Abstract
With the phasing-out of the polybrominated diphenyl ether (PBDE) flame retardants due to concerns regarding their potential developmental toxicity, the use of replacement compounds such as organophosphate flame retardants (OPFRs) has increased. Limited toxicity data are currently available to estimate the potential adverse health effects of the OPFRs. The toxicological effects of 4 brominated flame retardants, including 3 PBDEs and 3,3',5,5'-tetrabromobisphenol A, were compared with 6 aromatic OPFRs and 2 aliphatic OPFRs. The effects of these chemicals were determined using 3 biological endpoints in the nematode Caenorhabditis elegans (feeding, larval development, and reproduction). Because C. elegans development was previously reported to be sensitive to mitochondrial function, results were compared with those from an in vitro mitochondrial membrane permeabilization (MMP) assay. Overall 11 of the 12 flame retardants were active in 1 or more C. elegans biological endpoints, with only tris(2-chloroethyl) phosphate inactive across all endpoints including the in vitro MMP assay. For 2 of the C. elegans endpoints, at least 1 OPFR had similar toxicity to the PBDEs: triphenyl phosphate (TPHP) inhibited larval development at levels comparable to the 3 PBDEs; whereas TPHP and isopropylated phenol phosphate (IPP) affected C. elegans reproduction at levels similar to the PBDE commercial mixture, DE-71. The PBDEs reduced C. elegans feeding at lower concentrations than any OPFR. In addition, 9 of the 11 chemicals that inhibited C. elegans larval development also caused significant mitochondrial toxicity. These results suggest that some of the replacement aromatic OPFRs may have levels of toxicity comparable to PBDEs.
Keywords: Caenorhabditis elegans, flame retardants, mitochondrial toxicity.
To meet flammability standards, chemical flame retardants are extensively incorporated into commercial products including electronics, upholstered furniture, building materials, textiles, and baby products (Shaw et al., 2010; Stapleton et al., 2005). Prior to 2005, the polybrominated diphenyl ethers (PBDEs) were a major class of flame retardants used in the United States. These brominated flame retardants (BFRs) were produced as mixtures of penta-, octa-, and deca-brominated diphenyl ethers (BDEs) with 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) and 2,2′,4,4′,5-penta-bromodiphenyl ether (BDE-99) being the major congeners in the penta-BDE mixture. A widely used commercial mixture marketed as DE-71 contained PBDEs (BDE-47 and BDE-99) along with 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153), which were low molecular weight, lipophilic PBDEs that bioaccumulated in the environment and in human tissues (Birnbaum and Staskal, 2004; Hakk and Letcher, 2003).
The PBDEs were phased-out of use in 2005 due to concerns over their environmental persistence, bioaccumulation, and association with several adverse human health effects including altered circulating hormone levels, decreased fertility, and impaired neurodevelopment (Frederiksen et al., 2009; Harley et al., 2010; Herbstman et al., 2010; Meeker and Stapleton, 2010). They are increasingly being replaced by organophosphate flame retardants (OPFRs) (Stapleton et al., 2011, 2012). Use estimates of some OPFRs, which are also used in other manufacturing processes most commonly as PVC plasticizers (van der Veen and de Boer, 2012), have risen dramatically over the past 30 years from ∼14 000 to 38 000 tons per year (Schreder et al., 2016). Like PBDEs, OPFRs can leach out of treated materials and persist in the environment. They have been detected in indoor air, household dust, wastewater treatment plant effluent, drinking water, and wildlife samples (Barcelo et al., 1990; Meeker et al., 2013; Sundkvist et al., 2010; van der Veen and de Boer, 2012). In addition, they have been found in human tissues at levels similar to the PBDEs (Hoffman et al., 2014). Although OPFR usage is continually increasing, there is a paucity of information on the toxicity of this class of chemicals. Similar to PBDEs, exposures to OPFRs are associated with altered hormone levels, decreased reproductive function, and adverse developmental outcomes in humans and animal models (Farhat et al., 2013; Li et al., 2015; Liu et al., 2013, 2016; McGee et al., 2012; Meeker and Stapleton, 2010; Wang et al., 2013). Because structurally related compounds such as organophosphate pesticides (OPs) have been shown to adversely affect the development of the nervous system and alter reproductive parameters (Munoz-Quezada et al., 2013; Okamura et al., 2009; Roegge et al., 2008; Slotkin et al., 2006; Venerosi et al., 2012), the presence of a phosphate backbone in OPFRs suggests a potential for neurotoxicity and developmental toxicity (Crump et al., 2012; Dishaw et al., 2011; Egloff et al., 2014; Hendriks et al., 2014; McGee et al., 2012).
The nematode Caenorhabditis elegans was used as an alternative toxicological model because it offered a number of practical advantages similar to cell-based assays, but with the complexity of a whole organism (Leung et al., 2008). In addition, the effects of various environmental chemicals on a number of C. elegans outcomes have been well documented including development (Altun et al., 2002–2015; Anderson et al., 2001; Boyd et al., 2016), nervous system function (Boyd et al., 2003; Chen et al., 2015; Chisholm and Jin, 2005; Hobert, 2005; Leung et al., 2008; Mori et al., 2007; Silhankova and Korswagen, 2007), and reproduction (Harada et al., 2007; Hoss and Weltje, 2007). Of critical importance to this study was the ability to assess the effects of exposures across the lifespan of the nematode at multiple developmental stages within a few days.
The objective of the current study is to compare the developmental and neurotoxic potential of several phased-out PBDEs with representative replacement FRs. To do this, 3 medium-throughput C. elegans toxicological assays—larval development, feeding, and reproduction—were used to test the effects of 4 BFRs—including 3 phased-out PBDEs [2,2'4,4'-tetrabromodiphenyl ether (BDE-47), 2,2',4,4',5-pentabromodiphenyl ether (BDE-99), and the pentabromodiphenyl ether commercial mixture (DE-71)] and 3,3',5,5'-tetrabromobisphenol A (TBBPA)—to 6 aromatic OPFRs [triphenyl phosphate (TPHP), t-butylphenyl diphenyl phosphate (BPDP), isopropylated phenol phosphate (IPP), trimethyl phenyl phosphate (TMPP, aka tricresyl phosphate or TCP), isodecyl diphenyl phosphate (IDDP), and 2-ethylhexyl diphenyl phosphate (EHDP)], and 2 aliphatic OPFRs (tris(2-chloroethyl) phosphate (TCEP) and tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) (see Table 1). Two positive control compounds were also used for comparative purposes: the OP chlorpyrifos (CPF), which was previously tested using the 3 C. elegans assays (Boyd et al., 2010b), and tri-O-cresyl phosphate (TOCP), which was structurally similar to the aromatic OPFRs and was shown to cause delayed neurotoxicity in several species (reviewed in Weiner and Jortner, 1999). Because genetic mutations and exposures to environmental chemicals that affect mitochondrial function also alter development in C. elegans (Addo et al., 2010; Bess et al., 2012; Tsang and Lemire, 2002; Tsang et al., 2001), the effects observed in C. elegans were further compared with those from the Tox21 mitochondrial membrane permeabilizataion (MMP) dataset (Attene-Ramos et al., 2015).
TABLE 1.
Chemical Names, CASRNs, and Structures
| Chemical ID | Chemical Name | CASRN | Structure | Determined Purity (%) |
|---|---|---|---|---|
| BDE-47 | 2,2'4,4'-Tetrabromodiphenyl ether | 5436-43-1 | 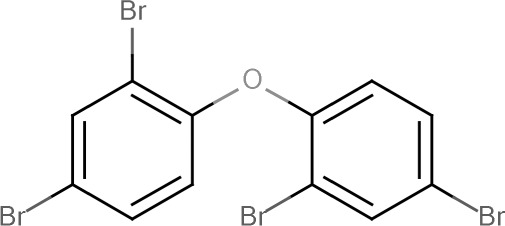 |
100 |
| BDE-99 | 2,2',4,4',5-Pentabromodiphenyl ether | 60348-60-9 | 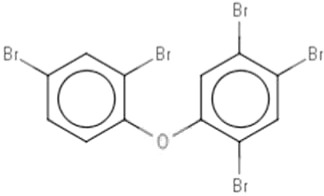 |
100 |
| BPDPa | t-Butylphenyl diphenyl phosphate | 56803-37-3 | 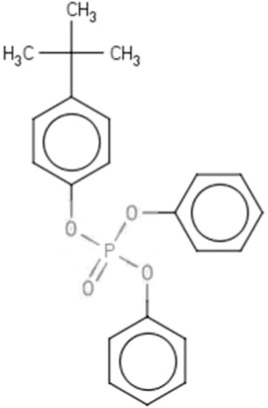 |
BPDP (64.5)TPHP (35.2) |
| CPF | Chlorpyrifos | 2921-88-2 | 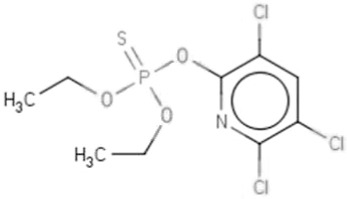 |
99.84 |
| DE-71b | Pentabromodiphenyl ether | 32534-81-9 | 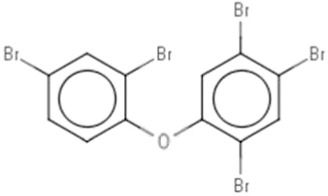 |
BDE-47(42.27)BDE-99 (38.72) |
| EHDP | 2-Ethylhexyl diphenyl phosphate | 1241-94-7 | 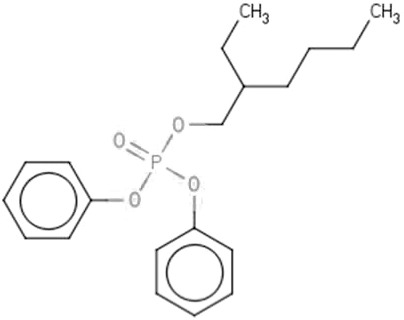 |
98.48 |
| IDDPc | Isodecyl diphenyl phosphate | 29761-21-5 | 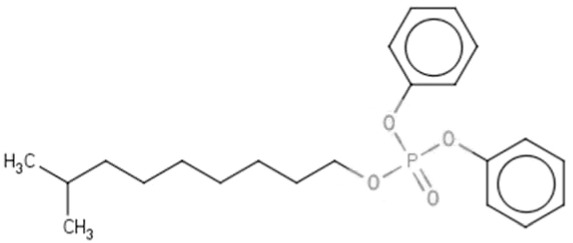 |
96.07 |
| IPPd | Isopropylated phenol phosphate | 68937-41-7 | 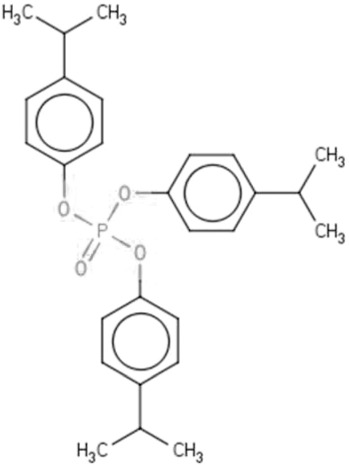 |
IPP (72.92)TPHP (26.01) |
| TBBPA | 3,3',5,5'-Tetrabromobisphenol A | 79-94-7 | 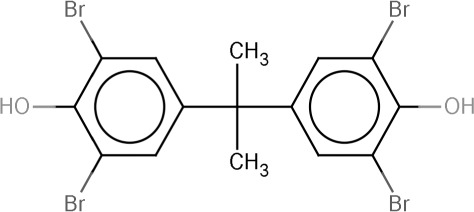 |
98.93 |
| TCEP | Tris(2-chloroethyl) phosphate | 115-96-8 | 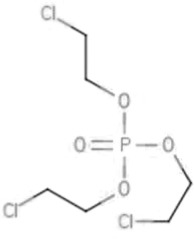 |
98.95 |
| TDCIPP | Tris(1,3-dichloro-isopropyl) phosphate | 13674-87-8 | 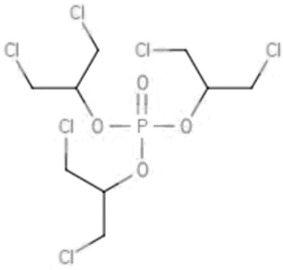 |
95.7 |
| TMPPe | Trimethyl phenyl phosphate | 1330-78-5 | 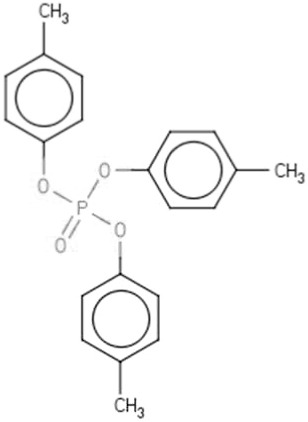 |
71.35 |
| TOCPf | Tri-o-cresyl phosphate | 78-30-8 | 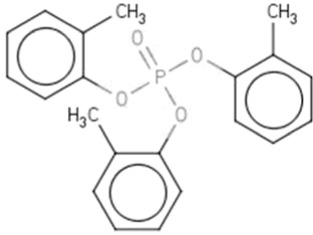 |
97.1 |
| TPHP | Triphenyl phosphate | 115-86-6 | 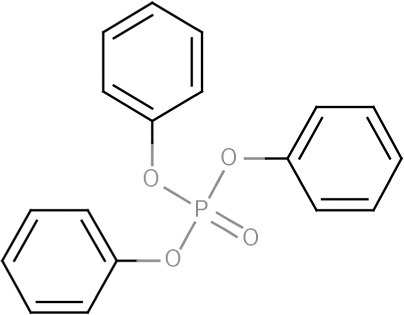 |
99.35 |
Mixture of 2-, 3-, and 4-tert-butylphenyl diphenyl phosphates; TPHP is a known byproduct of BPDP.
Mixture of phenoxybenzene derivatives; see discussion for individual constituents.
Mixture of isodecyl isomers.
Isomeric mixture including TPHP.
Mixture of isomeric tricresylphosphates; impurities consist of mixtures of methyl and ethyl tri-substituted compounds.
Isomer of TMPP.
MATERIALS AND METHODS
Flame Retardants
Full chemical names, IDs, CASRNs, and structures for all test compounds are presented in Table 1. Chemicals were purchased from the following vendors: BDE-47 and BDE-99 (Cerilliant Corp), BPDP (Ubichem Fine Chemicals), CPF (Toronto Research Chemicals), DE-71 (Great Lakes Chemical Company), EHDP (TCI America; Portland, OR), IDDP (Bayville Chemical Supply), IPP (Amfinecom Inc), TBBPA (Albemarle), DMSO, TPHP, TCEP and TDCIPP (Sigma Aldrich Chemical Co), TOCP and TMPP (Acros Organics).
Stock solutions were prepared in 100% DMSO at 100 mM (final concentration). Test solutions were then diluted from the stock solutions to final test concentrations in 1% DMSO. This allowed testing up to a maximum concentration of 1.0 mM. Chemical concentration ranges were adjusted for each in vivo assay to capture the full range of biological responses, from no observable to maximal sub-lethal effects. All concentration levels tested for each assay/chemical combination are provided in Supplementary Table 1.
Nematode Culture
Bristol N2 (wild-type) C. elegans (Caenorhabditis Genetic Center) were maintained at 20 °C on K-agar plates (2% bacto-agar, 0.25% bacto-peptone, 51 mM sodium chloride, 32 mM potassium chloride, 13 μM cholesterol) seeded with E. coli OP50 as the food source (Williams and Dusenbery, 1988). Age-synchronized cultures were prepared using a sodium hypochlorite solution (250 µM sodium hydroxide, 1% bleach) as previously described (Khanna et al., 1997). After a final rinse with K-medium (51 mM sodium chloride, 32 mM potassium chloride), embryos were transferred to a complete K-medium solution (K-medium plus 3 mM calcium chloride, 3 mM magnesium sulfate, 13 µM cholesterol) (Boyd et al., 2009) to hatch and arrest at the first larval stage (L1s) for the larval development assay, or were placed on K-agar plates with the OP50 strain of E. coli and allowed to develop to the appropriate life stage for the reproduction and feeding assays.
Larval Development, Feeding, and Reproduction Assays
Larval development, feeding and reproduction assays were performed as previously described using the COPAS Biosort (Union Biometrica Inc) (Boyd et al., 2007, 2010a; Rice et al., 2014). Age-synchronized nematodes were placed in 96-well plates containing the test compound, E. coli food source and either K-medium (reproduction and feeding) or complete K-medium (larval development). Following chemical exposure, 2 size characteristics for individual nematodes [length or “time of flight” (TOF) and optical density or “extinction” (EXT)] and fluorescence were measured. Three independent biological replicates with 6 technical replicate wells per treatment group were conducted for each chemical-assay combination on separate days.
To determine the effects of the chemicals on larval development, 50 synchronized L1 larvae were placed in each well of a 96-well plate. The sizes of individual nematodes, which were linked to specific C. elegans larval stages (Boyd et al., 2016), were measured after 48-h exposures using the COPAS Biosort. In the absence of developmental inhibition, larvae will develop from the first larval stage (L1) to the fourth and final larval stage (L4) within 48 h. C. elegans maintained in the presence of chemicals may exhibit a range of developmental inhibition, from those that develop to L4s to those that do not develop and arrest as L1s.
To evaluate reproduction, 5 L4 larvae were placed into each well of a 96-well plate and allowed to grow and lay embryos in the presence of toxicant for 48 h at 20 °C (Boyd et al., 2010a). Total numbers of adults and offspring were counted at the end of the exposure using the COPAS Biosort.
For the feeding assay, 25 young adult nematodes were placed into each well of a 96-well plate and incubated for 24 h at 20 °C in the presence of toxicant. To measure feeding rates, 5 µl fluoresbrite polychromatic red 0.5 µm microspheres (Polysciences Inc) in deionized water were added to each well (Boyd et al., 2007). Nematodes were allowed to feed for 15 min, after which 5 µl of 170 mM sodium azide was added to arrest feeding. The levels of fluorescence in the nematode pharynx and intestine were measured using the COPAS Bisort.
Statistical Analyses
Incubation of nematodes for 48 h in the growth assay leads to the accumulation of detritus, such as shed cuticles and other waste products that are smaller than nematodes. These noise observations were removed using statistical methods described previously (Boyd et al. 2010b). Observations [log(EXT)] were indicative of nematode size. Each observation was subtracted from the mean plate control response to adjust for differences between plates. The feeding assay observations [log(RED fluorescence)] of adult nematodes did not contain detritus and therefore no observations were removed. Large fluorescence intensity values indicate healthy feeding in the nematodes. Fluorescence intensity observations were subtracted from the plate control mean to adjust for plate effects. For development and feeding, the plate-adjusted values [log(EXT) or log(RED)] resulted in the observed effect, which increased with decreasing nematode size, indicating toxicity. These effects were fit to a Hill function that provided EC50 estimates (effective concentration for effect equal to 50% of the maximum effect) with confidence intervals. When needed, an upper bound limit, determined from previous experiments, was used in the estimation. The lowest effective concentration (LEC) was estimated using a t test with the Bonferroni correction to compare each dose group with the plate controls. Analyses were performed twice: once individually on the 3 biological replicates and a second time using the 3 combined replicates.
For the reproduction assay, number of offspring (L1 and L2 larva) was used in the analyses. Three biological replicates were fit by a Hill function individually, as well as together. The LEC values could only be estimated after combining the replicates, because a single observation (nematode count) was measured within each replicate, and were determined using Dunnett’s test to account for multiple testing of each dose group against the plate controls.
MMP Assay
The Tox21 MMP assay was used to identify chemically induced MMP disruption. This assay uses a water-soluble sensor, Mito-MPS (BD Biosciences), to detect chemically induced changes in MMP(Attene-Ramos et al., 2015). The dye produces red fluorescence when it accumulates and aggregates in the mitochondria of healthy cells. If the MMP is disrupted, then the dye does not accumulate and produces a green cytoplasmic fluorescence. To evaluate the specificity of chemical-induced mitochondrial toxicity, cell viability was also measured in the same compound well. The specificity of the MMP effect in relation to the cytotoxicity was quantified by the fold change of the weighted area under the curve (Hsieh et al., 2015). A threshold of 4 was chosen based on the activity variation.
Assay data were retrieved from PubChem [PubChem AID: 720637] and processed as previously described (Judson et al., 2010). Briefly, 3 types of activity calls (active, inactive, or inconclusive) were generated. For those compounds that were active or inconclusive due to cytotoxicity, the point-of-departure (POD) (ie the concentration at which the response was equivalent to the assay-dependent noise threshold) was reported. The relative order of toxicity was compared using the POD values in the MMP with the LEC values and effect size from the C. elegans assays (Table 2).
Table 2.
Comparisons of flame retardant toxicity to C.elegans with in vitro mitochondrial activity.
| Brominated | Aromatic OPFRs | Aliphatic OPFRs | Positive Controls | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBDE | Bisphenol | tri-phenyl OPFRs | di-phenyl OPFRs | Chlorinated | ||||||||||||
| Assay | BDE-99 | DE-71 | BDE-47 | TBBPA | TPHP | IPP | BPDP | TMPP | EHDP | IDDP | TDCIPP | TCEP | CPF | TOCP | ||
| C. elegans (LEC in µM) | Reproduction | 13a | 1.60b | 13a | 13a | 6.30b | 10b | 16a | 63 | - | 32a | 130 | - | 0.04c | 250 | |
| Larval Development | 0.16c | 0.16c | 0.40c | 3.20b | 0.16c | 1.60b | 2.50b | 100 | 1.60b | 2.50b | 13a | - | 0.25c | 10b | ||
| (1.72)c | (2.01)c | (1.75)c | (0.9)b | (1.62)c | (2.23)b | (2.17)b | (0.78) | (1.0)b | (2.02)b | (2.83)a | (2.38)c | (1.79)b | ||||
| Feeding | 7.90b | 5b | 6.30b | 20a | 40a | 63 | 63 | 630 | - | - | 50a | - | 0.12c | 400 | ||
| (1.22)b | (1.68)b | (2.08)b | (1.63)a | (1.08)a | (0.69) | (1.30) | (0.2) | (2.44)a | (1.38)c | (0.65) | ||||||
| qHTS (POD in µM) | MMP inhibition | 1.3b | 6.7b | 12.8a | 6.7b | 0.60c | 5.7b,c | 3.7b | 11.7a | - | - | 64.7c | - | 67.4c | 15.3a,c | |
| Cell Viability | - | - | - | - | 13.2a | 5.9b | 28.0a | 19.0a | 29.0a | 16.4a | 58.3 | - | 41.4a | 7.4b | ||
Lowest effective concentration (LEC) or point of departure (POD); 10 < a < 30 μM; 1 < b < 10 μM; c < 1 μm; Dashes (-) indicate no toxicity at concentrations tested
indicates cytoxicity at concurrent concentrations; numbers in parentheses indicate effect sizes of C. elegans endpoints; bolded text indicates effect size >1.5.
RESULTS
Toxicity of Flame Retardants to Caenorhabditis elegans
Larval Development
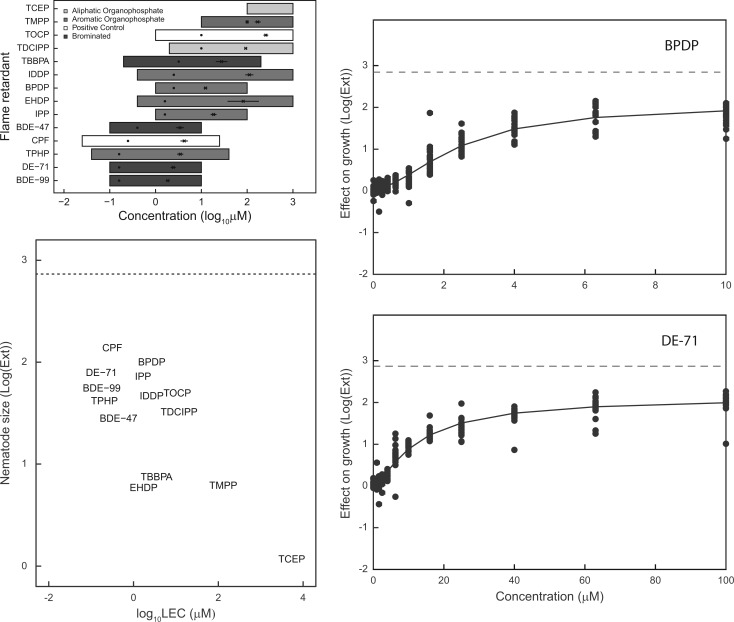
All chemicals were tested at concentrations designed to capture effects ranging from no effect to a maximal effect, with the highest concentration tested (1 mM) being limited to 1% DMSO tolerated by C. elegans (Boyd et al., 2010a). TCEP, TMPP, TDCIPP, IDDP, EHDP, and TOCP required exposures to concentrations that included the maximum 1 mM concentration in an attempt to observe maximal decreases in development (Figure 1A; Supplementary Table 1). PBDEs were among the most potent flame retardants affecting C. elegans larval development at the lowest concentrations including: BDE-99, DE-71, and BDE-47 with LECs of 0.16, 0.16, and 0.4 µM, respectively (Table 2; Supplementary Table 1). The aromatic OPFR, TPHP, showed comparable potencies to the PBDEs with an LEC of 0.16 µM. Chlorpyrifos, an OP pesticide used as a positive control and reference compound, had similar potency with an LEC = 0.25 µM. Four additional aromatic OPFRs; BPDP, EDHP, IDDP, and IPP; and the BFR, TBBPA, had LECs < 5 µM (Table 2).
FIG. 1.
Effects of flame retardants on C. elegans larval development and growth. A, Top left: Groups of 50 L1 nematodes were exposed for 48 h to various concentrations of flame retardants, illustrated by the horizontal bars. Flame retardants are grouped as: aliphatic organophospate flame retardants (OPFRs); aromatic OPFRs; brominated flame retardants including BDEs. The organophosphate pesticide chlorpyifos and aromatic OPFR TOCP were used as positive controls. Lowest effective concentrations (LECs) [dots] and EC50s [crosses] with 95% confidence intervals are also displayed. B, Bottom left: Scatterplots of observed maximum effect size (log(EXT)) and lowest effective concentration (LEC, in log scale). The horizontal dashed line refers to the maximum effect, no growth or development, for this assay. C, Top right: Sample concentration-effect plot for BPDP, a representative OPFR. D, Bottom left: Sample concentration-effect plot for DE-71, a representative PBDE.
Figure 1B shows the potency or LEC against the effect size in the larval development assay, where larger effect sizes correspond to smaller nematodes. Untreated nematodes correspond to an effect size of zero. Visual observations of larval stages were also performed by microscopy. The maximum effect size, designated by a dashed horizontal line in Figure 1B, is based upon the size of the nematodes at the first larval stage (L1) when loaded into the exposure plates at t = 0 h and would correspond to no growth over the 48 h exposure period. Likewise, treatments with insignificant effects would have an effect size near zero and nematodes would have developed to the fourth larval stage (L4). PBDEs and TPHP activities clustered together with low LECs and large effects on growth and development at the highest concentrations tested (Figure 1B). Most of the other OPFRs had slightly lower potencies with observed maximum effects similar to the PBDEs and TPHP, whereas TBBPA and EHDP had smaller maximum effects. TCEP was the only compound that did not affect larval development within the tested concentration range. Representative concentration response curves are presented in Figure 1C–D for a PBDE (DE-71), and OPFR (BPDP). All concentration response curves are provided in Supplementary Figure 1.
Feeding
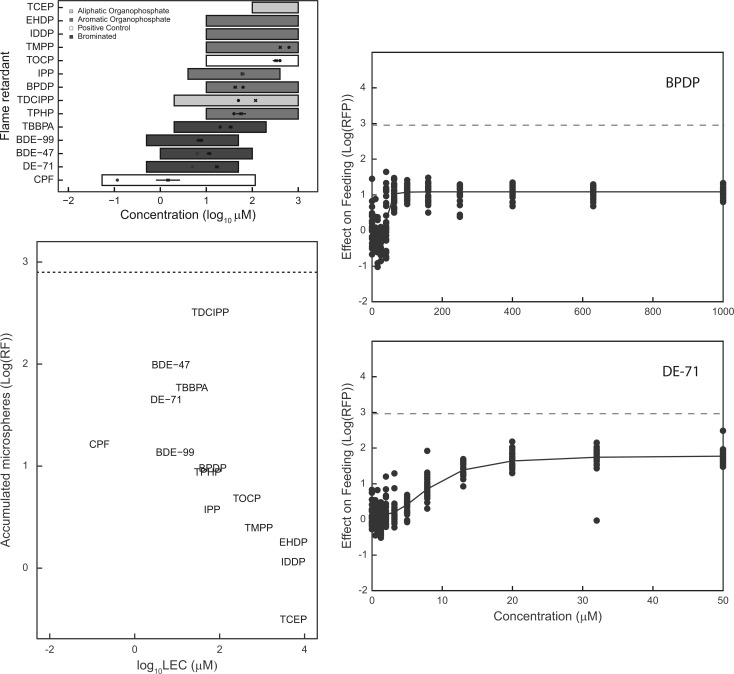
In general, the flame retardants had lesser effects on C. elegans feeding compared with larval development and growth (Figure 2A; Supplementary Table 1). Eight compounds (TCEP, EHDP, IDDP, TOCP, TMPP, BPDP, TDCIPP, and TPHP) were tested in the feeding assay up to the maximum testable concentration. TCEP, EHDP, and IDDP were inactive within the tested concentration ranges. PBDEs affected C. elegans feeding with LECs between 5 and 10 µM, whereas 4 aromatic OPFRs; BPDP, IPP, TDCIPP, and TPHP; and the BFR, TBBPA, had LECs ranging from 20 to 63 µM. Interestingly, the PBDEs, TDCIPP, and TBBPA produced effect sizes at least as large as CPF in the feeding assay but at much lower potencies (Figure 2B); whereas the isomers, TMPP and TOCP, had similar effect sizes and potencies. Representative concentration effect plots are presented in Figure 2C–D and all concentration effect curves are provided in Supplementary Figure 2.
FIG. 2.
Effects of flame retardants on C. elegans feeding. A, Top left: Groups of 25 adult nematodes were exposed for 24 h to various concentrations of flame retardants, illustrated by the horizontal bars. Flame retardants are grouped as: aliphatic organophospate flame retardants (OPFRs); aromatic OPFRs; brominated flame retardants including BDEs. The organophosphate pesticide chlorpyifos and aromatic OPFR TOCP were used as positive controls. Lowest effective concentrations (LECs) [dots] and EC50s [crosses] with 95% confidence intervals are also displayed. B, Bottom left: Scatterplots of observed maximum effect size (log(EXT)) and lowest effective concentration (LEC, in log scale). The horizontal dashed line refers to the maximum effect, no feeding, for this assay. C, Top right: Sample concentration-effect plot for BPDP, a representative OPFR. D, Bottom right: Sample concentration-effect plot for DE-71, a representative PBDE.
Reproduction
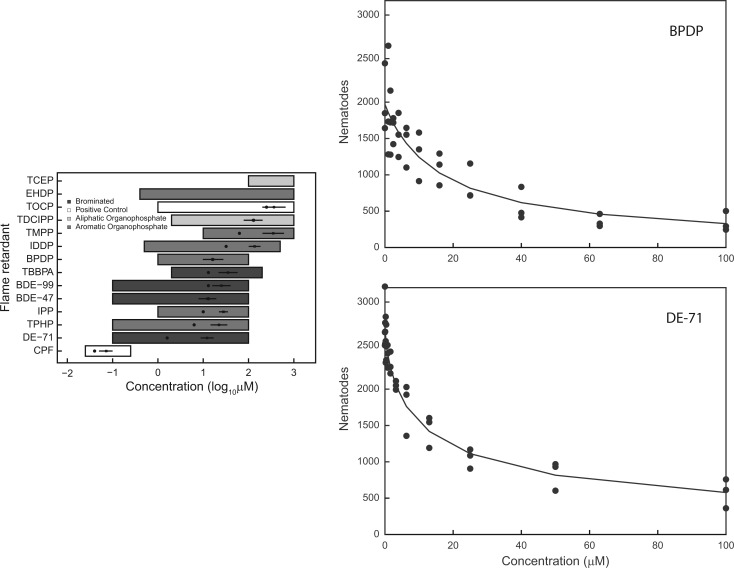
Similar to feeding, C. elegans reproduction was generally less sensitive than larval development. Five compounds (TCEP, EHDP, TOCP, TDCIPP, and TMPP) were tested up to the maximum testable concentration (Figure 3A). TCEP and EHDP also did not affect reproduction. Only DE-71 had an LEC less than 10 µM, whereas several flame retardants shared similar potencies ranging from 10 to 16 µM including BDE-47, BDE-99, TPHP, IPP, TBBPA, and BPDP (Table 2). IDDP, which was inactive in the feeding assay, was slightly less potent with an LEC of 32 µM compared with the other flame retardants. For the reproduction assay, the effect size of all active chemicals except TCEP and EHDP reached ∼100% or no offspring; thus potency verses effect size plots are not presented. Representative concentration response plots are presented in Figure 3B–C and all concentration response curves are provided in Supplementary Figure 3.
FIG. 3.
Effects of flame retardants on C. elegans reproduction. A, Left panel: Groups of 5 L4 nematodes were exposed for 48 h to various concentrations of flame retardants, illustrated by the horizontal bars. Flame retardants are grouped as: aliphatic organophospate flame retardants (OPFRs); aromatic OPFRs; brominated flame retardants including BDEs. The organophosphate pesticide chlorpyifos and aromatic OPFR TOCP were used as positive controls. Lowest effective concentrations (LECs) [dots] and EC50s [crosses] with 95% confidence intervals are also displayed. B, Top right: Sample concentration-effect plot for BPDP, a representative OPFR. C, Bottom right: Sample concentration-effect plot for DE-71, a representative PBDE.
Comparisons Between Caenorhabditis elegans and Tox21 Results
Approximately 8 300 unique compounds, including pharmaceuticals and environmental chemicals and the 14 compounds tested in C. elegans in this study, have been evaluated for toxicity in the Tox21 program using a suite of quantitative high-throughput screening (qHTS) assays (Huang et al., 2016). To test what biological activities might be associated with this group of chemicals, Fisher’s exact test was performed using all Tox21 assays. Only 3 types of activities were considered significantly enriched (α < 0.05) in this group of chemicals after applying the Bonferroni correction: MMP, increase of constitutive androstane receptor transcriptional factor activity (data not shown) and decrease of androgen receptor transcriptional factor activity (data not shown). Because MMP was more potent than the others when activities co-occurred and mitochondrial toxicity has been linked to developmental arrest in C. elegans, the results from C. elegans assays were compared with Tox21 in vitro MMP data (Table 2).
Our findings indicate that all of the PBDEs show strong effects on MMP (ie, POD < 15 µM) in the absence of cytotoxicity, whereas all of the OPFRs, except the completely inactive TCEP, cause some degree of cytotoxicity. Three tri-phenyl OPFRs (TPHP, BDHP, and TMPP) showed comparable effects to the PBDEs in altering MMP but with cytotoxicity at higher concentrations. The exception was the tri-phenyl OPFR IPP, which showed nonspecific effects on MMP with observations of cytotoxicity at a similar concentration. In total, 11 of the 14 chemicals tested (except EHDP, IDDP, and TCEP) caused significant MMP disruption with various degrees of cytotoxicity (Table 2). Sample concentration–response curves of the MMP data are presented in Supplementary Figure 4 for BDE-99 (selective for MMP), TPHP (selective for MMP), and TDCIPP (nonselective MMP inhibitor).
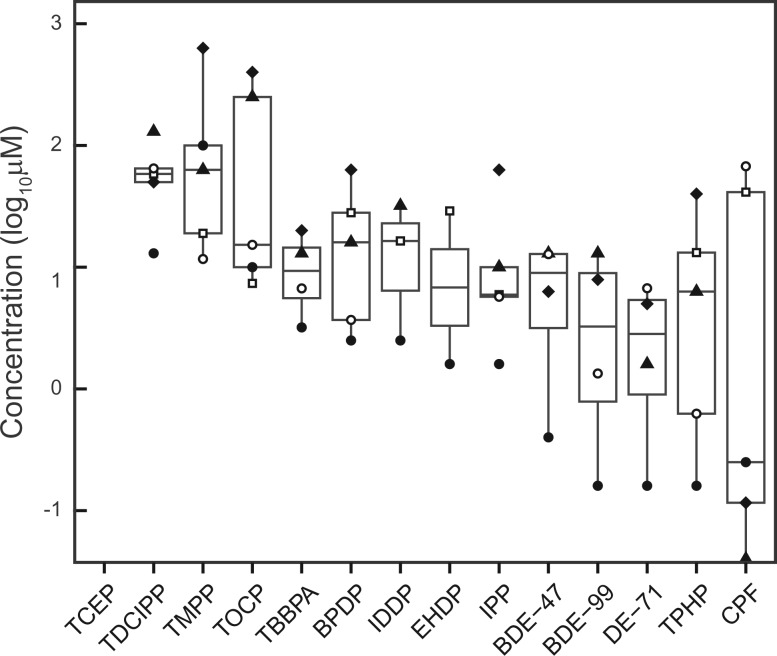
Figure 4 shows the flame retardants ranked according to the relative toxicity to C. elegans endpoint (larval development, feeding and reproduction) and the mammalian cell MMP endpoint from Tox21 qHTS. As seen in the figure, the C. elegans larval development assay was the most sensitive of all endpoints tested; whereas the phased-out BDEs and the positive control CPF were the most potent (as indicated by the lowest POD). Of the 13 chemicals that affected C. elegans larval development, 11 chemicals (except the di-phenyl OPFRs, EHDP, and IDDP) also caused significant MMP disruption; 7 of which showed specific effects on MMP (ie, in the absence of cytotoxicity or cytotoxicity observed at higher concentrations); whereas the remaining 4 nonspecifically altered MMP in the presence of cytotoxicity (Table 2). The POD for MMP disruption (either specific or nonspecific) and the LEC for C. elegans larval development closely tracked with each other: the fold change between the LEC and POD is less than 10 for 8 of the 11 chemicals (except CPF, DE-71, and BDE-47). TCEP was the only compound that did not cause toxicity as measured by any C. elegans endpoint or by the MMP assay.
FIG. 4.
Comparison of flame retardant activity in C. elegans and in vitro MMP assays. Box and whisker plot displaying the points of departure (PODs) for C. elegans larval development [filled circles], feeding [filled diamonds], and reproduction [filled triangles] as well as qHTS in vitro MMP assay [open circles] and cytotoxicity [open squares]. The x-axis displays chemicals in increasing order of activity from left to right.
DISCUSSION
With the phasing out of the PBDEs, the usage of and exposure to alternative flame retardants is increasing. As a result, the levels of exposure to a number of OPFRs are approaching those comparable to the PBDEs (Stapleton et al., 2014). The potential health effects of these chemicals however, remain largely unknown. An additional concern has been raised due to the structural similarity to OP pesticides, which are associated with developmental impairments in infants and children (Gonzalez-Alzaga et al., 2014).
In the current study, the relative toxicity of 8 OPFRs was compared with 4 BFRs and 2 positive controls using 3 C. elegans biological endpoints. These endpoints are designed to assess toxicity across the lifespan of the nematode and to monitor a range of adverse health effects including delayed larval development, decreased production of offspring or egg-laying, and neurotoxicity via the feeding. Our results indicated that several of the emerging OPFRs; namely TPHP, BPDP, and IPP; were consistently among the most active chemicals with comparable toxicity to the phased-out PBDEs and TBBPA (Table 2). TCEP was the only flame retardant that was inactive across all of the C. elegans endpoints (Table 2; Supplementary Figs. 1–3).
In all but 2 cases, larval development was the most sensitive endpoint in terms of potency; whereas reproduction was the most sensitive endpoint for TMPP and the positive control, CPF (Table 2; Figure 4). BDE 99, DE-71, and BDE-47 were the most active of the BFRs (LECs <1 μM) with the aromatic OPFR, TPHP, showing comparable activity (Table 2; Figure 4). Other aromatic OPFRs such as BPDP, IPP, and IDDP and the aliphatic OPFR, TDCIPP, closely followed with LECs less than 5 μM. DE-71 was the most active reproductive toxicant (LEC < 2 µM) closely followed by the OPFRs, TPHP, and IPP (LEC < 10 µM). Many of these compounds affected more than one C. elegans endpoint involving distinct biological processes, suggesting that these compound may alter multiple targets during development. Whereas the LEC determines the lowest concentration at which the compounds were active, the effect size determines the magnitude of the effect. For this class, the same compounds that had the lowest LEC also had the highest effect size thereby indicating that exposure to these compounds caused a significant arrest in the magnitude of response (Table 2; Figure 1B).
To determine the relationship between compound solubility and toxicity, correlations were calculated using Pearson’s correlation coefficient (R) for the active chemicals between the log KOW of each compound and the log10(LEC) for each C. elegans assay. No significant correlation was found between any of the pairs [R = 0.06 (Log KOW—Reproduction), 0.28 (Log KOW—Larval development, and 0.06 (Log KOW—Feeding)] suggesting that hydrophobicity alone did not drive the observed toxicities across these assays.
Although the OPFRs affected larval development and reproduction at concentrations similar to the PBDEs, feeding was affected by lower concentrations of the PBDEs than the OPFRs (Table 2). This suggests that neurotoxicity related to neuromuscular function may be of greater concern for PBDEs than for the OPFRs. These observations are consistent with previous findings that show associations between PBDE exposures and neurobehavioral deficits in rodents (Cheng et al., 2009; Kodavanti et al., 2010; Suvorov et al., 2009). It is interesting to note that while the OPFRs may not affect neuromuscular function, they appear to selectively inhibit neuronal firing and result in neuronal cell death at comparable concentrations in vitro (Behl et al., 2015). This highlights the need for a battery of assays to evaluate differences in activity between several models (rat and human in vitro models versus C. elegans whole organism models), differences in endpoints being assessed (neuromuscular function in C. elegans versus neuronal firing and neuronal death in vitro), differences in underlying bioavailability between the assays, and potential differences in metabolism.
Because the objective was to compare the toxicity of compounds used as flame retardants, several of the compounds tested are commercial mixtures such as DE-71, whereas others are single, pure chemicals such as BDE-47 (see footnotes in Table 1). Although complete chemical analyses of all compounds were outside the scope of the current work, purity analysis was completed for the DE-71 mixture. The following PBDEs were observed in DE-71 by gas chromatography: BDE-47 (42.27%), BDE-99 (38.72%), BDE-100 (8.32%), BDE-85 (2.19%), BDE-154 (2.26%), and BDE-153 (2.61%) for a total purity of 96.37%. Eleven additional peaks making up less than 1% of the total area of the chromatogram were not able to be identified (data not shown). Because dioxins and furans are known contaminants of DE-71 (Hanari et al., 2006), it cannot be completely ruled out that these compounds may be contributing to the observed toxicities. However, it is of note that the single chemicals BDE-47 and BDE-99, which are the major measured constituents, exhibited very similar toxicities to the DE-71 mixture across the 3 C. elegans endpoints, indicating that they may be acting via similar modes of action.
To identify a potential mechanism by which exposure to flame retardants may affect larval development, we compared C. elegans development with mammalian cell mitochondrial toxicity in vitro because mitochondrial dysfunction was previously associated with disrupted C. elegans larval development (Bess et al., 2012). Nine of the 11 flame retardants that affected C. elegans larval development also caused significant mitochondrial toxicity in mammalian cells (Figure 4; Table 2); TPHP, IPP, and BPDP were the most active OPFRs in the MMP assay with comparable activity to the phased-out PBDEs (POD < 10 μM) suggesting an association between disruption of mitochondrial function and altered larval development following FR exposure.
To understand the utility of C. elegans as a screening tool, we compared our findings with other in vivo studies. For DE-71, a highly active chemical in the C. elegans assays, a wide spectrum of effects were noted in rodents ranging from negative findings (Gonzalez-Alzaga et al., 2014) to selective lesions in the thyroid gland and reproductive tissues (Stoker et al., 2004) to complete reproductive failure in the mink model at comparable concentrations (Zhang et al., 2009). Another compound, TBBPA showed selective activity in all 3 C. elegans biological endpoints, but was found in a previous study to nonselectively alter endpoints of developmental neurotoxicity and acute neurotoxicity in the range of 10–12 μM in the presence of concomitant cytotoxicity (Behl et al., 2015), and resulted in mortality in developing zebrafish embryos (Jarema et al., 2015; Wu et al., 2015). Although the reasons for these differences remain unknown, these findings emphasize the importance of evaluating compounds in multiple species and models to better understand differences in species susceptibility, and ultimately impact on human health.
In conclusion, C. elegans was used as an in vivo phenotypic screening tool to evaluate the potential effects of a subset of flame retardants. The phased-out PBDEs and TBBPA were consistently among the most active chemicals in all 3 C. elegans biological endpoints and in vitro mitochondrial membrane potential assay. The replacement OPFRs TPHP, IPP, and BPDP showed comparable activity to the PBDEs thereby warranting further hazard characterization of these replacement OPFRs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors would like to thank Dr Raymond Tice, Dr Nisha Sipes, and Dr Jason Stanko for critical review of this manuscript. We also thank Mr Brad Collins for providing the chemical stock solutions used in this study and chemical purity information.
FUNDING
This work was supported in part by the National Toxicology Program Division and by the Intramural Research Program of the National Institute of Environmental Health Sciences (NIEHS)/National Institutes of Health (NIH) (Z01ES102045 and Z01ES102046).
REFERENCES
- Addo M. G., Cossard R., Pichard D., Obiri-Danso K., Rotig A., Delahodde A. (2010). Caenorhabditis elegans, a pluricellular model organism to screen new genes involved in mitochondrial genome maintenance. Biochim. Biophys. Acta 1802, 765–773. [DOI] [PubMed] [Google Scholar]
- Altun Z. F., Herndon L. A., Wolkow C. A., Crocker C., Lints R., Hall D. H. (2002–2015). WormAtlas. Available at: http://www.wormatlas.org/. Accessed September 2016.
- Anderson G. L., Boyd W. A., Williams P. L. (2001). Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 20, 833–838. [PubMed] [Google Scholar]
- Attene-Ramos M. S., Huang R., Michael S., Witt K. L., Richard A., Tice R. R., Simeonov A., Austin C. P., Xia M. (2015). Profiling of the Tox21 chemical collection for mitochondrial function to identify compounds that acutely decrease mitochondrial membrane potential. Environ. Health Perspect. 123, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo D., Porte C., Cid J., Albaiges J. (1990). Determination of organophosphorus compounds in Mediterranean Coastal Waters and Biota samples using gas-chromatography with nitrogen-phosphorus and chemical ionization mass-spectrometric detection. Int. J. Environ. Analyt. Chem. 38, 199–209. [Google Scholar]
- Behl M., Hsieh J. H., Shafer T. J., Mundy W. R., Rice J. R., Boyd W. A., Freedman J. H., Hunter E. S., Jarema K. A., Padilla S., et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 52 (Pt B), 181–193. [DOI] [PubMed] [Google Scholar]
- Bess A. S., Crocker T. L., Ryde I. T., Meyer J. N. (2012). Mitochondrial dynamics and autophagy aid in removal of persistent mitochondrial DNA damage in Caenorhabditis elegans. Nucleic Acids Res 40, 7916–7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Staskal D. F. (2004). Brominated flame retardants: cause for concern? Environ. Health Perspect. 112, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., Cole R. D., Anderson G. L., Williams P. L. (2003). The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ. Toxicol. Chem. 22, 3049–3055. [DOI] [PubMed] [Google Scholar]
- Boyd W. A., McBride S. J., Freedman J. H. (2007). Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS ONE 2, e1259.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., McBride S. J., Rice J. R., Snyder D. W., Freedman J. H. (2010a). A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 245, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., Smith M. V., Co C. A., Pirone J. R., Rice J. R., Shockley K. R., Freedman J. H. (2016). Developmental effects of the ToxCast Phase I and Phase II chemicals in Caenorhabditis elegans and corresponding responses in Zebrafish, rats, and rabbits. Environ. Health Perspect. 124, 586–593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., Smith M. V., Kissling G. E., Freedman J. H. (2010b). Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol. Teratol. 32, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd W. A., Smith M. V., Kissling G. E., Rice J. R., Snyder D. W., Portier C. J., Freedman J. H. (2009). Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PLoS One 4, e7024.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Chakraborty S., Peres T. V., Bowman A. B., Aschner M. (2015). Manganese-induced neurotoxicity: from to humans. Toxicol. Res. 4, 191–202. 10.1039/c4tx00127c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Gu J., Ma J., Chen X., Zhang M., Wang W. (2009). Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere 75, 963–968. [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., Jin Y. (2005). Neuronal differentiation in C. elegans. Curr. Opin. Cell Biol. 17, 682–689. 10.1016/j.ceb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Crump D., Chiu S., Kennedy S. W. (2012). Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol. Sci. 126, 140–148. [DOI] [PubMed] [Google Scholar]
- Dishaw L. V., Powers C. M., Ryde I. T., Roberts S. C., Seidler F. J., Slotkin T. A., Stapleton H. M. (2011). Is the PentaBDE replacement, tris (1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharmacol. 256, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff C., Crump D., Porter E., Williams K. L., Letcher R. J., Gauthier L. T., Kennedy S. W. (2014). Tris(2-butoxyethyl)phosphate and triethyl phosphate alter embryonic development, hepatic mRNA expression, thyroid hormone levels, and circulating bile acid concentrations in chicken embryos. Toxicol. Appl. Pharmacol. 279, 303–310. [DOI] [PubMed] [Google Scholar]
- Farhat A., Crump D., Chiu S., Williams K. L., Letcher R. J., Gauthier L. T., Kennedy S. W. (2013). In Ovo effects of two organophosphate flame retardants—TCPP and TDCPP—on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol. Sci. 134, 92–102. [DOI] [PubMed] [Google Scholar]
- Frederiksen M., Thomsen M., Vorkamp K., Knudsen L. E. (2009). Patterns and concentration levels of polybrominated diphenyl ethers (PBDEs) in placental tissue of women in Denmark. Chemosphere 76, 1464–1469. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B., Lacasana M., Aguilar-Garduno C., Rodriguez-Barranco M., Ballester F., Rebagliato M., Hernandez A. F. (2014). A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230, 104–121. [DOI] [PubMed] [Google Scholar]
- Hakk H., Letcher R. J. (2003). Metabolism in the toxicokinetics and fate of brominated flame retardants–a review. Environ. Int. 29, 801–828. [DOI] [PubMed] [Google Scholar]
- Hanari N., Kannan K., Okazawa T., Kodavanti P. R. S., Aldous K. M., Yamashita N. (2006). Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environ. Sci. Technol. 40, 4400–4405. [DOI] [PubMed] [Google Scholar]
- Harada H., Kurauchi M., Hayashi R., Eki T. (2007). Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol. Environ. Saf. 66, 378–383. [DOI] [PubMed] [Google Scholar]
- Harley K. G., Marks A. R., Chevrier J., Bradman A., Sjodin A., Eskenazi B. (2010). PBDE concentrations in women's serum and fecundability. Environ. Health Perspect. 118, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks H. S., Meijer M., Muilwijk M., van den Berg M., Westerink R. H. (2014). A comparison of the in vitro cyto- and neurotoxicity of brominated and halogen-free flame retardants: prioritization in search for safe(r) alternatives. Arch. Toxicol. 88, 857–869. [DOI] [PubMed] [Google Scholar]
- Herbstman J. B., Sjodin A., Kurzon M., Lederman S. A., Jones R. S., Rauh V., Needham L. L., Tang D., Niedzwiecki M., Wang R. Y., and., et al. (2010). Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect. 118, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. (2005). Specification of the nervous system. WormBook, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K., Fang M., Horman B., Patisaul H. B., Garantziotis S., Birnbaum L. S., Stapleton H. M. (2014). Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ. Health Perspect. 122, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoss S., Weltje L. (2007). Endocrine disruption in nematodes: effects and mechanisms. Ecotoxicology 16, 15–28. [DOI] [PubMed] [Google Scholar]
- Hsieh J. H., Sedykh A., Huang R., Xia M., Tice R. R. (2015). A data analysis pipeline accounting for artifacts in Tox21 quantitative high-throughput screening assays. J. Biomol. Screen. 20, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Xia M., Sakamuru S., Zhao J., Shahane S. A., Attene-Ramos M., Zhao T., Austin C. P., Simeonov A. (2016). Modelling the Tox21 10 K chemical profiles for in vivo toxicity prediction and mechanism characterization. Nat. Commun. 7, 10425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarema K. A., Hunter D. L., Shaffer R. M., Behl M., Padilla S. (2015). Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 52, 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, et al. (2010). In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ. Health Perspect. 118:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna N., Cressman C. P., 3rd, Tatara C. P., Williams P. L. (1997). Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch. Environ. Contam. Toxicol. 32, 110–114. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R., Coburn C. G., Moser V. C., MacPhail R. C., Fenton S. E., Stoker T. E., Rayner J. L., Kannan K., Birnbaum L. S. (2010). Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol. Sci. 116, 297–312. [DOI] [PubMed] [Google Scholar]
- Leung M. C., Williams P. L., Benedetto A., Au C., Helmcke K. J., Aschner M., Meyer J. N. (2008). Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol. Sci. 106, 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Su G., Zou M., Yu L., Letcher R. J., Yu H., Giesy J. P., Zhou B., Liu C. (2015). Effects of Tris(1,3-dichloro-2-propyl) phosphate on growth, reproduction, and gene transcription of daphnia magna at environmentally relevant concentrations. Environ. Sci. Technol. 49, 12975–12983. [DOI] [PubMed] [Google Scholar]
- Liu C., Su G., Giesy J. P, Letcher R. J., Li G., Agrawal I., Li J., Yu L., Wang J., Gong Z. (2016). Acute Exposure to Tris(1,3-dichloro-2-propyl) Phosphate (TDCIPP) Causes Hepatic Inflammation and Leads to Hepatotoxicity in Zebrafish. Sci. Rep. 6, 19045.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ji K., Jo A., Moon H. B., Choi K. (2013). Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol. 134–135, 104–111. [DOI] [PubMed] [Google Scholar]
- McGee S. P., Cooper E. M., Stapleton H. M., Volz D. C. (2012). Early Zebrafish embryogenesis is susceptible to developmental TDCPP exposure. Environ. Health Perspect. 120, 1585–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Cooper E. M., Stapleton H. M., Hauser R. (2013). Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 121, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Stapleton H. M. (2010). House dust concentrations of organophosphate flame retardants in relation to hormone levels and Semen quality parameters. Environ. Health Perspect. 118, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Sasakura H., Kuhara A. (2007). Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr. Opin. Neurobiol. 17, 712–719. [DOI] [PubMed] [Google Scholar]
- Munoz-Quezada M. T., Lucero B. A., Barr D. B., Steenland K., Levy K., Ryan P. B., Iglesias V., Alvarado S., Concha C., Rojas E., et al. (2013). Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology 39, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura A., Kamijima M., Ohtani K., Yamanoshita O., Nakamura D., Ito Y., Miyata M., Ueyama J., Suzuki T., Imai R., et al. (2009). Broken sperm, cytoplasmic droplets and reduced sperm motility are principal markers of decreased sperm quality due to organophosphorus pesticides in rats. J. Occup. Health 51, 478–487. [DOI] [PubMed] [Google Scholar]
- Rice J. R., Boyd W. A., Chandra D., Smith M. V., Den Besten P. K., Freedman J. H. (2014). Comparison of the toxicity of fluoridation compounds in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 33, 82–88. [DOI] [PubMed] [Google Scholar]
- Roegge C. S., Timofeeva O. A., Seidler F. J., Slotkin T. A., Levin E. D. (2008). Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res. Bull. 75, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreder E. D., Uding N., La Guardia M. J. (2016). Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere 150, 499–504. [DOI] [PubMed] [Google Scholar]
- Shaw S. D., Blum A., Weber R., Kannan K., Rich D., Lucas D., Koshland C. P., Dobraca D., Hanson S., Birnbaum L. S. (2010). Halogenated flame retardants: do the fire safety benefits justify the risks? Rev. Environ. Health 25, 261–305. [DOI] [PubMed] [Google Scholar]
- Silhankova M., Korswagen H. C. (2007). Migration of neuronal cells along the anterior–posterior body axis of C. elegans: Wnts are in control. Curr. Opin. Genet. Dev. 17, 320–325. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Levin E. D., Seidler F. J. (2006). Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 114, 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Dodder N. G., Offenberg J. H., Schantz M. M., Wise S. A. (2005). Polybrominated diphenyl ethers in house dust and clothes dryer lint. Environ. Sci. Technol. 39, 925–931. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Eagle S., Anthopolos R., Wolkin A., Miranda M. L. (2011). Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect. 119, 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Misenheimer J., Hoffman K., Webster T. F. (2014). Flame retardant associations between children's handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Sharma S., Getzinger G., Ferguson P. L., Gabriel M., Webster T. F., Blum A. (2012). Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker T. E., Laws S. C., Crofton K. M., Hedge J. M., Ferrell J. M., Cooper R. L. (2004). Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol. Sci. 78, 144–155. [DOI] [PubMed] [Google Scholar]
- Sundkvist A. M., Olofsson U., Haglund P. (2010). Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit 12, 943–951. [DOI] [PubMed] [Google Scholar]
- Suvorov A., Girard S., Lachapelle S., Abdelouahab N., Sebire G., Takser L. (2009). Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology 95, 203–209. [DOI] [PubMed] [Google Scholar]
- Tsang W. Y., Lemire B. D. (2002). Mitochondrial genome content is regulated during nematode development. Biochem. Biophys. Res. Commun. 291, 8–16. [DOI] [PubMed] [Google Scholar]
- Tsang W. Y., Sayles L. C., Grad L. I., Pilgrim D. B., Lemire B. D. (2001). Mitochondrial respiratory chain deficiency in Caenorhabditis elegans results in developmental arrest and increased life span. J. Biol. Chem. 276, 32240–32246. [DOI] [PubMed] [Google Scholar]
- van der Veen I., de Boer J. (2012). Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- Venerosi A., Ricceri L., Tait S., Calamandrei G. (2012). Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: the case of chlorpyrifos. Neurotoxicology 33, 1420–1426. [DOI] [PubMed] [Google Scholar]
- Wang J. Z., Hou Y., Zhang J., Zhu J., Feng Y. L. (2013). Transformation of 2,2′,4,4′-tetrabromodiphenyl ether under UV irradiation: potential sources of the secondary pollutants. J. Hazardous Mater. 263, Part 2, 778–783. [DOI] [PubMed] [Google Scholar]
- Weiner M. L., Jortner B. S. (1999). Organophosphate-induced delayed neurotoxicity of triarylphosphates. Neurotoxicology 20, 653–673. [PubMed] [Google Scholar]
- Williams P. L., Dusenbery D. B. (1988). Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health 4, 469–478. [DOI] [PubMed] [Google Scholar]
- Wu S., Ji G., Liu J., Zhang S., Gong Y., Shi L. (2015). TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and zebrafish larvae (Danio rerio). Environ. Toxicol. 31, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Zhang S., Bursian S. J., Martin P. A., Chan H. M., Tomy G., Palace V. P., Mayne G. J., Martin J. W. (2009). Reproductive and developmental toxicity of a pentabrominated diphenyl ether mixture, DE-71, to ranch mink (Mustela vison) and hazard assessment for wild mink in the Great Lakes region. Toxicol. Sci 110, 107–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.