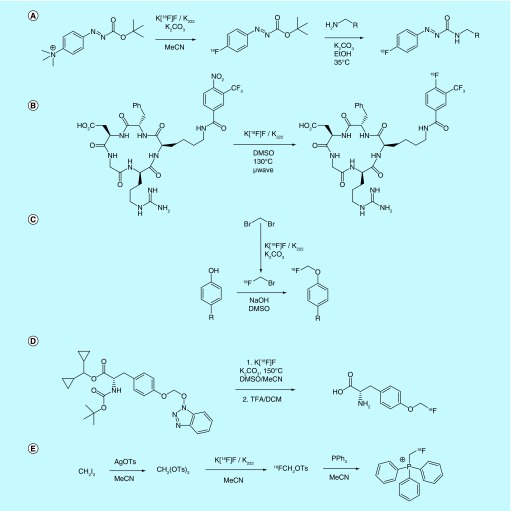
Several examples of novel aromatic prosthetic groups are also found in the patent literature. Investigators from the Friedrich-Alexander-Universitaet Erlangen-Nuernberg reported [115] and patented [116] the use of trimethylammonium phenyldiazocarboxylate esters as precursors for nucleophilic [18F]fluorination with [18F]fluoride (Figure 12A). A significant advantage of this particular type of precursor is that it is claimed to react rapidly under the described conditions, with RCCs ranging from 75 to 95% in reaction times of less than 1 min. The ester products were shown to act as efficient prosthetic groups for reaction with aliphatic amines, showing complete conversion after only 10 min when EtOH was used as solvent, and the inventors report that the resultant radiotracer had a specific activity of 0.3 Ci/µmol (12 GBq/µmol).
Figure 12. . Aromatic electrophilic prosthetic groups and [18F]fluoromethylation.
Nitroaryl precursors are commonly used for SNAr [18F]fluorination efforts, with [18F]fluorination proceeding much more smoothly when the aryl ring is appended with EWGs. To take advantage of this idea, a patent filed by inventors affiliated with the US NIH claims that the [18F]fluorination of 4-nitro-3-trifluoromethylbenzamides that are coupled to biomolecules can be accomplished under much milder conditions than the respective non-trifluoromethylated analogs [117], an idea that is also discussed in an accompanying publication [118]. The ortho-positioned trifluoromethyl functionality on this prosthetic group ‘activates’ the 4-nitro functionality toward SNAr [18F]fluorination under purportedly milder conditions than required for most nitroarene precursors (Figure 12B). The authors also claim that other EWGs can be used in place of and in addition to the trifluoromethyl moiety to yield similar results. Several benefits of this method in comparison to other biomolecule 18F-labeling methods are discussed, including a lower amount of precursor required for fluorination compared with other methods, as well as reduced synthesis time. The inventors also reported that the monomeric and dimeric [18F]-labeled RGD peptides labeled using this ‘one-step’ route were synthesized with specific activities of 2.1 ± 0.4 Ci/µmol (79 ± 13 GBq/µmol).
[18F]Fluoromethylation reactions
As fluorine can be considered to be a bioisostere of the hydrogen atom on aliphatic groups, [18F]fluoromethylation is an excellent method of introducing fluorine-18 into molecules bearing arylmethoxy (anisole) groups, as it does not tend to significantly change the molecule's properties. Unfortunately, it is a challenging group to incorporate, primarily due to difficulties in prosthetic group synthesis and purification. An improved method of generating this moiety from the [18F]fluoromethanebromide prosthetic group is claimed by Piramal Imaging (Figure 12C) [119]. Their patent describes an improved method of [18F]fluoromethylbromide synthesis wherein the dibromomethane precursor is more efficiently removed via an improved distillation technique based on boiling point differences between the two compounds (100 and 8°C for dibromomethane and [18F]fluoromethylbromide, respectively). Purification entails passing the heated reaction mixture through a solid-phase material with the product eluting first. Gas leakage issues can be avoided by using a single reverse-phase column fitted with a luer lock as the solid-state material, although multiple reverse-phase columns can likewise be used in series.
In an effort to entirely eliminate the use of gaseous [18F]fluoromethylbromide reagent in the [18F]fluoromethylation of tyrosine analogs, Piramal Imaging also described a method for the synthesis of O-fluoromethyl tyrosines and α-methyl tyrosines using hydroxybenzotriazle- or hydroxytriazole-modified precusrsors, taking advantage of the HOBt group as a leaving group (Figure 12D) [120]. They have patented a number of HOBt derivatives (including CF3, NO2, Cl and pyridinyl-substituted derivatives) and explored these as leaving groups for an SN2-type reaction.
A similar, albeit more substrate-specific method of introducing [18F]methylfluoride functionality to radioligands was patented by the Henan University of Traditional Chinese Medicine with the goal of synthesizing cardiac imaging probes related to [18F]fluoromethyl)triphenylphosphonium tosylate (Figure 12E) [121]. Their method utilizes diiodomethane as a precursor, which is converted to the corresponding ditosylmethane using silver tosylate. [18F]Fluorination of the ditosylate in MeCN (using a similar strategy to that reported by Berridge [122] and Scott [123]), followed by the addition of triphenylphosphine, yields the [18F]fluoromethyl)triphenylphosphonium salt as a product. In this case, the resultant [18F](fluroromethyl)triphenylphosponium salt was synthesized with a specific activity of 20 Ci/µmol (760 GBq/µmol).
Synthesis of aliphatic nucleophilic prosthetic groups
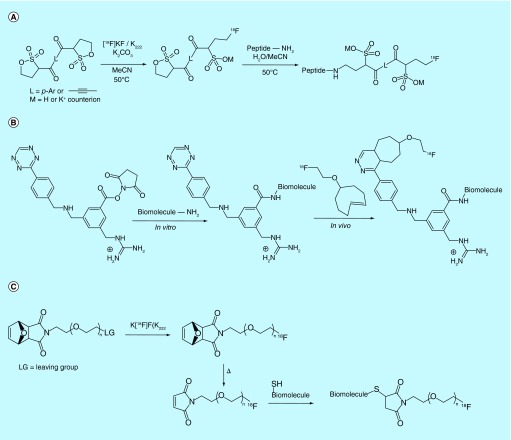
In addition to electrophilic prosthetic groups, there is also interest in the synthesis of [18F]labeled prosthetic groups bearing nucleophilic moieties. Amines are useful synthons for building more complex organic molecules, and consequently there is much interest in developing [18F]fluorinated amines as prosthetic groups. The inherent nucleophilicity of the amine, however, often precludes direct labeling, as precursors for radiolabeling with [18F]fluoride tend to also react intramolecularly to produce undesired by-products. In an effort to overcome this problem, researchers from Hammersmith Imanet Ltd. and GE Healthcare, Ltd. further developed a previously described method [124] for the synthesis of [18F]fluoroethylazide, and have demonstrated and patented [125] the efficient reduction of the azide to the amine by the action of metallic Cu under acidic conditions (Figure 13A). The inventors go on to show by way of example that the resultant [18F]fluoroalkylamine products can be used in amidation reactions with suitably activated acid derivatives. The inventors also claim the use of the [18F]fluoroalkylamines in reductive aminations through no examples of such reactions are provided.
Figure 13. . Aliphatic nucleophilic prosthetic groups.
Another approach to mildly introduce amines into molecular targets via azide prosthetic groups was taken by the Gouverneur laboratory [126], and patented by Isis Innovation Limited [127]. The Staudinger ligation was modified to provide a traceless means to form a 2-[18F]fluoroethyl amide from a diarylphosphanylmethyl thioester (Figure 13B). The reduction of the 2-[18F]fluoroethyl azide (produced from a perfluorosulfonate precursor) to the amine by the phosphine requires an electrophile to complete the reaction. The adjacent thioester provides an electrophilic trap to provide the formation of the desired 2-fluoroethyl amide and release of the phosphine thiol. While not reporting the specific activity of the resultant [18F]fluoroethylamides in the patent or publication, the latter describes that the 2-[18F]fluoroethyl azide isolated using the FSPE procedure had a specific activity of 0.27 Ci/µmol (10 GBq/µmol) and also provides greater detail on the examples and uses of the method [126].
In addition to problematic intramolecular reactivity, the conditions required for conjugation of nucleophilic prosthetic groups often require the use of bases, which may be a problem when radiolabeling base-sensitive target molecules, particularly biomolecules. To combat this limitation, the inventors affiliated with GE Healthcare Ltd. and Medi-Physics, Inc. filed a joint patent on the use of aminoxy-appended prosthetic groups for fluorine-18 labeling of biomolecules (Figure 13C) [128]. In the patented method, a biomolecule is first outfitted with a reactive group such as a Michael acceptor or organohalide. The aminoxy prosthetic group then reacts with the reactive moiety on the biomolecule at a range of pH values, although the main utility of this method comes from reactivity in a slightly acidic environment. The acidity enhances chemoselectivity of the aminoxy prosthetic group by reducing the reactivity of competing nucleophiles on the biomolecule, such as lysine residues. Alternately, a second approach is also claimed wherein the aminoxy group is instead introduced to the biomolecule and is reacted with an electrophilic prosthetic group to enable fluorination. An SPE method for the purification of such prosthetic groups is also patented by GE Healthcare Ltd. [129].
Synthesis of prosthetic groups using Huisgen cycloaddition substrates
Cu(I)-catalyzed Huisgen cycloaddition of alkynes and azides was one of the first reactions to be widely described as ‘click chemistry’ and has been adopted by the radiochemistry community since its inception. As such, several patents utilize this chemistry to enable mild [18F]fluorination of biomolecules via prosthetic groups and linkers.
Inventors affiliated with General Electric and Hammersmith Imanet Ltd. patented a bifunctional linker strategy wherein a thiol-reactive terminus on one end of the linker is used to bind to a biomolecule and an azide/alkyne moiety on the other end is used to ligate a [18F]fluorine-containing prosthetic group bearing the complementary functionality (Figure 14A) [130]. The linker is synthesized by coupling an alkyne/azide functionalized amine with a carboxylic acid/thiol-reactive group, although other chemistry can be used to form linkers as well, which allows tailoring of the linker's properties. The inventors alternately state that the alkyne/azide on the linker can be replaced by other fluoride-reactive functionalities, or other reactive groups that would couple with fluorine-18-containing prosthetic groups. The process of labeling involves reacting the target biomolecule with the linker thereby forming a sulfide linkage, then conjugating with [18F]labeled prosthetic group to form the azide/alkyne moiety using Cu-catalyzed dipolar cycloaddition.
Figure 14. . New developments in fluorine-18 click chemistry.
In the same year, inventors at Genentech patented a similar linker-based strategy for biomolecule labeling [131]. However, in their method longer polyethylene glycol chains are used as linkers in order to increase water solubility without diminishing solubility in organic solvents (Figure 14B). The inventors note that PEG chain length is conditionally dependant, and the conjugation of this prosthetic group to biomolecules may vary significantly. The authors provide several examples utilizing microwave heating for the synthesis. The inventors also include other examples of chemistry pertinent to this work including examples of peptide conjugation, fluoride labeling and click chemistry methods. Related work on the synthesis of ethynylmaleimide prosthetic groups was also patented by the Industry-Academy Cooperation Foundation of Sogang University [132].
Inventors at GE Healthcare Limited and Imperial Innovations Limited claim a more robust method of utilizing Huisgen cycloaddition to generate precursors that are reactive with [18F]fluoride [133]. Their method forgoes the synthesis of volatile [18F]fluoroethylazide and instead prereacts an alkyne-bearing targeting molecule with p-tosylalkylazide in the presence of Cu(I). The product of this reaction is then purified and reacted with [18F]fluoride using standard SN2 methods (Figure 14C). In addition to simplifying the overall radiolabeling process, this method is also claimed to improve the radiochemical yield of the final product and eliminates the presence of potentially deleterious reagents (both from a synthesis and release criteria standpoint), such as copper salts from the reaction medium.
A patent from Gifu University covers a method for the incorporation of ethynylbenzenedimethanol units into DNA, RNA and mixed nucleotide sequences through solid-phase synthesis [134]. While not demonstrating the process in the patent, a Cu(I)-catalyzed alkyne–azide cycloaddition reaction between the incorporated ethynyl unit and [18F]-fluorobenzyl azide for the generation of radiolabeled nucleotide sequences is claimed (Figure 14D). Additional related patents filed by other groups toward Huisgen cycloaddition include a method for producing [18F]azidofluoromethylbenzenes as aromatic azide analogs by Riken Corp. and Osaka University (Figure 14E) [135], and [18F]fluorinated azidoglycosides as bio-orthogonal prosthetic groups from Universite de Lorraine and Centre National de la Recherche Scientifique [136].
Biological methods for introducing prosthetic groups/synthesis of biogenic prosthetic groups
Several patents are also disclosed for the use of biological methods of introducing [18F]fluorinated prosthetic groups into biomolecules and/or the use of biogenic prosthetic groups. For example, Shimadzu Corporation and the University of Electro-Communications have disclosed a method for the enzymatic incorporation of fluorinated amino acids into peptides using an aminoacyl tRNA amino transferase [137]. They discuss that this method could be used for the incorporation of the [18F]-radiolabeled amino acids into proteins under mild conditions.
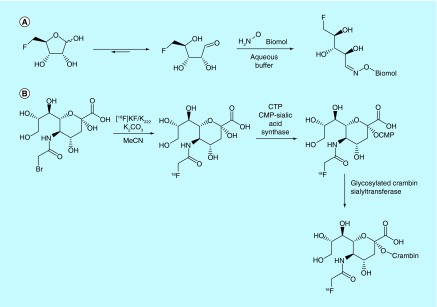
Li and O'Hagan from the University of St. Andrews and Turun Yliopisto have patented a method for the bioconjugation of 3- and 5-[18F]fluorodeoxypentoses with aminooxy-functionalized biological molecules as alternatives to non-bio-orthogonal prosthetic groups (Figure 15A) [138]. The [18F]fluorodeoxypentoses are available through a similar synthetic route to commonly available [18F]FDG. The inventors rationalized that a greater fraction of the pentose derivatives exist in solution as the open-chain aldehyde form, which is free to react to form the oxime with aminooxy residues. While the application of this work with [18F]-labeled fluorosugars is claimed, no such experiments were described in the patent. However, the utility of [18F]fluorodeoxyribose as a prosthetic group for RGD peptide is presented in the academic literature [139]. In this publication, the authors suggest that for this system the specific activity could not be measured as no product peak was observed in the UV HPLC chromatogram.
Figure 15. . Biological methods for introducing prosthetic groups.
Similarly, Otsuka Chemical Holdings Co. Ltd. has patented a method for the glycosylation of peptides and oligosaccharides using radiolabeled sialic acid derivatives, catalyzed by a sialyltransferase enzyme (Figure 15B) [140]. They have described the synthesis of an N-fluoroacetylsialic acid and its enzymatic conversion to N-fluorosialic acid–CMP conjugate (the substrate for the sialyltransferase). This prosthetic group was then successfully transferred by a sialyltransferase to a preglycosylated crambin, a simple 46-amino acid protein, as proof-of-principle.
The Whitehead Institute for Biomedical Research described an enzymatic method for site-specific radiolabeling of large proteins using a sortase enzyme [141]. A genetically modified sortase A was employed to couple a polyglycinyl-tetrazine or polyglyciny-NOTA unit to a sortase-tagged protein of interest; in this case, a murine MHC-specific single-domain antibody (SdAb) domain that had been engineered to contain the sortase recognition sequence LPXTG. After sortase-catalyzed peptide bond formation, the tetrazine- or NOTA-derivatized domains were labeled with either fluorine-18 or copper-64 by reaction with [18F]-transcyclooctene (TCO) or 64CuCl2, respectively, to provide the radiolabeled antibody domain. A published report of their work is also present in the academic literature, this time with TCO-modified small peptides installed onto proteins of interest and then labeled with tetrazine-appended [18F]FDG [142].
Synthesis of other novel linkers/prosthetic groups
A nonstandard electrophilic linker was patented by inventors affiliated with Advanced Accelerator Applications, who filed a patent on the development and use of polysultone derivatives as prosthetic groups for the radiolabeling of biomolecules (Figure 16A) [143]. [18F]Fluorination at one end of the bis-sultone linker proceeds via an opening of the sultone ring. Nucleophilic residues on the peptide then react with the opposing end of the linker to produce the desired radiolabeled peptide. This procedure enables rapid synthesis under mild conditions, and results in a charged linker that may be used to modulate the biological behavior of the conjugate.
Figure 16. . Other linkers and prosthetic groups.
A similar charged linker approach, albeit one functionalized more generally, was patented by inventors at Duke University [144]. In their patent, a charged moiety on the prosthetic group prevents radiolabeled metabolites from leaving the cell following degradation of the radiolabeled biomolecule. The prosthetic group consists of three main branches off a substituted aryl ring or small polypeptide that functions as the molecular ‘core’ of the prosthetic group (Figure 16B). These branches include a biomolecule-reactive succinimidyloxy carbonyl moiety or other electrophilic-reactive group, the radioactive tag (fluorine-18 or iodine-123/124), which can be introduced as a separate prosthetic group or be introduced via late-stage [18F]fluorination, and a charged group such as a guanidinium cation. This prosthetic group strategy is claimed to be applicable to bioorthogonal click chemistry in vivo, and a [18F]fluorinated linker displaying this chemistry is presented in the academic literature [145]. While not discussed in the patent, in this accompanying publication, the authors describe the specific activity of their protected prosthetic group to be 0.25 Ci/µmol (9.3 GBq/µmol). After bioconjugation of the deprotected prosthetic group to a SdAb fragments, a product with a specific activity of up to 0.01 Ci/mg (0.4 GBq/mg) was obtained, in contrast to specific activities of up to 0.02 Ci/mg (0.74 GBq/mg), when the SdAbs were bioconjugated to [18F]SFB. A similar idea was also patented by the inventors from Sogang University Research Foundation, presumably with the triazole moiety acting as both a site of conjugation during synthesis and as an analogous charged species [146].
Maleimide-based prosthetic groups are commonly used in the field, and allow radiochemists to selectively label biomolecules at thiol-containing residues, which spares the biologically important N-terminus of the biomolecule from modification. Synthesis of [18F]maleimides is, however, a multistep time-consuming process. A patent from the Hamamatsu Photonics Co. in Japan claims an improved synthetic method for producing maleimide-appended [18F]fluorinated linker [147]. In this method, a protected maleimide-containing linker is first [18F]fluorinated at moderate temperature and then deprotected by higher temperature heating (Figure 16C). This two-step synthesis can be carried out within 20–30 min and is adaptable for automation due to its simplicity.
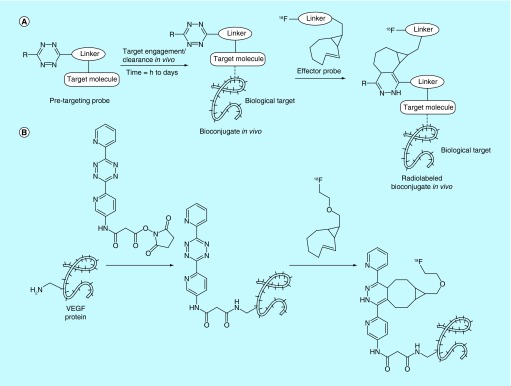
Tetrazine/TCO in vivo click chemistry
A recent interpretation of click chemistry that has featured significantly in both this section and in previous sections is in vivo click chemistry, more commonly referred to as ‘pretargeting’. In this approach, the first of two clickable units (the pretargeting probe that is not radiolabeled) is administered to a living system and allowed to incubate for a set period of time, usually on the order of hours to days. This ensures the necessary time for target engagement when using slow-binding targeting vectors (e.g., antibodies) as well as clearance from nontarget tissues. Following incubation, the second clickable unit (effector probe, which is radiolabeled) is administered to the living system and conjugates to the pretargeting probe via click chemistry. With respect to PET imaging, this method allows researchers to utilize short half-lived radionuclides such as fluorine-18 with slower targeting vectors such as antibodies, which typically show appropriate target binding over periods that are much longer than the half-lives of the radionuclide. This approach necessitates several design criteria: rapidity of the click reaction, long-term in vivo stability of the pretargeting probe and rapid clearance of unbound effector probe. For this reason, this area has been the subject of several patents over the last several years and a general review on the use of radiopharmaceutical pretargeting strategies was recently published [148].
Inventors affiliated with KoninKlijke Phillips Electronics patented a bio-orthogonal strategy wherein the rapid reaction between tetrazines and TCO is utilized for in vivo bio-orthogonal click chemistry with radioisotope-bearing effector probes (Figure 17A) [149]. Both tetrazines and TCO are abiotic and therefore undergo reasonably slow metabolism in vivo, so either of these groups can be used as the reactive group on the pretargeting/effector probe component. In later patents, the inventors specifically claim the use of additional exocyclic bonds to force the TCO (or other dienophilic alkenylene) into a planar arrangement, thereby increasing reactivity due to increased ring strain [150], and functionalization of the tetrazine moiety with electron-withdrawing substituents to increase reactivity [151].
Figure 17. . Strategies for tetrazine/transcyclooctene in vivo click chemistry.
Similarly, researchers from the university of Delaware patented a tetrazine/TCO system for radiolabeling of peptides, with a focus on fluorine-18, although carbon-11 and iodine-124 are also discussed (Figure 17B) [152]. The inventors who published the first academic report describing the tetrazine/TCO system [153] chose to utilize diaryl tetrazines, which show reasonable in vivo stability while maintaining rapid reactivity with TCO. The patent includes synthetic methods of producing fluorine-18-labeled effector probes consisting of both TCO and diaryltetrazine moieties as well as tetrazine prosthetic groups, including those appended by maleimide or active esters to label the biomolecule. Excellent yields of [18F]TCO effector probes were produced by reaction of [18F]fluoride with nosyl-substituted TCO over a relatively short timespan and used for the majority of the work presented in the patent. In an example where a tetrazine-bearing cRGD peptide was reacted with an [18F]-labeled TCO, the resultant dihydropyridazine product was determined to have a specific activity of 3–6 Ci/µmol (111–222 GBq/µmol).
Conclusion & future perspective
The number of patents containing fluorine-18 has risen steadily since the 1990s and now ≥100 are issued per year. Patents typically cover new radiotracers (see Part 1 of this review) or new radiochemical methods (reviewed in the present article). In the latter case, the last 5–6 years have seen significant work undertaken to develop new ways to work with fluorine-18 (e.g., new processing techniques, green radiosynthetic methods, novel purification strategies), as well as the introduction of many novel radiochemical reactions to label bioactive molecules and/or synthesize prosthetic groups. This article highlights many of the exciting developments in fluorine-18 radiochemistry from a patent literature perspective, including late-stage radiofluorination of aromatic rings, chelation or capture of [18F]fluoride, new developments in18F-labeled prosthetic groups, approaches to C–H fluorination and synthesis of [18F]trifluoromethylarenes. All of these developments provide the PET radiochemistry community with an impressive toolbox that allows labeling of very diverse bioactive molecules (and prosthetic groups) with fluorine-18. Reflecting this, many of these new methods are already being used to synthesize PET radiotracers for preclinical evaluation, with most of the large PET groups focused ultimately upon clinical translation of promising tracers, so that they can be used to support drug discovery efforts or applications in personalized medicine. Such clinical translation necessitates qualifying the new methods described herein for the production of PET radiotracer doses suitable for clinical use. Such process verification is beginning to be reported in the literature for the first of these methods [70], and it is expected will soon follow for many of the other new radiosynthetic methods described herein.
Executive summary.
Positron emission tomography (PET) imaging is a form of functional molecular imaging increasingly used in personalized medicine and drug discovery.
The most commonly utilized PET radionuclide is fluorine-18 because the convenient 110-min half-life enables commercial distribution from centralized nuclear pharmacies to satellite and/or mobile PET scanners, while its imaging properties provide exquisite images.
The growing popularity for incorporating fluorine into pharmaceutical scaffolds offers rich opportunities for employing fluorine-18 to adapting them into PET radiotracers for use as companion diagnostics.
Patent applications in the fluorine-18 space typically cover new radiotracers (see Part 1 of this review) or new radiochemical methods (reviewed in the present article).
New fluorine-18 methodology patents are generally focused in three priority areas: first, new strategies for working with fluorine-18 including novel processing methods, solvent systems (including green approaches), reaction strategies (e.g., solid-state) and purification approaches (including fluorous techniques); second, novel reactions with fluorine-18 including, for example, late-stage fluorination of aromatic rings and third, synthesis of new prosthetic groups labeled with fluorine-18 suitable for tagging macromolecules such as large peptides, proteins and antibodies.
Footnotes
Financial & competing interests disclosure
Financial support of this work by the US DOE/NIBIB (DE-SC0012484), NIH (T32-EB005172), Alzheimer's Association (NIRP-14–305669) and the University of Michigan (College of Pharmacy and Rackham Graduate School) is gratefully acknowledged. In addition to his employment at the University of Michigan, PJH Scott is an owner of SynFast Consulting, LLC, a consultant to JUAMA Medico and receives honoraria from Zevacor Molecular and John Wiley and Sons. His laboratory at the University of Michigan is supported through grants from the NIH, US DOE, Alzheimer's Association, Avid Radiopharmaceuticals/Eli Lilly, Bristol-Myers Squibb, Merck, Adeptio, GE Healthcare, Threshold Pharmaceuticals and Ionetix. PJH Scott and AF Brooks are co-inventors on patents mentioned in this review (Scott: US20150367005 A1 and WO2015157597 A1; Brooks: WO2015157597 A1). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.IMV Medical Information Division. IMV; IL, USA: 2014. 2014 PET imaging market summary report; p. 60018. [Google Scholar]
- 2.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015;58(21):8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Sánchez-Roselló M, Aceña JL, et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 2014;114(4):2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 4.Brooks AF, Drake LR, Stewart MN, et al. Fluorine-18 patents (2009–2015). Part 1: novel radiotracers. Pharm. Pat. Anal. 2016;5(1):17–47. doi: 10.4155/ppa.15.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008;47(47):8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 6.Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJH. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci. 2014;5(12):4545–4553. doi: 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann CN, Ritter T. Late-stage fluorination: fancy novelty or useful tool? Angew. Chem. Int. Ed. 2015;54(11):3216–3221. doi: 10.1002/anie.201410288. [DOI] [PubMed] [Google Scholar]
- 8.Preshlock S, Tredwell M, Gouverneur V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 2016;116(2):719–766. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- 9.Technische Universität München. 2011. WO2011141410 A1.
- 10.Industry-University Cooperation Foundation Sogang University. 2013. WO2013176522 A1.
- 11.Lee SJ, Hyun JS, Oh SJ, Yu KH, Ryu JS. Development of a new precursor-minimizing base control method and its application for the automated synthesis and SPE purification of [18F]fluoromisonidazole ([18F]FMISO) J. Labelled Comp. Radiopharm. 2013;56(14):731–735. doi: 10.1002/jlcr.3115. [DOI] [PubMed] [Google Scholar]
- 12.Naguib YMA, Naguib A, Naguib A. 2014. US20140051851 A1.
- 13.Bayer Pharma Aktiengesellschaft. 2012. WO2012032029 A1.
- 14.Lee S-J, Oh S-J, Chi D-Y, Moon D-H, Ryu J-S. High yielding [18F]fluorination method by fine control of the base. Bull. Korean Chem. Soc. 2012;33(7):2177–2180. [Google Scholar]
- 15.GE Healthcare Ltd. 2009. WO2009083530 A2.
- 16.Mayo Foundation for Medical Education and Research. 2015. WO2015143019 A3.
- 17.Jiang H, DiMagno SG, DeGrado TR. Production and transport of gaseous f-synthons: f-acyl fluorides. J. Fluor. Chem. 2015;180:181–185. doi: 10.1016/j.jfluchem.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trasis SA. Universite de Liege. 2009. EP2017359 A2.
- 19.Siemens Medical Solutions USA, Inc. 2011. WO2011127345 A1.
- 20.The Regents of the University of Michigan. 2015. US20150367005 A1.
- 21.Stewart MN, Hockley BG, Scott PJH. Green approaches to late-stage fluorination: radiosyntheses of [18F]-labelled radiopharmaceuticals in ethanol and water. Chem. Commun. 2015;51(79):14805–14808. doi: 10.1039/c5cc05919d. [DOI] [PubMed] [Google Scholar]
- 22.Technical University of Denmark. 2014. WO2014020035 A1.
- 23.Mathiessen B, Zhuravlev F. Automated solid-phase radiofluorination using polymer-supported phosphazenes. Molecules. 2013;18(9):10531–10547. doi: 10.3390/molecules180910531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokyo Institute of Technology; Niigata University. 2011. WO2011099480 A1.
- 25.Tokyo Institute of Technology. 2015. JP 2015054840.
- 26.Sogang University, Industry-Academy Cooperation Foundation. 2011. KR 2011130977.
- 27.Industry-University Cooperation Foundation Sogang University. 2012. WO2012033374 A2.
- 28.Isis Innovation Limited. 2010. WO 2010007363.
- 29.Bejot R, Fowler T, Carroll L, et al. Fluorous synthesis of 18F radiotracers with the [18f]fluoride ion: nucleophilic fluorination as the detagging process. Angew. Chem. Int. Ed. 2008;48(3):586–589. doi: 10.1002/anie.200803897. [DOI] [PubMed] [Google Scholar]
- 30.Bayer Schering Pharma AG. 2010. WO 2010000409.
- 31.Bayer Schering Pharma Aktiengesellschaft. 2011. WO 2011006610.
- 32.Laverman P, McBride WJ, Sharkey RM, Goldenberg DM, Boerman OC. Al18F labeling of peptides and proteins. J. Labelled Compd Radiopharm. 2014;57(4):219–223. doi: 10.1002/jlcr.3161. [DOI] [PubMed] [Google Scholar]
- 33.McBride WJ, Sharkey RM, Goldenberg DM. Radiofluorination using aluminum-fluoride (Al18F) EJNMMI Res. 2013;3(1):36. doi: 10.1186/2191-219X-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard-Gauthier V, Bailey JJ, Liu Z, et al. From unorthodox to established: the current status of 18F-trifluoroborate- and 18F-SiFA-based radiopharmaceuticals in PET nuclear imaging. Bioconjugate Chem. 2016;27(2):267–279. doi: 10.1021/acs.bioconjchem.5b00560. [DOI] [PubMed] [Google Scholar]
- 35.Perrin DM. [18F]-organotrifluoroborates as radioprosthetic groups for PET imaging: from design principles to preclinical applications. Acc. Chem. Res. 2016;49(7):1333–1343. doi: 10.1021/acs.accounts.5b00398. [DOI] [PubMed] [Google Scholar]
- 36.University of Kent. 2009. WO2009027707 A2.
- 37.Ting R, Harwig C, auf dem Keller U, et al. Toward [18F]-labeled aryltrifluoroborate radiotracers: in vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J. Am. Chem. Soc. 2008;130(36):12045–12055. doi: 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 38.Ting R, Harwig CW, Lo J, et al. Substituent effects on aryltrifluoroborate solvolysis in water: implications for Suzuki−miyaura coupling and the design of stable 18F-labeled aryltrifluoroborates for use in PET imaging. J. Org. Chem. 2008;73(12):4662–4670. doi: 10.1021/jo800681d. [DOI] [PubMed] [Google Scholar]
- 39.The University of British Columbia. 2009. WO2009012596 A1.
- 40.The University of British Columbia. 2014. WO2014134716 A1.
- 41.University of Southern California. 2013. WO2013012754 A1.
- 42.Liu S, Li D, Zhang Z, Surya Prakash GK, Conti PS, Li Z. Efficient synthesis of fluorescent-PET probes based on [18F]BODIPY dye. Chem. Commun. 2014;50(55):7371–7373. doi: 10.1039/c4cc01411a. [DOI] [PubMed] [Google Scholar]
- 43.University of South Florida, H Lee Moffitt Cancer Center and Research Institute, Inc. 2014. WO2014117028 A1.
- 44.Aptenia SRL. 2014. WO2014135590 A1.
- 45.Scabini M. 2011. WO2011095150.
- 46.Immunomedics, Inc. 2009. WO2009079024.; •• Strategies for chelating Al-18F.
- 47.Immunomedics, Inc. 2009. WO2009135015 A2.
- 48.Immunomedics, Inc. 2011. US20110110854.
- 49.Immunomedics, Inc. 2012. US20120134920.
- 50.Immunomedics, Inc. 2013. US20130315821.
- 51.Immunomedics, Inc. 2012. WO2012075361.
- 52.Immunomedics, Inc. 2012. US20120076727.
- 53.GE Healthcare Limited; University of Southampton. 2013. WO2013110615 A1.
- 54.GE Healthcare Limited; University of Southampton. 2014. WO2014177689 A1.
- 55.GE Healthcare Limited; University of Southampton. 2014. WO2014177690 A1.
- 56.University of Pennsylvania; University of Florida. 2010. WO2010135180 A1.
- 57.Kachur AV, Dolbier WR, Jr, Xu W, Koch CJ. Catalysis of fluorine addition to double bond: an improvement of method for synthesis of 18F PET agents. Appl. Radiat. Isot. 2010;68(2):293–296. doi: 10.1016/j.apradiso.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin LL, Silvoniemi A, Stubbs JB, et al. Radiation dosimetry and biodistribution of the hypoxia tracer 18F-EF5 in oncologic patients. Cancer Biother. Radiopharm. 2012;27(7):412–419. doi: 10.1089/cbr.2011.1130. [DOI] [PubMed] [Google Scholar]
- 59.Isis Innovation Limited. 2012. WO2012004567.
- 60.Gao Z, Lim YH, Tredwell M, et al. Metal-free oxidative fluorination of phenols with [18F]Fluoride. Angew. Chem. Int. Ed. 2012;51(27):6733–6737. doi: 10.1002/anie.201201502. [DOI] [PubMed] [Google Scholar]
- 61.University of California. 2014. WO2014183130.
- 62.He Q, Alfeazi I, Sadeghi S. No-carrier-added electrochemical nucleophilic radiofluorination of aromatics. J. Radioanal. Nucl. Chem. 2015;303(1):1037–1040. [Google Scholar]
- 63.President and Fellows of Harvard College. 2012. WO2012024604 A3. [PubMed]; •• Synthesis of [18F]arenes using a transition-metal-mediated radiofluorination reaction.
- 64.Lee E, Kamlet AS, Powers DC, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science. 2011;334(6056):639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamlet AS, Neumann CN, Lee E, et al. Application of palladium-mediated 18F-fluorination to PET radiotracer development: over-coming hurdles to translation. PLoS ONE. 2013;8:e59187. doi: 10.1371/journal.pone.0059187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.President and Fellows of Harvard College. 2014. WO2014052622 A1. [PubMed]
- 67.Lee E, Hooker JM, Ritter T. Nickel-mediated oxidative fluorination for PET with aqueous [18F] fluoride. J. Am. Chem. Soc. 2012;134(42):17456–17458. doi: 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren H, Wey H-Y, Strebl M, Neelamegam R, Ritter T, Hooker JM. Synthesis and imaging validation of [18F]MDL100907 enabled by Ni-mediated fluorination. ACS Chem. Neurosci. 2014;5(7):611–615. doi: 10.1021/cn500078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoover AJ, Lazari M, Ren H, et al. A transmetalation reaction enables the synthesis of [18F]5-Fluorouracil from [18F]Fluoride for human PET imaging. Organometallics. 2016;35(7):1008–1014. doi: 10.1021/acs.organomet.6b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanford MS, Scott PJH. Moving metal-mediated 18F-fluorination from concept to clinic. ACS Cent. Sci. 2016;2(3):128–130. doi: 10.1021/acscentsci.6b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.University of California. 2013. WO2013188554 A1.
- 72.Fier PS, Hartwig JF. Copper-mediated fluorination of aryl iodides. J. Am. Chem. Soc. 2012;134(26):10795–10798. doi: 10.1021/ja304410x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks A, Ichiishi N, Topczewski J, Sanford M, Scott P. Synthesis of PET radiotracers by copper-mediated 18F-fluorination of (mesityl)(aryl)iodonium salts and aryl iodides. J. Nucl. Med. 2015;56(Suppl. 3):218. [Google Scholar]
- 74.Ye Y, Schimler SD, Hanley PS, Sanford MS. CuOTf2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J. Am. Chem. Soc. 2013;135(44):16292–16295. doi: 10.1021/ja408607r. [DOI] [PubMed] [Google Scholar]
- 75.The Regents of the University of Michigan. 2015. WO2015157597 A1.; • Synthesis of [18F]arenes by copper-mediated fluorination of (mesityl)(aryl)iodonium salts.
- 76.Isis Innovation Limited. 2015. WO2015140572.; • Synthesis of [18F]arenes by copper-mediated fluorination of organoborons.
- 77.Tredwell M, Preshlock SM, Taylor NJ, et al. A general copper-mediated nucleophilic 18F fluorination of arenes. Angew. Chem. Int. Ed. 2014;53(30):7751–7755. doi: 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]
- 78.Preshlock S, Calderwood S, Verhoog S, et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem. Commun. 2016;52(54):8361–8364. doi: 10.1039/c6cc03295h. [DOI] [PubMed] [Google Scholar]
- 79.Zlatopolskiy BD, Zischler J, Krapf P, et al. Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chem. Eur. J. 2015;21:5972–5979. doi: 10.1002/chem.201405586. [DOI] [PubMed] [Google Scholar]
- 80.Mossine AV, Brooks AF, Makaravage KJ, et al. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org. Lett. 2015;17(23):5780–5783. doi: 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neumann CN, Hooker JM, Ritter T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature. 2016 doi: 10.1038/nature19311. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.President and Fellows of Harvard College. 2015. WO2015058047 A2. [PubMed]; •• Concerted nucleophilic aromatic substitution via 18F-deoxyfluorination of phenols.
- 83.GE Healthcare Limited. 2012. WO2012117069 A1.
- 84.Pike VW, Aigbirhio FI. Reactions of cyclotron-produced [18F]Fluoride with diaryliodonium salts – a novel single-step route to no-carrier-added [18F]fluoroarenes. J. Chem. Soc. Chem. Commun. 1995;(21):2215–2216. [Google Scholar]
- 85.Yusubov MS, Svitich DY, Larkina MS, Zhdankin VV. Applications of iodonium salts and iodonium ylides as precursors for nucleophilic fluorination in positron emission tomography. ARKIVOC. 2013;(1):364–395. 332. [Google Scholar]
- 86.University of Newcastle upon Tyne. 2009. WO2009138763 A1.
- 87.Carroll MA, Jones C, Tang S-L. Fluoridation of 2-thienyliodonium salts. J. Labelled Compd Radiopharm. 2007;50(5–6):450–451. [Google Scholar]
- 88.GE Healthcare Limited; Medi-Physics, Inc. 2009. WO2009073273 A2.
- 89.GE Healthcare Ltd. 2010. WO2010018218.
- 90.NUtech Ventures; University of Nebraska. 2011. US20110313170.
- 91.NUtech Ventures. 2013. WO2013003734 A2.
- 92.Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH. Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett. 2014;16(12):3224–3227. doi: 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.University of California. 2010. WO2010008522 A2.
- 94.University of California. 2010. WO2010117435 A2.
- 95.The General Hospital Corporation. 2015. US20150252007 A1.; • Synthesis of [18F]arenes by fluorination of iodonium ylides.
- 96.Rotstein BH, Stephenson NA, Vasdev N, Liang SH. Spirocyclic hypervalent iodine(III)-mediated radiofluorination of non-activated and hindered aromatics. Nat. Commun. 2014;5:4365. doi: 10.1038/ncomms5365. [DOI] [PubMed] [Google Scholar]
- 97.Bayer Schering Pharma Aktiengesellschaft. 2010. WO2010066380 A1.
- 98.GE Healthcare Limited. 2013. WO2013144301 A2.
- 99.UCL Business PLC. 2014. WO2014057291 A1.
- 100.Sander K, Gendron T, Yiannaki E, et al. Sulfonium salts as leaving groups for aromatic labelling of drug-like small molecules with Fluorine-18. Sci. Rep. 2015;5:9941. doi: 10.1038/srep09941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang X, Liu W, Ren H, Neelamegam R, Hooker JM, Groves JT. Late stage benzylic C-H fluorination with [18F]Fluoride for PET imaging. J. Am. Chem. Soc. 2014;136(19):6842–6845. doi: 10.1021/ja5039819. [DOI] [PubMed] [Google Scholar]
- 102.Princeton University. 2015. WO2015134467 A1.; •• Benzylic C–H radiofluorination with [18F]fluoride.
- 103.Leland Stanford Junior University. 2010. US20100152502 A1.
- 104.University of Oslo. 2015. US20150239796 A1.; • Synthesis of trifluoromethylarenes using [18F]CuCF3.
- 105.Institut National des Sciences Appliquees de Rouen (INSA); Centre National de la Recherche Scientifique (CNRS) 2014. EP2784055 A1.
- 106.Ruhl T, Rafique W, Lien VT, Riss PJ. Cu(i)-mediated 18F-trifluoromethylation of arenes: rapid synthesis of 18F-labeled trifluoromethyl arenes. Chem. Commun. 2014;50(45):6056–6059. doi: 10.1039/c4cc01641f. [DOI] [PubMed] [Google Scholar]
- 107.Huiban M, Tredwell M, Mizuta S, et al. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 2013;5(11):941–944. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- 108.Verhoog S, Pfeifer L, Khotavivattana T, et al. Silver-mediated 18F-labeling of aryl-CF3 and aryl-CHF2 with 18F-fluoride. Synlett. 2016;27(01):25–28. [Google Scholar]
- 109.van der Born D, Sewing C, Herscheid JDM, Windhorst AD, Orru RVA, Vugts DJ. A universal procedure for the [18F]trifluoromethylation of aryl iodides and aryl boronic acids with highly improved specific activity. Angew. Chem. Int. Ed. Engl. 2014;53(41):11046–11050. doi: 10.1002/anie.201406221. [DOI] [PubMed] [Google Scholar]
- 110.GE Healthcare Limited; Medi-Physics, Inc. 2015. WO2015027012 A1.
- 111.GE Healthcare Ltd.; Medi-Physics, Inc. 2009. WO2009029633 A1.
- 112.Gachon University of Medicine & Science Industry. 2010. US20100292478 A1.
- 113.Bayer Schering Pharma Aktiengesellschaft. 2011. WO2011006621 A1.
- 114.Hammersmith Imanet Limited; Medi-Physics, Inc. 2010. WO2010117557 A1.
- 115.Fehler SK, Maschauer S, Hoefling SB, et al. Fast and efficient 18F-labeling by [18F|fluorophenylazocarboxylic esters. Chem. Eur. J. 2014;20(2):370–375. doi: 10.1002/chem.201303409. [DOI] [PubMed] [Google Scholar]
- 116.Friedrich-Alexander-Universitaet Erlangen-Nuernberg. 2015. DE102014017352 A1.
- 117.United States Dept. of Health and Human Services. 2012. WO2012094334 A1.
- 118.Jacobson O, Zhu L, Ma Y, et al. A rapid and simple one-step F-18 labeling of peptides. Bioconjugate Chem. 2011;22(3):422–428. doi: 10.1021/bc100437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piramal Imaging SA. 2013. WO2013026941 A1.
- 120.Piramal Imaging SA. 2013. WO2013001088 A1.
- 121.Henan University of Traditional Chinese Medicine. 2015. CN 105153227 A.
- 122.Neal TT, Apana S, Berridge MS. Improved synthesis of [18F]fluoromethyl tosylate, a convenient reagent for radiofluoromethylations. J. Labelled Comp. Radiopharm. 2005;48(8):557–568. [Google Scholar]
- 123.Rodnick ME, Brooks AF, Hockley BG, Henderson BG, Scott PJH. A fully-automated one-pot synthesis of [18F]fluoromethylcholine with reduced dimethylaminoethanol contamination via [18F]fluoromethyl tosylate. Appl. Radiat. Isot. 2013;78(1):26–32. doi: 10.1016/j.apradiso.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glaser M, Aarstad E. “Click labeling” with 2-[18F]fluoroethylazide for positron emission tomography. Bioconjugate Chem. 2007;18(3):989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 125.Hammersmith Imanet Ltd.; GE Healthcare Ltd. 2010. WO2010060694 A1.
- 126.Carroll L, Boldon S, Bejot R, Moore JE, Declerck J, Gouverneur V. The traceless staudinger ligation for indirect 18F-radiolabelling. Org. Biomol. Chem. 2011;9(1):136–140. doi: 10.1039/c0ob00564a. [DOI] [PubMed] [Google Scholar]
- 127.Isis Innovation Limited. 2010. WO2010133851.
- 128.GE Healthcare Limited; Medi-Physics, Inc. 2009. WO2009035959 A2.
- 129.GE Healthcare Limited. 2015. WO2015091881 A1.
- 130.General Electric Company; Hammersmith Imanet Limited. 2009. WO2009080561 A.
- 131.Genentech, Inc. 2010. WO2010099273 A1.
- 132.Sogang University, Industry-Academy Cooperation Foundation. 2011. KR2011131022 A.
- 133.GE Healthcare Limited; Imperial Innovations Limited. 2013. WO2013092790 A1.
- 134.Gifu University. 2010. JP2010195698 A.
- 135.Riken Corp.; Osaka University. 2010. WO2010131745 A1.
- 136.Universite de Lorraine; Centre National de la Recherche Scientifique. 2014. WO2014006022 A1.
- 137.Shimadzu Corporation; The University of Electro-Communications. 2015. WO2015045052 A1.
- 138.University Court of the University of St Andrews, UK; Turun Yliopisto. 2012. GB2491950 A.
- 139.Dall'Angelo S, Zhang Q, Fleming IN, et al. Efficient bioconjugation of 5-fluoro-5-deoxy-ribose (FDR) to RGD peptides for positron emission tomography (PET) imaging of αvβ3 integrin receptors. Org. Biomol. Chem. 2013;11(27):4551–4558. doi: 10.1039/c3ob40550h. [DOI] [PubMed] [Google Scholar]
- 140.Otsuka Chemical Holdings Co. Ltd. 2011. JP2011116736 A.
- 141.Whitehead Institute for Biomedical Research. 2015. WO2015073746 A2.
- 142.Rashidian M, Keliher EJ, Dougan M, et al. Use of 18F-2-fluorodeoxyglucose to label antibody fragments for immuno-positron emission tomography of pancreatic cancer. ACS Cent. Sci. 2015;1(3):142–147. doi: 10.1021/acscentsci.5b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Advanced Accelerator Applications. 2011. WO2011018467 A1.
- 144.Duke University. 2014. WO2014179715 A1.
- 145.Vaidyanathan G, McDougald D, Choi J, et al. N-succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate ([18F]SFBTMGMB): a residualizing label for 18F-labeling of internalizing biomolecules. Org. Biomol. Chem. 2016;14(4):1261–1271. doi: 10.1039/c5ob02258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sogang University Research Foundation. 2015. KR2015141816 A.
- 147.Hamamatsu Photonics Co. Ltd. 2013. JP2013216618 A.
- 148.Kraeber-Bodere F, Rousseau C, Bodet-Milin C, et al. A pretargeting system for tumor PET imaging and radioimmunotherapy. Front. Pharmacol. 2015;6:1–9. doi: 10.3389/fphar.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koninklijke Philips Electronics N.V. 2010. WO2010119389 A2.
- 150.Koninklijke Philips Electronics N.V. 2012. WO2012153254 A1.
- 151.Koninklijke Philips Electronics N.V. 2012. WO2012049624 A1.
- 152.University of Delaware. 2012. WO2012012612 A2.; • Tetrazine/transcyclooctene ligation for radiolabeling of peptides.
- 153.Blackman ML, Royzen M, Fox JM. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand diels-alder reactivity. J. Am. Chem. Soc. 2008;130(41):13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]