Abstract
Sulfur mustard (bis 2-chloroethyl ethyl sulfide, SM) is a powerful bi-functional vesicating chemical warfare agent. SM tissue injury is partially mediated by the overproduction of reactive oxygen species resulting in oxidative stress. We hypothesized that using a catalytic antioxidant (AEOL 10150) to alleviate oxidative stress and secondary inflammation following exposure to SM would attenuate the toxic effects of SM inhalation. Adult male rats were intubated and exposed to SM (1.4 mg/kg), a dose that produces an LD50 at approximately 24 h. Rats were randomized and treated via subcutaneous injection with either sterile PBS or AEOL 10150 (5 mg/kg, sc, every 4 h) beginning 1 h post-SM exposure. Rats were euthanized between 6 and 48 h after exposure to SM and survival and markers of injury were determined. Catalytic antioxidant treatment improved survival after SM inhalation in a dose-dependent manner, up to 52% over SM PBS at 48 h post-exposure. This improvement was sustained for at least 72 h after SM exposure when treatments were stopped after 48 h. Non-invasive monitoring throughout the duration of the studies also revealed blood oxygen saturations were improved by 10% and clinical scores were reduced by 57% after SM exposure in the catalytic antioxidant treatment group. Tissue analysis showed catalytic antioxidant therapy was able to decrease airway cast formation by 69% at 48 h post-exposure. To investigate antioxidant induced changes at the peak of injury, several biomarkers of oxidative stress and inflammation were evaluated at 24 h post-exposure. AEOL 10150 attenuated SM-mediated lung lipid oxidation, nitrosative stress and many proinflammatory cytokines. The findings indicate that catalytic antioxidants may be useful medical countermeasure against inhaled SM exposure.
Keywords: sulfur mustard, chemical weapons, antioxidant.
Sulfur mustard (SM) is a chemical warfare agent possessing strong alkylating properties that induces inflammation and tissue necrosis. SM exposure produces delayed and highly incapacitating injuries that can be lethal (Anderson, 2015). SM was used during World War I and is still maintained in the chemical weapons arsenals of several countries (Kehe and Szinicz, 2005). A specific and effective antidote is not available for use after SM exposure despite research conducted for nearly 100 years (Kehe and Szinicz, 2005). The population most at risk for exposure to SM is comprised of soldiers and individuals working or living near storage depots, but SM is also considered to be a potential terrorist threat due to its low cost of production and simple synthesis.
Sulfur mustard (SM) vapor adversely affects the skin, eyes, and the respiratory tract. The respiratory tract is highly susceptible to SM toxicity, with the majority of historical mortality being attributed to pulmonary injury (Keyser et al., 2014). Inhaled SM may cause death or the development of severe chronic airway disorders in survivors (Poursaleh et al., 2012). Inhaled SM causes acute sloughing of the epithelial surfaces, formation of occlusive casts, and can lead to chronic respiratory problems such as bronchiolitis obliterans and COPD (Ghanei and Harandi, 2011; Veress et al., 2013). A hindrance in developing treatments for SM inhalation is due to our poor understanding of its pathogenesis. Previous studies suggest that SM induces oxidative stress, inflammation, apoptosis, DNA damage, and airway coagulation that are all known to play a role in damage progression (Keyser et al., 2014). Many therapeutics targeting these pathways have been tested and have shown some promise in in vitro models (Weinberger et al., 2011). Replication of therapeutic success in most physiologically relevant in vivo models has been less successful. Expanding our knowledge of the mechanism through which SM exerts its toxic effects is critical for further development of rescue therapies.
A major component of SM toxicity is oxidative stress. The oxidative stress observed in SM toxicity is the result of 3 major pathways contributing to the disturbance in redox balance. These are the direct depletion of cellular thiols such as glutathione (GSH), alkylation or oxidation of DNA strands, and lipid peroxidation (Dacre and Goldman, 1996; Debiak et al., 2009; Inturi et al., 2011). DNA damage is considered to be an important aspect of SM toxicity and has been noted in all models of SM injury. It is known that DNA can be directly alkylated by SM or its analogs on guanine residues, and bi-functional sulfur mustard is capable of cross linking DNA strands (Dacre and Goldman, 1996). One theory of SM toxicity holds that cellular attempts to repair these DNA adducts/cross-linked strands results in the over activation of poly(adenosine diphosphate ribose) polymerase (PARP) leading to rapid NAD+/ATP depletion leading to cell death (Korkmaz et al., 2006). Another theory prevalent in the literature is that inducible nitric oxide synthase (iNOS) is activated by mustard agent exposures leading to increased formation of intracellular peroxynitrite (ONOO−) which can damage lipids, proteins, and DNA (Sunil et al., 2012). Oxidative stress mechanisms may also reinforce tissue inflammation in the progression of SM induced toxicity.
AEOL 10150 is a metalloporphyrin class of catalytic antioxidants that has shown promising therapeutic potential for suppressing oxidative stress in relevant SM analog, 2-chloroethyl ethyl sulfide (CEES) models (Gould et al., 2009; O'Neill et al., 2010, 2011; Tewari-Singh et al., 2014). The aim of this study is to characterize the efficacy of this catalytic antioxidant in improving survival by attenuating the progression of oxidative stress, inflammation, and injury following SM inhalation. AEOL 10150 when given after SM inhalation improved survival, blood oxygenation, and clinical scores while diminishing markers of oxidative stress and inflammation.
MATERIALS AND METHODS
Chemicals
The sulfur mustard agent used in these exposures was provided by the U.S. Army Edgewood Research, Development and Engineering Center (Aberdeen Proving Ground, Maryland) and was military grade. The catalytic antioxidant AEOL 10150 (Mn(III) tetrakis(N,N'-diethylimidazolium-2-yl) porphyrin) was administered subcutaneously as a 5 mg/ml solution in sterile water for injection and was greater than 98% purity (Aeolus Pharmaceuticals, Mission Viejo, California). Unless otherwise stated, all other chemicals used were from purchased from Sigma-Aldrich (St. Louis, Missouri).
Animals and exposures
The Institutional Animal Care and Use Committee (IACUC) of United States Army Medical Research Institute of Chemical Defense (USAMRICD) approved these studies. Adult male Sprague Dawley rats (200–300 g) from Charles River Laboratories (Wilmington, Massachusetts) were used. Animals were exposed to SM or vehicle (ethanol, EtOH) at the USAMRICD Aberdeen Proving Ground using a vapor generator based model as previously reported (Anderson et al., 1996, 2000, 2009;; Gao et al., 2011; Malaviya et al., 2010; Veress et al., 2015). Rats were anesthetized with a combination of ketamine (80 mg/kg, IM) and xylazine (10 mg/kg, IM). Anesthetized rats were intubated with a polished glass Pasteur pipette and a PE90 guide tube. A glass endotracheal tube was used to minimize absorption of SM. SM (0.35 mg) in absolute ethanol (100 µl) was placed in a water-jacketed (37 °C) glass vapor generator (Atmar Glass, Kennett Square, Pennsylvania). The length of the glass endotracheal tube (∼5 cm) was determined based on length between the larynx and the bifurcation of the trachea. Spontaneously breathing anesthetized rats were connected to these devices and exposed accordingly for 50 min. By the end of the exposure period, the SM in ethanol was completely vaporized and inhaled. This passive exposure system includes an inlet one-way respiratory check valve (Hans Rudolph, Kansas City, Missouri) to ensure that the only source of air for the animal during the exposure was through the vapor generator. Exhaled air passes through a two-way non-rebreathing Rudolph valve and then through a charcoal-filtered bleach trap to decontaminate any exhaled SM. At the conclusion of the 50-min exposure, the rats were disconnected from the vapor generator, the endotracheal tube was removed, and the rats were returned to their cage for recovery. A limitation of this exposure system is the inability to monitor SM vapor concentrations over the 50-min exposure period.
Animals were placed in the following treatment groups for the 48 h catalytic antioxidant dose-finding studies; SM/Phosphate Buffered Saline (PBS) (n = 67), SM/10150 every (q) 4 h (n = 32), SM/10150 q6 hours (n = 12), SM/10150 q8 hours (n = 25), EtOH/PBS (n = 6), EtOH/10150 (n = 6). Animals were placed in the following treatment groups for the 72-h study; SM/PBS (n = 13) and SM/10150 q4 hours (n = 14). Animals were placed in the following treatment groups for 24 h studies (lungs frozen/lavage fluid collected); SM/10150 (n = 7), SM/PBS (n = 8). Animals were placed in the following treatment groups for airway cast scoring (lungs fixed) over time 6 h SM/PBS (n = 5), 6 h SM/10150 (n = 5), 12 h SM/PBS (n = 5), 12 h SM/10150 (n = 5), 18 h SM/PBS (n = 6), 18 h SM/10150 (n = 6), 24 h SM/PBS (n = 6), 24 h SM/10150 (n = 7). SM/PBS groups (q4 hours, q6 hours, and q8 hours) in 48 h studies were determined to not differ statistically and were pooled for presentation. Survival times were measured for each animal as the time from the exposure apparatus to euthanasia. To meet euthanasia criteria, an animal required an oxygen saturation lower than 70% and clinical score greater than 7, as previously described (Veress et al., 2013). For euthanasia, a cocktail of ketamine (75 mg/kg)/xylazine (7.5 mg/kg)/acepromazine (1.5 mg/kg) was administered by intraperitoneal (IP) injection. Once the animal was toe-pinch unresponsive, exsanguination and collection of blood for complete blood count (CBC) measurements preceded lung lavage or fixation. Lavage was performed with two 5 mL washes of a PBS solution containing 0.03% bovine serum albumin (BSA). Collected lavage fluid was centrifuged at 2000 g for 8 min; aliquots of supernatants were frozen at −80°C until use. Whole lung tissues were perfused with heparinized saline delivered into the pulmonary artery, after which lungs were collected and snap frozen. Left lung lobes were pulverized in liquid nitrogen for assays requiring lung tissue.
Pulse oximetry and clinical scores
A pulse oximeter (Starr Life Sciences, Oakmont, Pennsylvania) was used to collect heart rates (HR) and oxygen saturation (POx) data from animals prior to SM exposure and immediately prior to each catalytic antioxidant injection. HR and POx values represent the combined mean of 3 running average measurements recorded by the monitoring software (Starr Life Sciences). Clinical Scores (CS) were recorded concurrently with each of the heart rate and pulse oximetry measurements as a subjective determination of distress. Respiratory quality was scored on a scale of 0–6, and activity on a scale of 0–3 using a previously described method (Veress et al., 2013). CS represents the summation of both respiratory quality and activity scores where greater distress is indicated by a higher CS score. The highest score possible in a live animal is 9, whereas a clinical score of 10 is assigned to dead animals.
Oxidative and nitrosative stress measurements
To quantify DNA oxidation, DNA was extracted from 25 mg of pulverized left lung tissue using DNeasy Blood and Tissue Kit (QIAGEN, Valencia, California). DNA yield was determined using a NanoDrop Spectrophotometer ND1000 (Thermo Fisher Scientific, Waltham, Massachusetts), digested using 4U of nuclease P1, then 4U of alkaline phosphatase. Samples were analyzed for 8-hydroxy-2-deoxyguanosine (8OHdG) and 2-deoxyguanosine (2dG) using HPLC-UV/EC (CoulArray Model 5600; ESA, Chelmsford, Massachusetts) as previously reported (O'Neill et al., 2010). Lipid oxidation levels in lung tissues were quantified by analysis of 4-hydroxynonenal (4-HNE) by GC/MS as previously described (O'Neill et al., 2010). 4-HNE was extracted from 150 mg of pulverized lung tissue, spiked with 4-hydroxynonenal-d3 as an internal standard, and derivatized for analysis using GC/MS. Nitrosative stress assessments were made using the total nitrate/nitrite kit (Cayman Chemical, Cat 780001, Ann Arbor, Michigan). Nitrate was converted to nitrite using nitrate reductase and then Greiss Reagent was used to quantify total nitrate/nitrite concentrations in BALF samples spectrophotometrically at 540 nm. Glutathione (GSH) concentrations were measured in lung tissue homogenized in 0.1 N perchloric acid. Samples were then quantified using HPLC-EC as previously described (Chandler et al., 2013).
Cytokine quantification
Analysis of proinflammatory cytokines in BALF samples was performed with the Proinflammatory Panel (2) rat V-Plex Multi-Spot Assay System (Meso Scale Discovery, Rockville, Maryland). The electrochemiluminescent array was used to simultaneously analyze each proinflammatory cytokine. An ELISA assay was used for analysis of TFGβ1 in BALF (Elisa Tech, LLC, Aurora, Colorado). Briefly, 100 µl of BALF sample was acid activated with 4 µl of 1 N HCl, allowed to incubate at room temperature for 10 min, then returned to pH 7.2 with the addition of 4 µl 1.2 N NaOH. Samples were then analyzed for TGFβ1 using conventional ELISA methods. Multiplex and conventional ELISA assays were performed according to manufacturer’s protocols and using supplied reagents. Sample values appearing below the lower limit of detection for each assay were considered to be 0 pg/ml.
Hematology
Blood samples were collected at euthanasia for analysis of complete blood cell count (CBC). An aliquot of whole blood was transferred to a CBC collection tube containing EDTA. Samples were kept on a rocker until tested using a hematology analyzer (Cell Dyne 3500, Abbott Diagnostics, Santa Clara, California).
Airway cast scoring
Upon euthanasia, tracheas were cannulated using 15 gauge Luer stub adapters and the lungs were inflation-fixed at 20 cm H2O with 4% paraformaldehyde for 30 min. After this time the cannula was removed and the trachea tied closed. Lungs were stored in specimen containers filled with 4% paraformaldehyde until microdissection. Lungs were micro-dissected under a light microscope and major airways were assigned cast scores as previously described (Veress et al., 2015).
Statistical analysis
All statistical analyses were carried out with Graphpad Prism Version 7.0 software (La Jolla, California). Values are expressed as mean ± standard error of the mean. Log-rank (Mantel-Cox) tests were performed to compare survival groups. Two-way ANOVA with multiple comparisons were performed to determine differences between groups, where applicable. Dunnetts’ post-test was performed to determine differences between groups over time and corrected for multiple comparisons. For 24 h analysis of oxidative stress or cytokine markers, two-way ANOVA with Newman–Keuls post-test was used to compare each group and corrected for multiple comparisons (P < .05). Data is presented as mean ± SEM. Statistical tests performed are described within each figure legend.
RESULTS
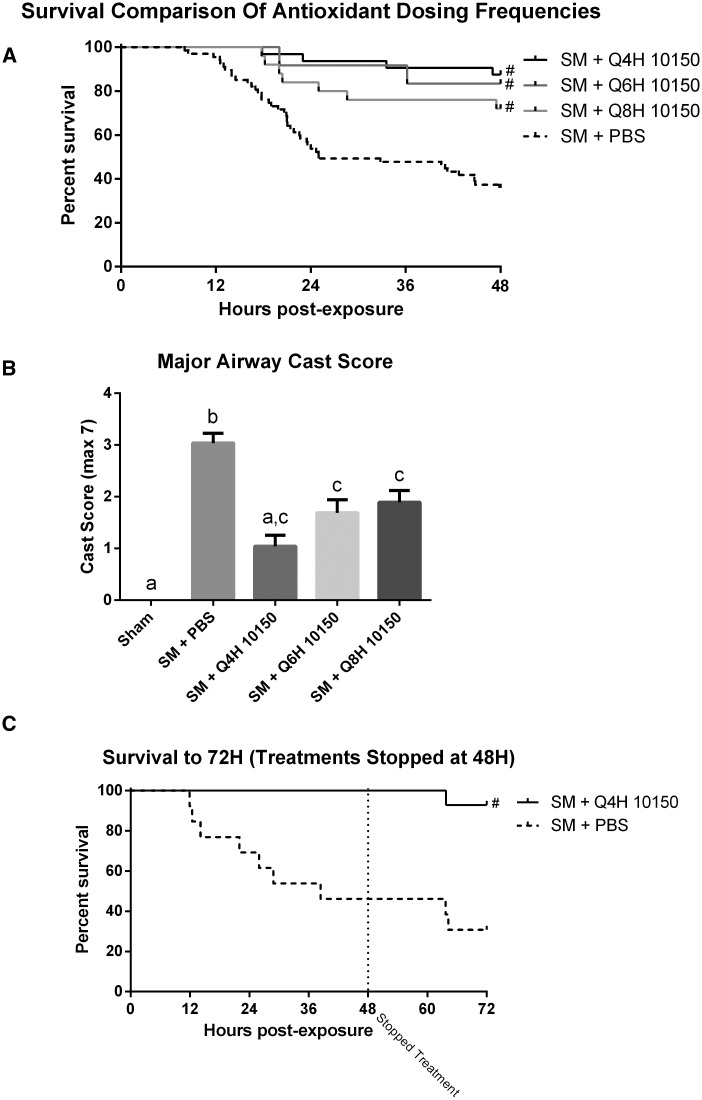
Sulfur mustard (SM) inhalation (1.4 mg/kg) resulted in an LD50 at approximately 24 h post-exposure. Most of the animal deaths due to SM exposure occurred between 12 and 24 h post-exposure. Animals receiving subcutaneous injections of catalytic antioxidant (5 mg/kg) 1-h post-SM exposure displayed greatly improved survival over animals receiving an equivalent volume of sterile PBS (Fig. 1A). The catalytic antioxidant rescue effect was dose-dependent, with the q4 hours dosing regimen (30 mg/kg/day) showing the greatest improvement in survival as compared with the q8 hours dosing regimen (15 mg/kg/day). The catalytic antioxidant regimens improved survival at 48 h from 36% in the SM PBS group to 72–88% in the catalytic antioxidant treatment groups. Fixed lung tissue was also collected at euthanasia and airway casts were scored. The catalytic antioxidant treatments caused a decrease in airway cast formation in a dose-dependent manner (Fig. 1B) that mirrored observed survival effects. To examine the effects of catalytic antioxidant cessation following SM inhalation, an additional experiment with all treatments stopped at 48 h following exposure was performed. Continued monitoring of animals out to 72 h revealed a sustained protective effect in the q4 hours catalytic antioxidant treatment group (Fig. 1C).
FIG. 1.
Effect of catalytic antioxidant treatment on acute SM survival and airway cast formation. Animals exposed to 1.4 mg/kg SM were treated with either sterile PBS or 5 mg/kg of AEOL 10150 subcutaneously. Injections began 1 h after animals were disconnected from the exposure apparatus, and repeated every 4, 6, or 8 h, with control (PBS injected) animal data pooled for comparison (median SM PBS survival time was 25 h) (A). At euthanasia, lungs were fixed to quantify airway obstruction (B). Major airway cast scores are presented as mean ± SEM. One-way ANOVA with Newman–Keuls post-test was performed for statistical analysis; significant differences are represented by different letters. To observe the effects of treatment cessation at 48 h post-exposure, animals were treated with the most efficacious dose of antioxidant (5 mg/kg, q4 hours) or PBS (q4 hours) for 48 h, then monitored for an additional 24 h (C) without any treatments (median SM PBS survival time was 38.4 h). Log-rank (Mantel-Cox) tests were performed to compare survival groups, # Significant difference from SM + PBS. The median survival for each 150 treatment group was >48 h (A), and >72 h (C). (A) SM PBS n = 67, SM 10150 q4 hours n = 32, SM 10150 q6 hours n = 12, SM 10150 q8 hours n = 25. (B) Sham n = 12, SM PBS n = 67, SM 10150 q4 hours n = 32, SM 10150 q6 hours n = 12, SM 10150 q8 hours n = 25. (C) SM PBS n = 13, SM 10150 n = 14.
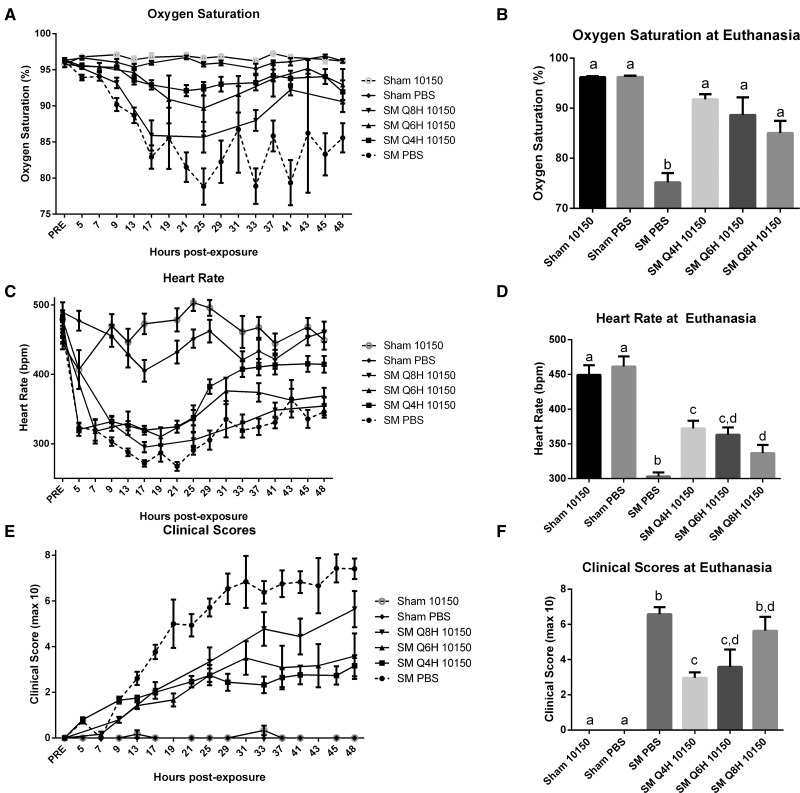
Sham and SM exposed rats were monitored non-invasively for changes in cardiopulmonary function by assessing blood oxygenation using pulse oximetry, heart rate, and clinical scoring every 4, 6, or 8 h, just before dosing. Clinical score assessments took into consideration animal activity levels and difficulty in breathing assessments where a higher number correlates to worsening clinical outcome. SM-mediated decline in pulse oximetry, heart rate, and clinical score assessments illustrate progressively deteriorating arterial oxygen saturations and vitality after SM inhalation. The SM/PBS group displayed reduced oxygen saturations, heart rates and clinical score assessments beginning shortly after exposure and remaining altered for the duration of the study compared with sham exposed animals (Fig. 2A, C, and E). Variations in data collection times and animal deaths did not allow for direct comparison of each treatment group over time. Instead, comparison of oxygen saturation, heart rate, and clinical score values are presented by their final measured value at the end of the study (or at euthanasia if animals did not survive until the end of the study). Each marker showed significant damage in SM PBS groups which was rescued with antioxidant treatments in a dose-dependent manner (Fig. 2B, D, and F).
FIG. 2.
Effect of catalytic antioxidant treatment on SM-mediated changes in physiologic and clinical outcomes. Animals exposed to 1.4 mg/kg SM or EtOH vehicle (Sham) and treated with either q4 hours AEOL 10150 or q4 hours PBS. Data from sham exposed animals treated with either PBS or 10150 were pooled for comparison. Prior to exposure (PRE) and immediately prior to each injection, animals were tested for oxygen saturation (A) and heart rates (B) using a MouseOx pulse oximeter. Each animal was also scored at these time points for respiratory quality and activity levels, the combination of which is presented as clinical scores (C). Varying treatment times and animal mortality precludes direct comparisons between groups over time (A, C, E). Statistical analysis of oxygen saturations, heart rates, and clinical scores were performed on data collected at euthanasia (B, D, F). Data is presented as mean ± SEM. Statistical comparisons were performed by one-way ANOVA with Newman–Keuls post-test corrected for multiple comparisons, with significant differences between groups represented as different letters (P < .05). (A–F) Sham 10150 n = 6, Sham PBS n = 6, SM PBS n = 67, SM 10150 q4 hours n = 32, SM 10150 q6 hours n = 12, SM 10150 q8 hours n = 25.
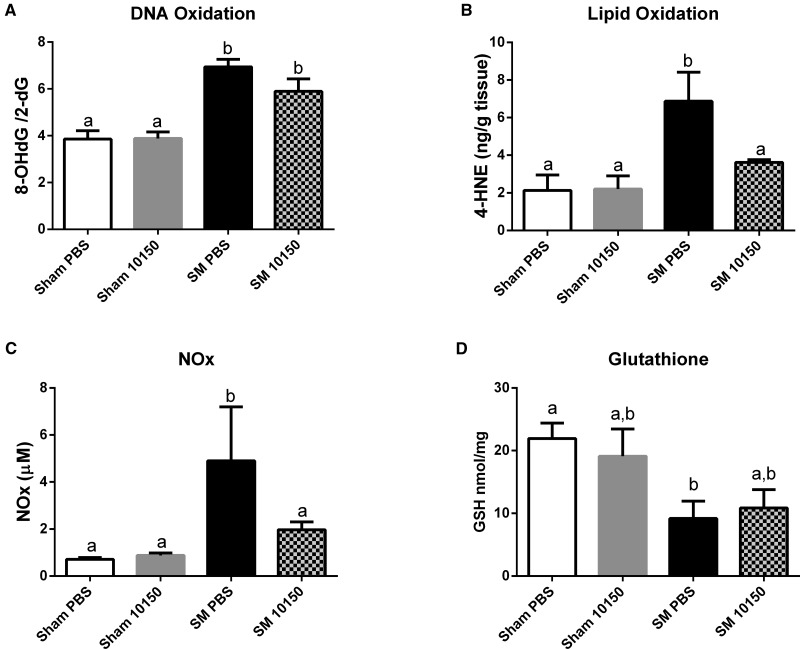
Oxidative DNA damage was measured using the ratio of 8-oxo-2-deoxyguanosine (8OHdG) to 2-deoxyguanosine (2dG) as a marker of oxidative stress in this model. Oxidative DNA damage was increased 75% from sham values at 24 h post-exposure to SM (Fig. 3A). The catalytic antioxidant treatment group showed a small decrease in DNA damage compared with the SM group, but this did not reach statistical significance. Lipid peroxidation was measured at 24 h post-exposure by analyzing 4-HNE levels in lung tissue. SM exposure increased lung lipid oxidation 3-fold and antioxidant administration was able to reduce SM’s effect on this biomarker back to sham exposed levels (Fig. 3B). Nitrosative stress can be monitored by measuring changes in the nitrate and nitrite levels which are a surrogate measure for nitric oxide production. Lung nitrosative stress was also observed to increase 3-fold 24 h after SM exposure (Figure 3C). The catalytic antioxidant treatment greatly attenuated the SM-mediated increase in nitrosative stress seen at 24 h. Lung glutathione (GSH) levels were assessed by HPLC as an additional indicator of oxidative stress. SM exposures significantly diminished GSH lung levels by 55%, which were not significantly improved with antioxidant treatment (Fig. 3D).
FIG. 3.
Effect of catalytic antioxidant treatment on SM-mediated increase in oxidative/nitrosative stress biomarkers. Animals exposed to 1.4 mg/kg SM or EtOH vehicle (Sham) and treated with either q4 hours AEOL 10150 or q4 hours PBS. Groups of animals were euthanized 24 h post-SM exposure. Pulverized left lung tissue samples were analyzed using HPLC for DNA oxidation (A). Lipid peroxidation was analyzed in lung tissue homogenate by measuring 4-HNE by GC/MS (B). BALF samples were analyzed for Total NO2/NO3 levels using a plate reader assay (C). Reduced glutathione concentrations were measured in lung tissue by HPLC. Data is presented as mean ± SEM. Two-way ANOVA with Newman–Keuls post-test corrected for multiple comparisons was performed to determine differences between groups, bars with different letters are significantly different from one another (P < .05). (A–D) Sham PBS n = 17, Sham 10150 n = 19, SM PBS n = 8, SM 10150 n = 7.
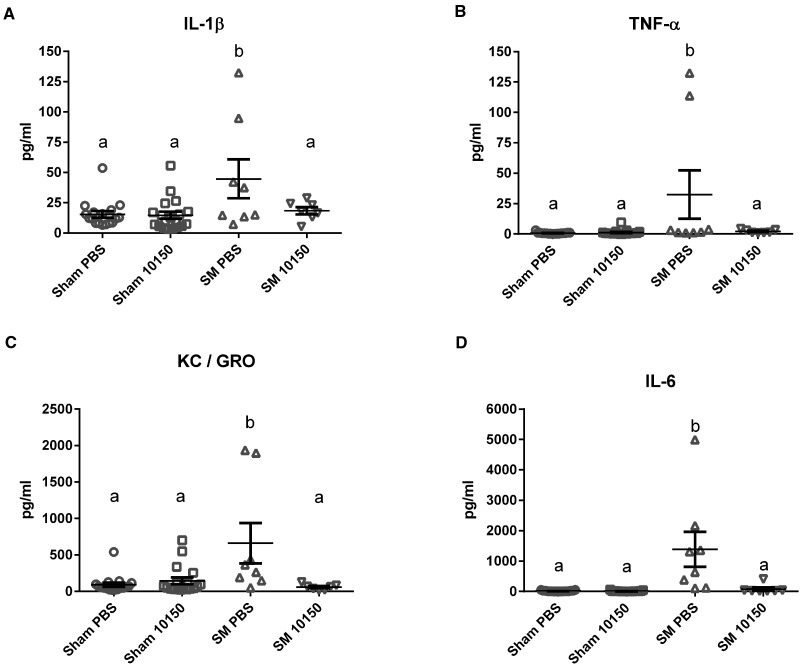
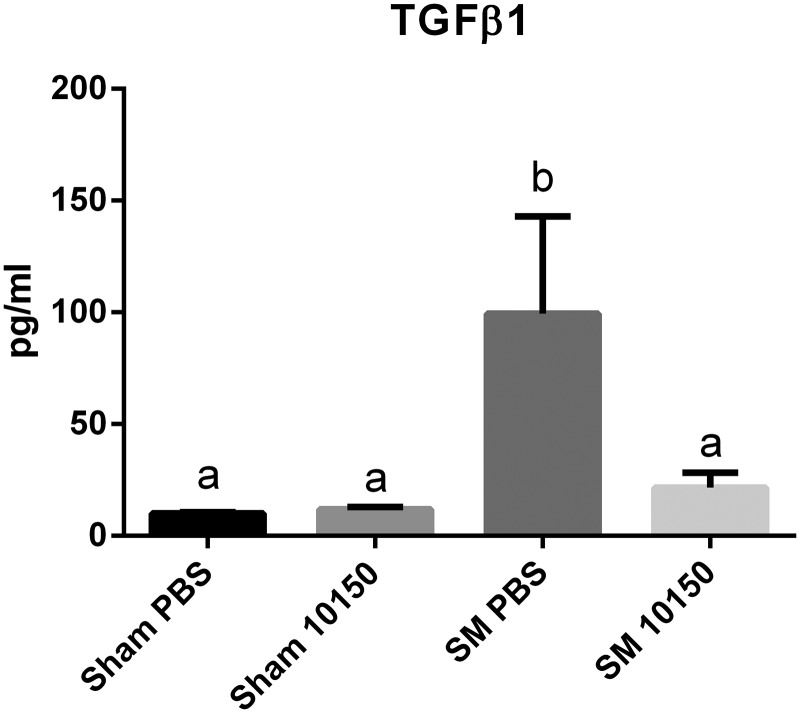
Sulfur mustard (SM)-mediated lung inflammation was assessed by measuring a panel of cytokines in bronchoalveolar lavage fluid (BALF) 24 h after SM inhalation. The inflammatory cytokine panel included IFN-ɣ, IL-10, IL-13, IL-1β, IL-4, IL-5, IL-6, KC/GRO and TNF-α. SM inhalation resulted in significant elevation of TNF-α, IL-6, KC/GRO, and IL-1β levels in BALF 24 h after exposure compared with sham exposed groups (Fig. 4A–D). The other cytokines measured in this array were not significantly affected at 24 h by SM exposures (data not shown). The catalytic antioxidant treatment significantly attenuated SM-mediated increases in TNF-α, IL-6, KC/GRO, and IL-1β at 24 h. Transforming growth factor β1 (TGFβ1) is commonly associated with lung injury and repair responses (Liu, 2008; Tatler and Jenkins, 2012). TGFβ1 has been implicated in the development of pulmonary fibrosis in chronic models of SM exposure (Aghanouri et al., 2004; Emad and Emad, 2007; Ghanei and Harandi, 2011; Poursaleh et al., 2012). SM exposure significantly increased BALF TGFβ1 levels nearly 10-fold over Sham PBS at 24 h post-exposure (Fig. 5). The catalytic antioxidant treatment group significantly abolished SM-mediated increase in TGFβ1 BALF levels.
FIG. 4.
Effect of catalytic antioxidant treatment on SM-mediated increase in bronchoalveolar lavage fluid (BALF) proinflammatory cytokine levels. BALF samples collected 24 h post-exposure were analyzed for inflammatory cytokines using a proinflammatory panel. Levels of IL-1β (A), TNF-α (B), KC/GRO (C), and IL-6 (D) were assessed using a multiplex array. Two-way ANOVA with multiple comparisons were performed to determine differences between groups. Data is presented as mean ± SEM. Two-way ANOVA with Newman–Keuls post-test corrected for multiple comparisons was performed to determine differences between groups, symbols with different letters are significantly different from one another (P < .05). (A–D) Sham PBS n = 17, Sham 10150 n = 19, SM PBS n = 8, SM 10150 n = 7.
Fig. 5.
Effect of catalytic antioxidant treatment on SM-mediated increase in bronchoalveolar lavage fluid (BALF) TGFβ1 levels. Concentrations of the pro-fibrotic growth factor TGFβ1 were quantified in BALF samples at 24 h post-exposure to SM using conventional ELISA. Data is presented as mean ± SEM. Two-way ANOVA with Newman–Keuls post-test corrected for multiple comparisons was performed to determine differences between groups, bars with different letters are significantly different from one another (P < .05). Sham PBS n = 17, Sham 10150 n = 19, SM PBS n = 8, SM 10150 n = 7.
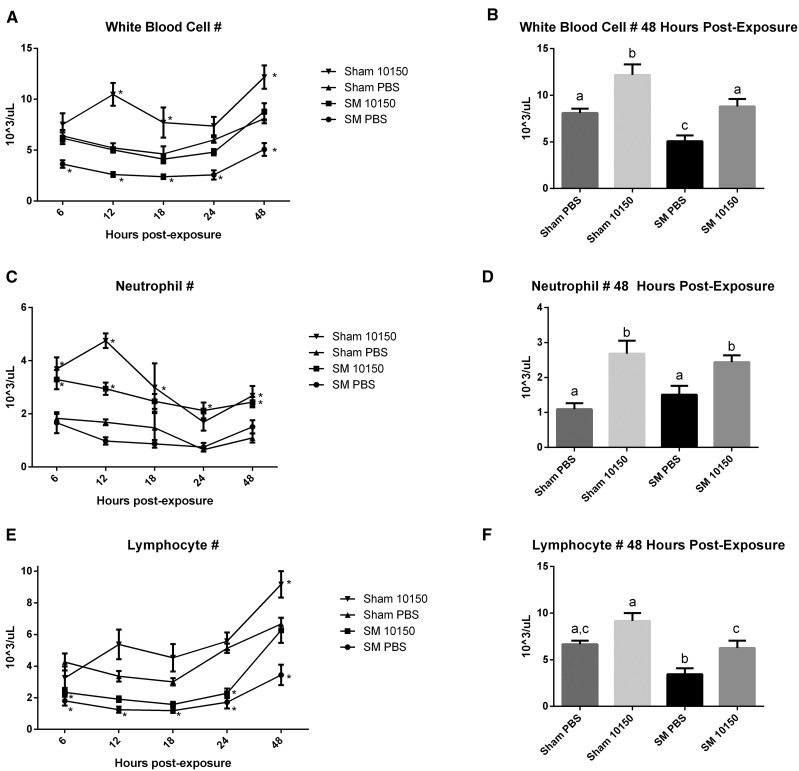
Sulfur mustard (SM) exposure produces a well-recognized suppressive effect on circulating white blood cells. Analysis of circulating white blood cells using a hematology analyzer revealed a prolonged and pronounced leukopenia by SM exposure (Fig. 6A). This effect was evident as early as 6 h after SM exposure and remained depressed out to 48 h. The catalytic antioxidant treatment blunted this effect over the observed time course. Comparison of white blood cell (WBC) counts at 48 h post-SM exposure showed that antioxidant treatment restored WBCs back to control levels. Differential cell counts showed that the catalytic antioxidant caused increases in neutrophils at several time points after exposure (Fig. 6C and D). Circulating lymphocytes were found to be significantly suppressed by SM exposure over the 48-h time course, but antioxidant treatment improved lymphocyte counts (Fig. 6E and F).
FIG. 6.
Effect of catalytic antioxidant treatment on SM-mediated leukopenia. Whole blood was analyzed using a hematology analyzer for WBC levels. WBC counts in whole blood samples were measured at euthanasia. White blood cell counts over time are presented in panels A, C, and E and were analyzed by two-way ANOVA with Dunnetts’ post-test, comparing each group over time to Sham PBS *Significantly differs from sham exposed. Total WBC, neutrophil, and lymphocyte counts collected at euthanasia from 48 h experiments are presented in panels B, D, and F. Two-way ANOVA with Newman–Keuls multiple comparisons test corrected for multiple comparisons were performed to determine differences between these groups, different letters represent significant differences (P < .05). (A–F) Sham PBS/Sham 10150 n = 5/time point, SM PBS 6 h n = 15, SM PBS 12 h n = 14, SM PBS 18 h n = 17, SM PBS 24 h n = 13, SM PBS 48 h n = 13, SM 10150 6 h n = 14, SM 10150 12 h n = 14, SM 10150 18 h n = 16, SM 10150 24 h n = 11, SM 10150 24 h n = 13.
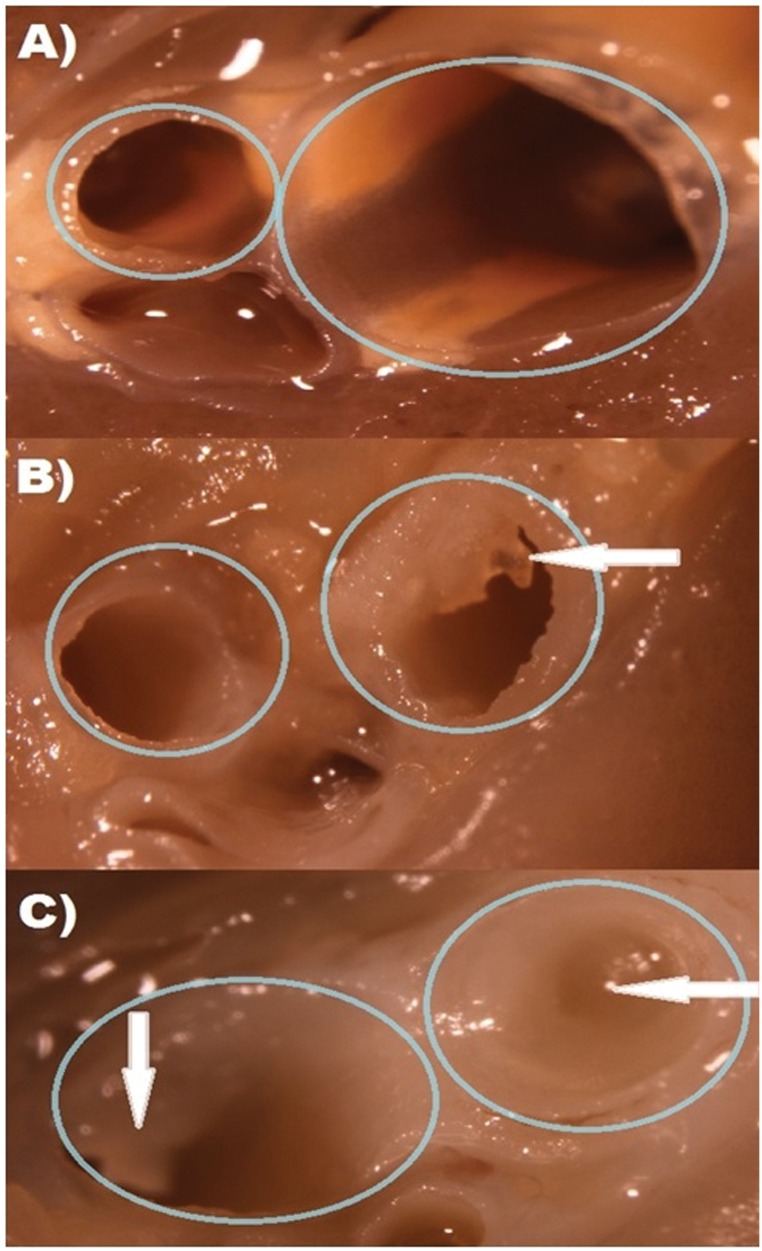
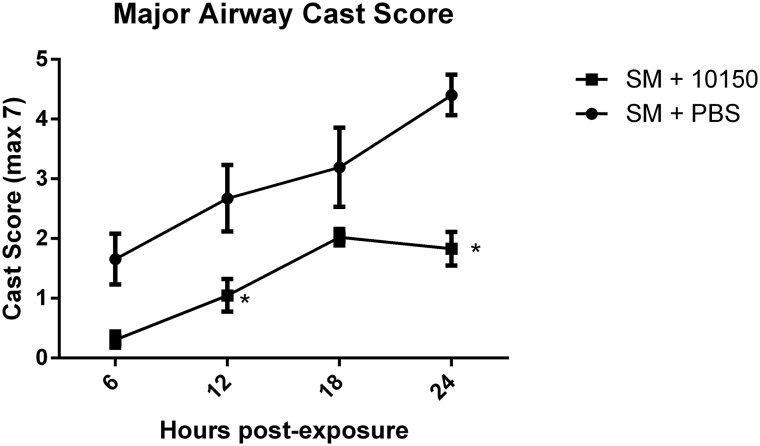
Fixed rat lung tissue was micro-dissected under a light microscope for visual assessment and scoring of cast occlusion in major airways. SM-mediated airway occlusions were scored for percent occlusion as previously described (Veress et al. 2015). Airway casts were clearly visible in animals exposed to SM compared with sham treatment group and were diminished in the catalytic antioxidant treatment group (Fig. 7). SM-mediated increases in airway cast scoring were significantly evident at 6 h after exposure and increased linearly over 24 h (Fig. 8A). Rats receiving catalytic antioxidant had much lower airway cast formation at 6 h than the SM group and cast progression was inhibited over time. Airway cast size was significantly decreased by 58% at the 24-h time point in the catalytic antioxidant treatment group.
FIG. 7.
Visual depiction of SM–mediated cast formation upon lung microdissection. Large airways were sectioned for visual inspection of airway cast formation. Airway sections from the left lung are shown above from representative treatment groups, depicting visible airway blockages. Sham exposed animals representing normal airways (A) are shown in comparison to SM + 10150 (B) and SM + PBS (C). Circles indicate airways and arrows identify pseudomembranes.
FIG. 8.
Effect of catalytic antioxidant treatment on SM-induced early airway cast formation. Following microdissection, airway sections were scored for percent occlusion, which was converted to a weighted score. Cast scores were evaluated at 6, 12, 18, and 24 h post-exposure to evaluate cast formation over time with or without (q4 hours) antioxidant administration. Data is presented as mean ± SEM. Two-way ANOVA with Bonferroni’s multiple comparisons test was performed to determine differences between groups over time where * indicates significantly different from SM +PBS (P < .05). SM PBS 6 h n = 5, SM PBS 12 h n = 5, SM PBS 18 h n = 6, SM PBS 24 h n = 6, SM 10150 6 h n = 5, SM 10150 12 h n = 5, SM 10150 18 h n = 6, SM 10150 24 h n = 7.
DISCUSSION
Many oxidative stress and inflammatory pathways have been associated with SM exposure and its resulting toxicity. Isolating a single mechanism of toxicity for SM is difficult because its high reactivity allows it to alkylate many cellular macromolecules that in turn can affect many biological processes. Many diverse therapies have been tested as countermeasures against SM toxicity including anti-inflammatory drugs, antioxidants, protease inhibitors, and anti-apoptotic compounds (Keyser et al., 2014; Tang and Loke, 2013; Weinberger et al., 2011). These treatments have been met with mixed results, and the search continues to find a feasible drug therapy for use in treating humans exposed to SM. One recurring theme in SM toxicity models is the presence of an oxidative stress component which may be responsible for propagating the deleterious effects of SM (Brimfield et al., 2012; Inturi et al., 2011; Jain et al., 2011). Many antioxidants have been evaluated against different models of SM toxicity with some beneficial effect in the treatment of SM toxicity (Anderson et al., 2000; Bobb et al., 2005; Korkmaz et al., 2008; Kumar et al., 2001). It is difficult to compare many of the results of these studies because they occur across many different models of SM exposure, time points, toxicity endpoints, and are often performed with surrogate SM agents such as 2-chloroethyl ethyl sulfide (CEES). The primary aim of these studies was to characterize the role played by oxidative stress in injury progression after SM inhalation and the potential of a catalytic antioxidant, AEOL 10150, as a medical countermeasure. AEOL 10150 is a manganese-containing porphyrin which functions as a catalytic antioxidant, capable of scavenging superoxide, hydrogen peroxide, peroxynitrite, and lipid peroxides (Kachadourian et al., 2004). Catalytic antioxidants can scavenge multiple reactive oxygen and nitrogen species without being consumed in the reaction (Day, 2004). This antioxidant has shown promising effects in other studies of SM induced toxicity in both in vitro and in vivo models (Gould et al., 2009; O'Neill et al., 2010, 2011; Tewari-Singh et al., 2014). This catalytic antioxidant has also displayed therapeutic benefits in other models of lung injury such as exposure to fractionated radiation and chlorine gas inhalation (McGovern et al., 2011; Rabbani et al., 2007).
In our model of SM inhalation, approximately 50% of the animals exposed to SM and administered PBS q4 hours did not survive to 24 h post-exposure. Administration of the catalytic antioxidant AEOL10150 beginning 1 h post-SM exposure produced a robust improvement in survival. The extent of survival improvement provided by antioxidant administration was dose-dependent and resulted in nearly complete protection against acute SM lethality at the highest catalytic antioxidant dosing regimen (q4 hours, 30 mg/kg/day). The catalytic antioxidant improvement in SM survival continued to 72 h even after treatment cessation at 48 h post-SM exposure. Inhaled SM has been recently shown to produce obstructive airways fibrin casts and these are believed responsible for acute deaths (Veress et al., 2010). Direct intratracheal administration of tissue plasminogen activator (tPA) has been previously shown to improve survival of animals after exposure to CEES and SM through the reduction of obstructive airway casts. Stoichiometric antioxidants including the water soluble vitamin E analog trolox, and the bioflavonoid quercetin have been shown to improve median survival times in mice exposed to SM (Kumar et al., 2001; Veress et al., 2013, 2015). This is the first published study to report significant improvements in survival using a catalytic antioxidant in a rat model of SM inhalation.
It is not known what the human exposures to SM have been because they have occurred on battlefields, during terrorist events, or due to accidental exposures. However, soldiers exposed to sulfur mustard on the battlefield experienced similar respiratory symptoms and airway casts as we observed in our rat studies at the 1.4mg/kg sulfur mustard dose (Khazdair et al., 2015). The sulfur mustard dose we used was identified previously as one that produces around an LD50 at 24 h post-exposure in our rat model. This was necessary to satisfy FDA requirements of preclinical survival efficacy under the animal rule. Any therapeutic that is intended to be developed and approved as a medical countermeasure for chemical agents needs to meet the requirements of the FDA animal rule. Under this rule it is important to not only show that the countermeasure can improve a significant outcome such as survival, but also improve function and quality of life (Jett, 2016). Diminished arterial oxygen saturations following SM exposure have previously been reported in this model system (Rancourt et al., 2013; Veress et al., 2015). In addition to the pulse oximetry for live-animal surveillance, each animal was assigned a clinical score at each time point. The clinical scoring parameters provide a quantitative assessment of respiratory distress and general activity levels. Following SM exposure, there was a marked increase in clinical scores over time in our model. Our continued monitoring of exposed animals throughout the experiment revealed that the catalytic antioxidant treatments were able to effectively suppress the SM induced deterioration of oxygen saturations, heart rates, and adverse clinical scores over time. Protective effects on these markers of injury have also been reported in tPA rescue from CEES and SM inhalation (Veress et al., 2015).
To measure oxidative and nitrosative stress conditions after SM inhalation, several biomarkers of oxidative stress were evaluated. These markers included DNA oxidation (8-OHdG), lipid peroxidation (4-HNE), reduced glutathione (GSH) concentrations, and total nitrate/nitrite levels (NOx). Our results indicate that each of these markers of oxidative/nitrosative stress was worsened at 24 h post-exposure to SM. Treatment with the catalytic antioxidant was able to significantly reduce oxidative and nitrosative stress at 24 h, as measured by 4-HNE and NOx levels, respectively. Antioxidant treated groups appeared to have lower oxidative DNA damage levels than SM PBS groups, but this effect was not significant, possibly due to the small sample size being evaluated. Although AEOL 10150 did not appear to improve GSH levels, this effect may be complicated by the formation of SM-GSH adducts which would not have been detected by the HPLC methods used here (Batal et al., 2015; Mol et al., 2008). Other publications investigating oxidative stress and vesicants have concluded that reductions in the measured markers of oxidative stress are linked to lowered toxicity (Gould et al., 2009; Laskin et al., 2010; O'Neill et al., 2010; Tewari-Singh et al., 2011; Ucar et al., 2007). Our results support the hypothesis that AEOL 10150 lowered oxidative stress in this model, which correlated with attenuating SM toxicity.
Oxidative stress and inflammation often occur together and some propose that each enhances the formation of each other (Conner and Grisham, 1996; Gill et al., 2010; Khansari et al., 2009; Nathan and Cunningham-Bussel, 2013). Proinflammatory cytokines which had been observed to be elevated after SM exposure were selected to determine if antioxidant treatments would affect their presence in bronchoalveolar lavage fluid (BALF) (Emmler et al., 2007; Malaviya et al., 2010; Ricketts et al., 2000; Seagrave et al., 2010; Zhu et al., 2014). The cytokines were analyzed using a proinflammatory multiplex panel that included cytokines previously reported to be elevated by SM exposure. Of the cytokines tested in this panel, only TNF-α, IL-6, KC/GRO, and IL-1β were significantly increased by SM exposure at 24 h over control values. For each marker tested, treatment with AEOL 10150 was able to restore BALF cytokine concentrations back to control values. Published research linking inflammatory responses to injury progression after exposure to SM analogs support the idea that an indirect reduction in inflammation from antioxidant treatment may reinforce the protective effect it displays against SM toxicity (Arroyo et al., 1995; Emmler et al., 2007; Kehe et al., 2009; O'Neill et al., 2010; Ricketts et al., 2000).
It has been suggested that TFGβ1 plays a role in the pathogenesis of chronic SM toxicity (Ghanei and Harandi, 2007; Poursaleh et al., 2012). Generation of reactive oxygen species have been previously shown to influence expression of TFGβ1 (Jain et al., 2013). TFGβ1 levels were measured in BALF samples to investigate whether they are elevated after acute SM exposure as well as to determine if catalytic antioxidant treatment can alter their expression. It was discovered that this growth factor is elevated in the acute model of SM toxicity, and that antioxidant dosing acutely inhibits this overexpression. TGFβ1 levels are heavily associated with the development of idiopathic pulmonary fibrosis, and it has previously been described that levels of TGFβ1 can be upregulated long after exposure to SM (Aghanouri et al., 2004; Tatler and Jenkins, 2012). The antioxidant induced suppression of TGFβ1 levels could indicate that antioxidants may be beneficial for chronic pulmonary conditions following SM inhalation as well as resolving acute toxicity.
Depletion of white blood cells is a well reported facet of SM toxicity, and it has been suggested that the SM-mediated immune suppression may be one of the most threatening effects of exposure (Anderson et al., 2006; Hassan et al., 2006; Yue et al., 2015). These studies also found SM exposure to produce a leukopenia that was primarily influenced by depressed lymphocyte counts. Catalytic antioxidant administration attenuated leukopenia at each time point studied with the largest effects on the neutrophil and lymphocyte counts. The finding that white blood cells are restored with catalytic antioxidant treatments is highly significant due to the previous research which has shown bone marrow to be an important target of SM damage (Yue et al., 2015). Leukocytes are believed to have an important role in the pathogenic response to inhaled vesicants (McClintock et al., 2002; Weinberger et al., 2011). The protective effect observed with antioxidant therapy on WBC counts may be a vital component of its protective capabilities. Although significant neutropenia was not observed in these studies, lowered neutrophil counts have been noted in models of both SM and nitrogen mustard (NM) injury. The elevated neutrophil counts produced by AEOL 10150 (independent of SM exposure) may be a beneficial outcome considering the use of granulocyte colony stimulating factor has been recommended as a therapeutic to increase neutrophils after injury from SM and NM (Anderson et al., 2006).
The presence of airway casts in this model, as well as the therapeutic benefit of their removal have been previously reported (Veress et al., 2010, 2013). This study further these findings by quantifying the development of the casts over time as well as the first to show that catalytic antioxidant therapy reduces SM-induced airway cast formation. The physical disruption of airflow into the lungs from the cast occlusions appears to be the driving force in acute SM inhalation lethality (Veress et al., 2010, 2015). The reduction of airway cast size, and subsequent improvements in lung function from catalytic antioxidant therapy are likely responsible for the improved pulse oximetry values, clinical scores, and diminished biomarkers reported herein. The mechanism by which catalytic antioxidants mediated airway cast suppression is not well understood at this time, and merits further investigation. Possible explanations for a reduction in cast size may include reducing oxidant-induced vascular permeability and thereby preventing plasma proteins from seeping into the lung or an effect on clotting factors which may prevent coagulation (Rancourt et al., 2012, 2013).
Oxidative stress may not be the only toxic pathway involved in SM toxicity, but also our results indicate that treatment with the catalytic antioxidant AEOL 10150 was capable of affording greatly improved survival against acute SM inhalation. The extent of protection afforded to animals receiving catalytic antioxidants supports that idea that oxidative stress plays a central role in inhaled SM toxicity, and that injectable catalytic antioxidants may be a viable therapeutic option for emergency management of SM exposures. The suppression of airway cast formation and related improvements in lung function/survival show that further research into catalytic antioxidant therapeutics to treat SM toxicity is merited. Further studies are required to investigate if catalytic antioxidants may be valuable in reducing negative outcomes in chronic models of SM injury.
ACKNOWLEDGMENTS
B.J.D. is a consultant for and holds equity in Aeolus Pharmaceuticals that is developing metalloporphyrins as potential therapeutic agents.
FUNDING
National Institute of Environmental Health Sciences(Grant/Award Number: ′U54ES015678′).
REFERENCES
- Aghanouri R., Ghanei M., Aslani J., Keivani-Amine H., Rastegar F., Karkhane A. (2004). Fibrogenic cytokine levels in bronchoalveolar lavage aspirates 15 years after exposure to sulfur mustard. Am. J. Physiol. Lung. Cell Mol. Physiol. 287, L1160–L1164. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Byers S. L., Vesely K. R. (2000). Treatment of sulfur mustard (HD)-induced lung injury. J. Appl. Toxicol. 20 Suppl 1, S129–S132. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Holmes W. W., Lee R. B., Dalal S. J., Hurst C. G., Maliner B. I., Newmark J., Smith W. J. (2006). Sulfur mustard-induced neutropenia: treatment with granulocyte colony-stimulating factor. Mil. Med. 171, 448–453. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Taylor S. L., Fetterer D. P., Holmes W. W. (2009). Evaluation of protease inhibitors and an antioxidant for treatment of sulfur mustard-induced toxic lung injury. Toxicology 263, 41–46. [DOI] [PubMed] [Google Scholar]
- Anderson D. R., Yourick J. J., Moeller R. B., Petrali J. P., Young G. D., Byers S. L. (1996). Pathologic changes in rat lungs following acute sulfur mustard inhalation. Inhal. Toxicol. 8, 285–297. [Google Scholar]
- Anderson P. D. (2015). Emergency management of chemical weapons injuries. J. Pharm. Pract. 25, 61–68. [DOI] [PubMed] [Google Scholar]
- Arroyo C. M., Von Tersch R. L., Broomfield C. A. (1995). Activation of alpha-human tumour necrosis factor (TNF-alpha) by human monocytes (THP-1) exposed to 2-chloroethyl ethyl sulphide (H-MG). Hum. Exp. Toxicol. 14, 547–553. [DOI] [PubMed] [Google Scholar]
- Batal M., Rebelo-Moreira S., Hamon N., Bayle P. A., Mouret S., Clery-Barraud C., Boudry I., Douki T. (2015). A guanine-ethylthioethyl-glutathione adduct as a major DNA lesion in the skin and in organs of mice exposed to sulfur mustard. Toxicol. Lett. 233, 1–7. [DOI] [PubMed] [Google Scholar]
- Bobb A. J., Arfsten D. P., Jederberg W. W. (2005). N-acetyl-L-Cysteine as prophylaxis against sulfur mustard. Mil. Med. 170, 52–56. [DOI] [PubMed] [Google Scholar]
- Brimfield A. A., Soni S. D., Trimmer K. A., Zottola M. A., Sweeney R. E., Graham J. S. (2012). Metabolic activation of sulfur mustard leads to oxygen free radical formation. Free Radic. Biol. Med. 52, 811–817. [DOI] [PubMed] [Google Scholar]
- Chandler J. D., Min E., Huang J., Nichols D. P., Day B. J. (2013). Nebulized thiocyanate improves lung infection outcomes in mice. Br. J. Pharmacol. 169, 1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner E. M., Grisham M. B. (1996). Inflammation, free radicals, and antioxidants. Nutrition 12, 274–277. [DOI] [PubMed] [Google Scholar]
- Dacre J. C., Goldman M. (1996). Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol. Rev. 48, 289–326. [PubMed] [Google Scholar]
- Day B. J. (2004). Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov. Today 9, 557–566. [DOI] [PubMed] [Google Scholar]
- Debiak M., Kehe K., Burkle A. (2009). Role of poly(ADP-ribose) polymerase in sulfur mustard toxicity. Toxicology 263, 20–25. [DOI] [PubMed] [Google Scholar]
- Emad A., Emad Y. (2007). Levels of cytokine in bronchoalveolar lavage (BAL) fluid in patients with pulmonary fibrosis due to sulfur mustard gas inhalation. J. Interferon Cytokine Res. 27, 38–43. [DOI] [PubMed] [Google Scholar]
- Emmler J., Hermanns M. I., Steinritz D., Kreppel H., Kirkpatrick C. J., Bloch W., Szinicz L., Kehe K. (2007). Assessment of alterations in barrier functionality and induction of proinflammatory and cytotoxic effects after sulfur mustard exposure of an in vitro coculture model of the human alveolo-capillary barrier. Inhal. Toxicol. 19, 657–665. [DOI] [PubMed] [Google Scholar]
- Gao X., Anderson D. R., Brown A. W., Lin H., Amnuaysirikul J., Chua A. L., Holmes W. W., Ray P. (2011). Pathological studies on the protective effect of a macrolide antibiotic, roxithromycin, against sulfur mustard inhalation toxicity in a rat model. Toxicol. Pathol. 39, 1056–1064. [DOI] [PubMed] [Google Scholar]
- Ghanei M., Harandi A. A. (2007). Long term consequences from exposure to sulfur mustard: a review. Inhal. Toxicol. 19, 451–456. [DOI] [PubMed] [Google Scholar]
- Ghanei M., Harandi A. A. (2011). Molecular and cellular mechanism of lung injuries due to exposure to sulfur mustard: a review. Inhal. Toxicol. 23, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R., Tsung A., Billiar T. (2010). Linking oxidative stress to inflammation: toll-like receptors. Free Radic. Biol. Med. 48, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N. S., White C. W., Day B. J. (2009). A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J. Pharmacol. Exp. Ther. 328, 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Z. M., Ebtekar M., Ghanei M., Taghikhani M., Noori Daloii M. R., Ghazanfari T. (2006). Immunobiological consequences of sulfur mustard contamination. Iran. J. Allergy Asthma Immunol. 5, 101–108. [PubMed] [Google Scholar]
- Inturi S., Tewari-Singh N., Gu M., Shrotriya S., Gomez J., Agarwal C., White C. W., Agarwal R. (2011). Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free Radic. Biol. Med. 51, 2272–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A. K., Tewari-Singh N., Gu M., Inturi S., White C. W., Agarwal R. (2011). Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicol. Lett. 205, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M., Rivera S., Monclus E. A., Synenki L., Zirk A., Eisenbart J., Feghali-Bostwick C., Mutlu G. M., Budinger G. R., Chandel N. S. (2013). Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J. Biol. Chem. 288, 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett D. A. (2016). The NIH Countermeasures Against Chemical Threats Program: overview and special challenges. Ann. N. Y. Acad. Sci. 1374, 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachadourian R., Johnson C. A., Min E., Spasojevic I., Day B. J. (2004). Flavin-dependent antioxidant properties of a new series of meso-N,N'-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem. Pharmacol. 67, 77–85. [DOI] [PubMed] [Google Scholar]
- Kehe K., Szinicz L. (2005). Medical aspects of sulphur mustard poisoning. Toxicology 214, 198–209. [DOI] [PubMed] [Google Scholar]
- Kehe K., Thiermann H., Balszuweit F., Eyer F., Steinritz D., Zilker T. (2009). Acute effects of sulfur mustard injury–Munich experiences. Toxicology 263, 3–8. [DOI] [PubMed] [Google Scholar]
- Keyser B. M., Andres D. K., Holmes W. W., Paradiso D., Appell A., Letukas V. A., Benton B., Clark O. E., Gao X., Ray P., et al. (2014). Mustard gas inhalation injury: therapeutic strategy. Int. J. Toxicol. 33, 271–281. [DOI] [PubMed] [Google Scholar]
- Khansari N., Shakiba Y., Mahmoudi M. (2009). Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 3, 73–80. [DOI] [PubMed] [Google Scholar]
- Khazdair M. R., Boskabady M. H., Ghorani V. (2015). Respiratory effects of sulfur mustard exposure, similarities and differences with asthma and COPD. Inhal. Toxicol. 27, 731–744. [DOI] [PubMed] [Google Scholar]
- Korkmaz A., Tan D. X., Reiter R. J. (2008). Acute and delayed sulfur mustard toxicity; novel mechanisms and future studies. Interdiscip. Toxicol. 1, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz A., Yaren H., Topal T., Oter S. (2006). Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Arch. Toxicol. 80, 662–670. [DOI] [PubMed] [Google Scholar]
- Kumar O., Sugendran K., Vijayaraghavan R. (2001). Protective effect of various antioxidants on the toxicity of sulphur mustard administered to mice by inhalation or percutaneous routes. Chem. Biol. Interact. 134, 1–12. [DOI] [PubMed] [Google Scholar]
- Laskin J. D., Black A. T., Jan Y. H., Sinko P. J., Heindel N. D., Sunil V., Heck D. E., Laskin D. L. (2010). Oxidants and antioxidants in sulfur mustard-induced injury. Ann. N. Y. Acad. Sci. 1203, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. M. (2008). Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid. Redox Signal. 10, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Sunil V. R., Cervelli J., Anderson D. R., Holmes W. W., Conti M. L., Gordon R. E., Laskin J. D., Laskin D. L. (2010). Inflammatory effects of inhaled sulfur mustard in rat lung. Toxicol. Appl. Pharmacol. 248, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock S. D., Till G. O., Smith M. G., Ward P. A. (2002). Protection from half-mustard-gas-induced acute lung injury in the rat. J. Appl. Toxicol. 22, 257–262. [DOI] [PubMed] [Google Scholar]
- McGovern T., Day B. J., White C. W., Powell W. S., Martin J. G. (2011). AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic. Biol. Med. 50, 602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M. A., van den Berg R. M., Benschop H. P. (2008). Proteomic assessment of sulfur mustard-induced protein adducts and other protein modifications in human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 230, 97–108. [DOI] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A. (2013). Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill H. C., Orlicky D. J., Hendry-Hofer T. B., Loader J. E., Day B. J., White C. W. (2011). Role of reactive oxygen and nitrogen species in olfactory epithelial injury by the sulfur mustard analogue 2-chloroethyl ethyl sulfide. Am. J. Respir. Cell Mol. Biol. 45, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill H. C., White C. W., Veress L. A., Hendry-Hofer T. B., Loader J. E., Min E., Huang J., Rancourt R. C., Day B. J. (2010). Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic. Biol. Med. 48, 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poursaleh Z., Harandi A. A., Vahedi E., Ghanei M. (2012). Treatment for sulfur mustard lung injuries; new therapeutic approaches from acute to chronic phase. Daru 20, 27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani Z. N., Batinic-Haberle I., Anscher M. S., Huang J., Day B. J., Alexander E., Dewhirst M. W., Vujaskovic Z. (2007). Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int. J. Radiat. Oncol. Biol. Phys. 67, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt R. C., Veress L. A., Ahmad A., Hendry-Hofer T. B., Rioux J. S., Garlick R. B., White C. W. (2013). Tissue factor pathway inhibitor prevents airway obstruction, respiratory failure and death due to sulfur mustard analog inhalation. Toxicol. Appl. Pharmacol. 272, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancourt R. C., Veress L. A., Guo X., Jones T. N., Hendry-Hofer T. B., White C. W. (2012). Airway tissue factor-dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L82–L92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts K. M., Santai C. T., France J. A., Graziosi A. M., Doyel T. D., Gazaway M. Y., Casillas R. P. (2000). Inflammatory cytokine response in sulfur mustard-exposed mouse skin. J. Appl. Toxicol. 20 Suppl 1, S73–S76. [DOI] [PubMed] [Google Scholar]
- Seagrave J., Weber W. M., Grotendorst G. R. (2010). Sulfur mustard vapor effects on differentiated human lung cells. Inhal. Toxicol. 22, 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil V. R., Shen J., Patel-Vayas K., Gow A. J., Laskin J. D., Laskin D. L. (2012). Role of reactive nitrogen species generated via inducible nitric oxide synthase in vesicant-induced lung injury, inflammation and altered lung functioning. Toxicol. Appl. Pharmacol. 261, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F. R., Loke W. K. (2013). Sulfur mustard and respiratory diseases: Revisit with special reference to the “Comments on ′Sulfur Mustard and Respiratory Diseases′, Tang and Loke (2012) and a prepared Integrated Mechanism for Chronic Pulmonary Disease from Exposure to Sulfur Mustard” by Saburi and Ghanei (2013). Crit. Rev. Toxicol. 43, 277–281. [DOI] [PubMed] [Google Scholar]
- Tatler A. L., Jenkins G. (2012). TGF-beta activation and lung fibrosis. Proc. Am. Thorac. Soc. 9, 130–136. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N., Agarwal C., Huang J., Day B. J., White C. W., Agarwal R. (2011). Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. J. Pharmacol. Exp. Ther. 336, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N., Inturi S., Jain A. K., Agarwal C., Orlicky D. J., White C. W., Agarwal R., Day B. J. (2014). Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free Radic. Biol. Med. 72, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar M., Korkmaz A., Reiter R. J., Yaren H., Oter S., Kurt B., Topal T. (2007). Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol. Lett. 173, 124–131. [DOI] [PubMed] [Google Scholar]
- Veress L. A., Anderson D. R., Hendry-Hofer T. B., Houin P. R., Rioux J. S., Garlick R. B., Loader J. E., Paradiso D. C., Smith R. W., Rancourt R. C., et al. (2015). Airway tissue plasminogen activator prevents acute mortality due to lethal sulfur mustard inhalation. Toxicol. Sci. 143, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress L. A., Hendry-Hofer T. B., Loader J. E., Rioux J. S., Garlick R. B., White C. W. (2013). Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. Am. J. Respir. Cell Mol. Biol. 48, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress L. A., O'Neill H. C., Hendry-Hofer T. B., Loader J. E., Rancourt R. C., White C. W. (2010). Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am. J. Respir. Crit. Care Med. 182, 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B., Laskin J. D., Sunil V. R., Sinko P. J., Heck D. E., Laskin D. L. (2011). Sulfur mustard-induced pulmonary injury: therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther. 24, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Zhang Y., Chen J., Zhao Z., Liu Q., Wu R., Guo L., He J., Zhao J., Xie J., and., et al. (2015). Distribution of DNA adducts and corresponding tissue damage of Sprague-Dawley rats with percutaneous exposure to sulfur mustard. Chem. Res. Toxicol. 28, 532–540. [DOI] [PubMed] [Google Scholar]
- Zhu X. J., Xu R., Meng X., Chu H. B., Zhao C., Lian C. J., Wang T., Guo W. J., Zhang S. M. (2014). Mechanistic insights of sulfur mustard-induced acute tracheal injury in rats. Int. J. Toxicol. 33, 382–392. [DOI] [PubMed] [Google Scholar]