Abstract
Salmon exposed to waterborne metals can experience olfactory impairment leading to disrupted chemosensation. In the current study, we investigated the effects of cadmium (Cd) on salmon olfactory function by modeling an exposure scenario where juvenile salmon transiently migrate through a polluted waterway. Coho were exposed to environmentally relevant concentrations of waterborne Cd (2 and 30 µg/L) for 48 h and (0.3 and 2 μg/L) for 16 days, followed by a 16-day depuration associated with outmigration. Cadmium exposures inhibited behavioral responses towards L-cysteine and conspecific odorants, with effects persisting following the depuration. Behavioral alterations following the 30 µg/L exposure were associated with increased olfactory epithelial gene expression of metallothionein (mt1a) and heme oxygenase (hmox1); reduced expression of olfactory signal transduction (OST) molecules; and reduced expression of mRNAs encoding major coho odorant receptors (ORs). Salmon OR array analysis indicated that Cd preferentially impacted expression of OST and OR markers for ciliated olfactory sensory neurons (OSNs) relative to microvillus OSNs, suggesting a differential sensitivity of these two major OSN populations. Behavioral alterations on exposure to 0.3 and 2 µg/L Cd were associated with increased mt1a, but not with major histological or OR molecular changes, likely indicating disrupted OST as a major mechanism underlying the behavioral dysfunction at the low-level Cd exposures. Laser-ablation mass spectrometry analysis revealed that the OSN injury and behavioral dysfunction was associated with significant Cd bioaccumulation within the olfactory sensory epithelium. In summary, low-level Cd exposures associated with polluted waterways can induce differential and persistent olfactory dysfunction in juvenile coho salmon.
Keywords: cadmium, coho salmon, olfactory injury, LA-ICP-MS, behavior, olfactory signal transduction
Olfaction is a critical sensory system in fish species (Hara, 1992). For salmon, the olfactory system serves a central role in their life cycle (Quinn, 2011) due to their reliance on detecting chemosensory cues in locating prey, avoiding predators, and in migration (Cooper et al., 1976; Dittman and Quinn, 1996; Hara, 1992; Quinn, 2011; Sutterlin and Gray, 1973). However, the salmon olfactory system is also a potential target for waterborne pollutants (Tierney et al., 2010). The close contact of the olfactory sensory epithelium with the surrounding water allows for interaction with dissolved pollutants (Hamdani el and Doving, 2007), which can lead to a loss of olfactory function. This pollutant-based disruption of olfactory-driven neurobehavioral function is a factor implicated in the declining Pacific salmon populations (Sandahl et al., 2007; Tierney et al., 2008), many of which are threatened or endangered.
Dissolved metals, such as cadmium (Cd) and copper (Cu), are common pollutants in urban and agricultural waterways, and are potent fish olfactory toxicants (Atchison et al., 1987; Baldwin et al., 2011; Green et al., 2010; Sandahl et al., 2007; Sloman, 2007; Tierney et al., 2010). Cd has been shown to disrupt important cellular processes that modulate olfactory function and signaling (Choong et al., 2014; Thévenod, 2009). In salmon, Cd exposures can lead to disruption of olfactory function and critical olfactory-driven behaviors (Scott et al., 2003; Sloman et al., 2003; Williams and Gallagher, 2013). Furthermore, even a brief disruption in olfactory function can increase susceptibility to predation (McIntyre et al., 2012), which emphasizes the importance of characterizing the effects of common environmental pollutants on inhibition of olfactory-driven neurobehavior.
At the cellular level, odorants are detected by olfactory sensory neurons (OSNs) located within the olfactory sensory epithelium (Hamdani el and Doving, 2007). Two main categories of OSNs, microvillus and ciliated OSNs (Hamdani el and Doving, 2007), comprise the OSN population in salmonids. Ciliated and microvillus OSNs differ in morphology and location within the olfactory epithelium, and express different odorant receptors (OR), the signal transduction molecules activated by different odorants (Hansen et al., 2003, 2004; Zippel et al., 1997). Each individual OSN expresses a single type of OR, of which there are four major classes: Trace Amine Associated Receptor (TAAR), Major Olfactory Receptor (MOR), Olfactory receptor class A-related (ora), and Vomeronasal type 2 receptor-like (OlfC). Ciliated OSNs express TAAR and MOR classes of ORs, whereas microvillus OSNs express ora and OlfC receptors (Johnstone et al., 2008, 2009, 2012; Tessarolo et al., 2014). Odorants combinatorially activate these different ORs, which result in their perception by the olfactory bulb and brain (DeMaria and Ngai, 2010). In salmonids, approximately 81 putative ORs have currently been identified (Johnstone et al., 2008, 2009, 2012; Malnic et al., 1999; Tessarolo et al., 2014). Alteration of an odorant’s unique combinatorial OR activation (i.e., “fingerprint”) can lead to altered odorant perception and behavioral responses (DeMaria and Ngai, 2010; Troemel et al., 1997). Although there is evidence that certain waterborne metals can differentially affect the ciliated and microvillus OSN populations expressing the various OR classes (Dew et al., 2014; Kolmakov et al., 2009), the effects of this differential toxicity has not been characterized in detail.
Historically, studies of Cd inhibition of fish olfactory function have primarily focused on acute exposures and responses to single odorants (reviewed in Tierney et al., 2010). In this study, we investigated the effects of Cd on salmon olfaction at times and concentrations relevant to environmental exposures, and analyzed behavior in response to several odorants. Our goal was to increase our understanding of the molecular and cellular mechanisms of Cd-induced olfactory injury by testing our hypothesis that acute and sub-chronic Cd exposures would induce differential and long-lasting impairment of olfaction. Our laboratory exposure paradigm modeled an environmental scenario in which juvenile salmon migrating through contaminated waterways were transiently exposed to Cd followed by their transition to uncontaminated water (Ruggerone and Volk, 2003). We analyzed olfactory-driven behaviors in response to environmentally relevant odorants, and correlated our findings with the extent of Cd uptake by the salmon olfactory system using laser ablation mass spectrometry, histological analysis of cellular injury, molecular biomarker expression, and gene analysis of a diverse set of salmon ORs spanning the 4 OR families (Johnstone et al., 2012).
MATERIALS AND METHODS
Chemicals
MS-222 (Tricaine methanesulfonate) was purchased from Western Chemical (Ferndale, Washington). Analytical grade cadmium chloride was purchased from Mallinckrodt Baker (Phillipsburg, New Jersey). Bradford reagent was purchased from Bio-Rad (Hercules, California). L-cysteine, Harris Hematoxylin solution-modified, bovine serum albumin and Alcian blue 8GX were purchased from Sigma-Aldrich (St. Louis, Missouri). Eosin stain was purchased from VWR (Radnor, Pennsylvania). Triton X-100 was purchased from MP Biomedicals (Cleveland, Ohio). Tween-20 was purchased from Fisher Chemicals (Pittsburg, Pennsylvania).
Animals
Juvenile coho salmon (1 year of age, 15.0 g ± 5.7 g) were housed at the University of Washington salmon hatchery in 8–12 °C flow-through freshwater from Lake Washington, Seattle. Fish were kept under natural photoperiod conditions and were fed BioVita Fry Feed (Bio-Oregon). Water quality conditions were typically at 80–120 mg/L total hardness (calcium carbonate concentration), pH 7.2, 2.5 mg/L dissolved organic carbon, and 9.1 mg/L dissolved oxygen content. Water chemistry was similar to those of other salmon-bearing rivers and streams in the Pacific Northwest (McIntyre et al., 2008).
Cd exposures
All animal welfare and experimental procedures were carried out in accordance with the University of Washington Institutional Animal Care and Use Committee (IACUC) guidelines. Tracking of individual fish over the course of the experiments was accomplished by implantation of Biomark HPT12-12 mm Passive Integrated Transponder (PIT) tags. Coho were placed within 6 designated exposure groups (0, 2, and 30 ppb Cd for 48 h; 0, 0.3, and 2 ppb Cd for 16 days), with n = 30 juvenile coho per exposure group (24 PIT-tagged fish and 6 non-tagged fish). Two of the concentrations of waterborne Cd used in this study (0.3 and 2 ppb) were below or similar to the fresh water ambient water quality criteria (AWQC) for Cd set by the US Environmental Protection Agency (1.8 ppb acute freshwater AWQC, 0.72 ppb chronic freshwater AWQC) (Environmental Protection Agency, 2016) and representative of Cd pollution in some urban waterways (Washington Department of Ecology, 2008). A third exposure group (30 ppb Cd) was representative of point source emissions and heavily polluted waterways (Srinivasa Gowd and Govil, 2008). Total waterborne Cd concentrations were analyzed before and after Cd exposures by ICP-MS (Williams and Gallagher, 2013). All background levels of total Cd in lake water were <0.01 ppb. Although the nominal Cd concentrations were 0.6, 3, and 30 ppb, the measured total waterborne Cd concentrations (0.3, 2, and 30 ppb) are used in all tables and figures.
Fish were transferred from holding tanks 24 h prior to the initiation of the exposures to 120 L glass exposure aquaria containing control water. During Cd exposures, tank water was maintained at ambient lake water temperatures (10°C) via a large chilled water bath. A static renewal approach was used in all exposures with full water renewal after a 24 h period to avoid ammonia buildup. Coho were fed 2% W/W daily for a 20 min period prior to the daily water changes, after which any remaining food was removed. Cd exposures were staggered for each experimental period and exposure group so that equal numbers of fish received Cd exposures. Nitrate and nitrite levels were below detection for all treatment groups, and ammonia levels were ≤ 0.25 ppm during the exposures. To control for handling and housing effects on behaviors, all exposure experiments were conducted in three stages (pre-exposure, exposure, and depuration), with behavioral testing conducted on the PIT-tagged fish following each stage. In the first pre-exposure stage, all fish (in all groups) were exposed to control water, followed by behavior testing on the PIT-tagged fish. This pre-exposure allowed the individual PIT-tagged fish to act as their own controls for the Cd exposure experiments to follow. After the control exposures and behavioral testing, all fish in each group were returned to glass aquaria containing control water and allowed to re-acclimate for 24 h before exposing them to Cd according to their designated concentration and duration. Following the Cd exposures, non-PIT tagged fish (n = 6 per group) were euthanized and analyzed for olfactory gene expression and histological injury. PIT-tagged fish underwent behavioral testing, and then transferred back into the glass aquaria containing control water for another 24 h of re-acclimation before a 16-day depuration in control water. Following depuration, the PIT-tagged coho (n = 24 per group) underwent a final round of behavioral testing, and were then euthanized for tissue collection.
Odorant preparation and neurobehavioral analysis of olfactory function
Three sets of salmon odorants were used for behavioral analysis. A coho skin extract was prepared as previously described (Williams and Gallagher, 2013) and adjusted to a final protein stock concentration of 3 mg/ml in DI water and stored at −20 °C. Salmon conspecific odorant was prepared by placing 8 juvenile coho (∼15 g each) in a 35 L glass tank filled with lake water overnight. Animals were then removed and the water was used as the conspecific odor stock (3.4 g of fish/L). L-cysteine stocks were used at 10 mM and prepared daily.
Olfactory-mediated behavioral analysis was conducted using a 2-choice maze constructed from acrylic. The size of the maze was 100 × 40 × 25 cm (l, w, h), and consisted of 2 arms (50 cm × 20 cm) that terminated at a 40 × 40 cm holding chamber. A transparent screen separated the maze arms from the holding chamber, and a black curtain surrounded the maze to minimize outside disturbances. Illumination for the 2 overhead mounted video cameras (JVC Everio camcorders) was provided by a low-level red light. The maze received a continuous unidirectional flow of fresh lake water from the arms into the holding chamber, with no mixing of water in between the arms.
Passive integrated transponder (PIT)-tagged coho from each exposure group (n = 24) were tested individually. Coho were placed into the holding chamber and were allowed to acclimate for 15 min prior to odorant addition (10−6 M L-cysteine, 6 mg/L conspecific odor, and 300 μg protein/L of the skin extract) into a single arm. Conspecific odor and L-cysteine were delivered via a peristaltic pump at 10 mL/min, with the flow of lake water into each arm at 5 L/min. The skin extract was delivered via a slow injection directly into the holding chamber without lake water inflow. Video recordings for behavioral responses were initiated 10 min prior to the odorant addition to establish baseline behavior, and 5 min after odorant addition to establish the behavioral response to odorants. The maze was flushed with clean water for 20 min between each behavioral trial. The maze arm of odorant addition and the order of L-cysteine and conspecific odor delivery was randomized for each behavioral trial. The skin extract was introduced lastly in all behavioral tests to minimize confounding effects of potential fright responses. Fish odorant responses were quantified from video recordings using EthoVision-XT (Noldus, Leesburg, Virginia) software. Maze arm preference was the designated response for l-cysteine and the conspecific odorant, whereas reduced swimming activity (“freezing” fear response) was measured for the response to the skin extract.
Histological analysis of the olfactory sensory epithelium
Histological analyses of the olfactory epithelia were conducted using a subset of fish (n = 6) from post- and depurated exposure groups. Briefly, coho head tissues were fixed overnight in 4% paraformaldehyde (PFA) at 4°C. Olfactory rosettes were excised and embedded in a 2:1 mixture of 20% sucrose to Tissue-Tek OCT and stored at −80°C. Tissue sections (6 µm) were mounted on VWR glass Superfrost Plus micro-slides. Hemotyxylin and eosin (H&E) staining of olfactory epithelial tissue sections was conducted using a slight modification of our previously established method (Williams and Gallagher, 2013) in that a step to stain goblet cells using Alcian blue 8GX (Sigma-Aldrich, Missouri) was added. All images were captured using a Nikon Labophot-2 microscope (Nikon, Inc., New York) and Nuance 3.0 imaging software (PerkinElmer, Inc., Massachusetts). Histological measurements and scoring of injury on tissues sections were done blind using the ImageJ software (NIH, USA) as previously described (Williams and Gallagher, 2013).
Immunohistochemistry
Fluorescent immunohistological analysis of olfactory epithelial tissue sections was performed using the following primary antibodies: adenylyl cyclase-III (ACIII; sc-588 Santa Cruz); G alpha subunit (Gαo; sc-387 Santa Cruz); and sex determining region Y-box 2 (sox2; AB5603 Millipore). On day of use, slides were thawed at room temperature and rehydrated through serial methanol washes (100-25%) finalizing with 100% phosphate buffered saline (PBS). Antigen retrieval was performed using 10 mM sodium citrate (pH 6.0) with 0.05% Tween-20 at 95°C for 20 min (for sox2 antigen retrieval), and using 0.9% Triton X-100 at 27°C for 30 min (for ACIII and Gαo antigen retrieval). Sections were incubated with the primary antibody overnight at 4°C, followed by additional PBS washes and secondary antibody incubation. Secondary antibodies used were Cy3 and Alexa-Fluor 488 (Invitrogen, California). Nuclei were stained using DAPI in Prolong Diamond anti-fade media (Invitrogen). All slides were visualized using a Nikon Labophot-2 microscope using Nuance 3.0 imaging software. Fluorescence intensities were analyzed using MetaMorph image analysis software (Molecular Devices, California). Fluorescent intensities from the negative controls (i.e., absence of primary antibody) were used for normalization.
Laser ablation inductively coupled mass spectrometry
Olfactory tissue sections used for Laser Ablation Inductively Coupled Mass Spectrometry (LA-ICP-MS) analysis were prepared as described above with minor modifications. Briefly, tissues used for metal analysis were sectioned at 20 µm thickness and mounted on 1 × 0.5-inch glass slides. Tissue sections used for imaging were 6 μm in thickness and dried overnight in a desiccator before analysis. LA-ICP-MS analysis was conducted using an NWR213 laser ablation system (ESI, Portland, Oregon) connected to an Agilent 7500ce ICP-MS (Agilent Technologies, Santa Clara, California). The Nd:YAG laser operated at a wavelength of 213 nm. Quantitative measurements of Cd were made with a laser energy output of 35%, firing frequency of 20 Hz, laser beam spot size of 110 µm, and translation speed of 75 µm/s. Line scans were programmed to ablate around the borders of the olfactory rosette lamellae, whereas line scans between the lamellae were included to measure background Cd from the tissue preparation and the glass mounting slide. Data for the heat map image were collected using a laser energy output of 40%, firing frequency of 20 Hz, laser beam spot size of 25 µm, and translation speed of 25 µm/s following a raster pattern. All ICP-MS measurement were performed with the following parameters: rf power 1500 W, argon carrier gas flow 0.5 L/min, helium reaction cell gas flow 1.3 mL/min, helium gas flow from the laser cell 0.8 L/min, and dwell time 400 ms.
Quantification of Cd was conducted using dried, spiked gelatin films as external calibration standards. A linear relationship (R2 >0.99) was obtained from 0.5 to 250 µg/g, using the same LA-ICP-MS parameters as described above for the quantitative measurements. Bioimages were prepared using Igor Pro 6.37 (WaveMetrics, Inc., Oregon) and Iolite 3.32 (Iolite, Victoria, Australia) software applications.
Quantitative PCR (qPCR) analysis of olfactory gene expression
Eight coho were collected for qPCR analysis. The fish were euthanized with MS-222 prior to cervical dislocation, and the olfactory rosettes were excised, transferred to Qiazol reagent, immediately frozen in liquid nitrogen, and stored at −80°C. Total RNA isolation was performed using the Qiagen RNeasy kit (Hilden, Germany). Isolated RNA was treated with DNase, and cDNA synthesis was conducted using the BioRad iScript cDNA synthesis kit (Hercules, California). Candidate olfactory biomarker gene primers were generated by multiple sequence alignment of rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), and zebrafish (Danio rerio) target sequences. To assess the effects of Cd on the mature OSN cell populations, we analyzed gene expression of omp (a marker of mature olfactory neurons) and nrn1 (a marker of neuronal regrowth and axonal growth) (Kudo et al., 2009; Naeve et al., 1997). Additionally, to assess the effects of Cd on markers of cellular stress and metal exposures, we analyzed the expression of metallothionein 1a (mt1a; a sensitive metal response marker) and heme oxygenase-1 (hmox1; a sensitive marker of oxidative and cellular stress) (Coyle et al., 2002; Ryter and Choi, 2002; Tallkvist et al., 2002; Wang et al., 2008). Primers for hmox1, nrn1, omp, and mt1a (Supplementary Table 1) were designed using Primer 3 software program (San Diego Biology Workbench 3.2, http://workbench.sdsc.edu/). All PCR products were confirmed by sequencing. Quantitative PCR analysis was conducted in a 96-well format with BioRad SsoAdvanced SYBR Green supermix using the relative standard curve method (Espinoza et al., 2012). ß-actin expression did not differ between treatments and was used for normalization.
Multiplex PCR specific target amplification of salmon olfactory receptors
A target pool of coho salmon olfactory receptors was identified based upon previously identified putative Atlantic salmon ORs (Johnson and Banks, 2011; Johnstone et al., 2008, 2009, 2011, 2012). From the total pool of putative ORs, a subset of 6-8 ORs from each of the four major receptor classes (MOR, TAAR, OlfC, and ora) were selected based upon sequence dissimilarity (i.e., genes with more sequence dissimilarity within the same major class), relative expression levels, and ability to specifically amplify the target OR mRNA. Primers were designed using the Primer 3 software program and are shown in Supplementary Table 1. All PCR products were sequenced and used for the design of the TaqMan probes. cDNA samples (synthesis described above) were pre-amplified following the Fluidigm (South San Francisco, California) Specific Target Amplification (STA) protocol to increase target gene template cDNA for gene expression reactions.
Following design and validation of OR probes, the pool of PCR primers and TaqMan probes (Supplementary Table 1) were multiplexed in a single PCR for each diluted aliquot of DNA. Reactions were conducted in triplicate incubations according to the manufacturer’s TaqMan protocol (Applied Biosystems Inc., California) on an Applied Biosystems 2720 thermal cycler. Pre-amplified samples were then diluted 1:5 with DNA Suspension Buffer. Fluidigm GE Dynamic Array 96.96 plates were primed on the Fluidigm IFC Controller prior to loading. The assays were prepared by adding equal volumes of Assay Loading Reagent (Fluidigm) to TaqMan Gene Expression Assays (Applied Biosystems). Each 10X assay mix was then loaded onto each assay well on the GE Dynamic Array (Fluidigm). Sample Pre-Mix was prepared by combining TaqMan Universal PCR Master Mix with GE Sample Loading Reagent (Fluidigm) and STA cDNA. Each Sample of Pre-Mix was then added to each sample well on the GE Dynamic Array. The GE Dynamic Array was then loaded onto the IFC Controller before thermocycling following the manufacturer’s established protocol. Data were collected using the Fluidigm BioMark Data Collection Software and analyzed using the Fluidigm Real-Time PCR Analysis Software 4.1.2.
Statistical analysis
Experimental data for the effects of Cd on olfactory gene expression, immunohistological fluorescence, and Cd concentrations by LA-ICP-MS were inspected for homogeneity of variances using D'Agostino and Pearson omnibus normality testing (D'Agostino and Pearson, 1973), and treatment-related effects were assessed using Kruskal-Wallis one-way ANOVA test followed by Dunn’s non-parametric post-hoc test. Behavioral data consisted of repeated measures on the same set of fish within Cd exposure groups. These data were analyzed within exposure levels using conventional ANOVA, with a blocking factor for each fish to account for fish-specific behavioral effects. Statistical testing was conducted using GraphPad software (Prism, La Jolla, CA) and R software and treatment-related effects were considered significant at P < .05.
RESULTS
Behavioral Effects of Waterborne Cd
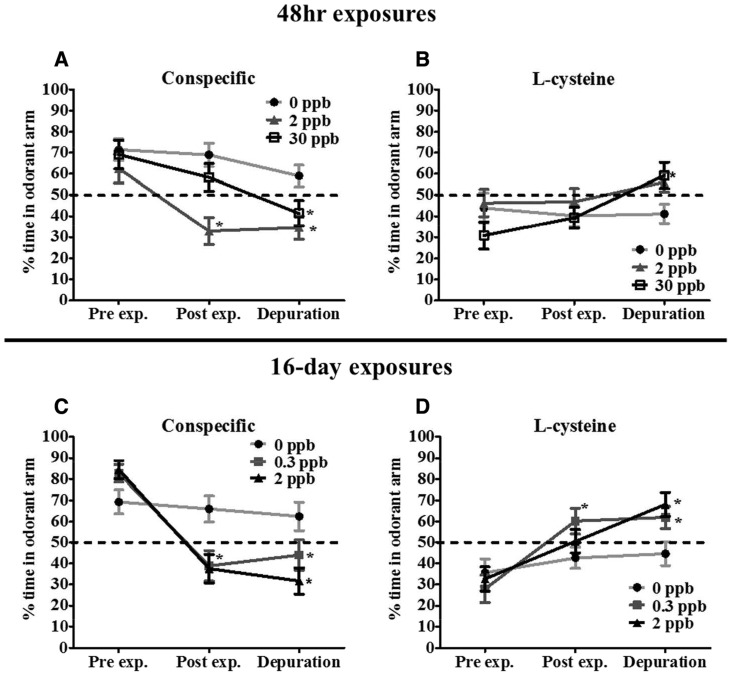
To investigate the relationship between different Cd exposure scenarios and changes to multiple olfactory driven behaviors, we utilized attractive (conspecific), aversive (L-cysteine) and alarm (skin extract) odorants. Our results revealed an initial preference by coho for conspecific odorants prior to Cd exposure (Fig. 1A and C). Coho spent between 60–80% of their time in the arm receiving the conspecific odorant compared with the non-scented arm (Fig. 1A and C). However, following a 48-h exposure to 2 ppb Cd, coho spent only 30% of their time in the conspecific-scented arm, half the time compared with pre-exposure behavior. In contrast, coho exposed to 30 ppb Cd for 48 h spent 60% of their time in the conspecific arm, a 10% decrease compared with pre-exposure behavior (Fig. 1A). As observed in Fig. 1A, this decrease continued following depuration. After the 16-day depuration, coho previously exposed to 30 ppb Cd spent ∼40% of their time in the odorant arm, which was similar to the avoidance behavior of coho previously exposed to 2 ppb Cd following depuration (∼35% time spent in odorant arm), P < .05. These observations indicate that coho actively avoided the conspecific odor, marking a reversal of pre-exposure behavior. Similarly, coho exposed to 0.3 and 2 ppb Cd for 16 days actively avoided the conspecific scented arm, spending only ∼35% of the time in the conspecific scented arm compared with their pre-exposure behavior of ∼85% in this arm (Fig. 1C). This behavioral reversal persisted following the 16-day depuration. Coho previously exposed to 0.3 and 2 ppb Cd for 16 days spent ∼45 and ∼30% of their time in the conspecific scented arms, respectively (Fig. 1C), whereas the control groups showed no significant changes in odorant attraction (Fig. 1A and C).
FIG. 1.
Behavioral responses to conspecific (A, C) and L-cysteine (B, D) odorants following Cd exposures. Percent time juvenile coho spent in the arm of a two-choice maze receiving conspecific odorant or L-cysteine odorant before exposures (Pre exp.), after exposure (Post exp.), and after a 16-day depuration (Depuration). All data represent mean ± SEM of n = 24 individuals. Asterisks indicates statistically significant differences between pre-exposure and post-exposure/depuration behaviors within exposure groups (*P ≤.05).
In contrast to the attractant conspecific odorant, coho showed an initial aversion response towards the arm receiving the L-cysteine odorant prior to Cd exposures, spending 30–45% of their time in the arm receiving L-cysteine (Fig. 1B and D). However, following exposure to either 2 or 30 ppb Cd for 48 h, coho did not show an aversion response towards L-cysteine (Fig. 1B). Following the 16-day depuration, coho in these 48-h exposure groups exhibited an increase in attraction behavior towards L-cysteine, with the 30 ppb exposure group spending ∼60% of their time in the odorant arm (Fig. 1B). Coho exposed to lower concentrations of 0.3 and 2 ppb Cd for 16 days also exhibited a decreased aversion towards L-cysteine, with the 0.3 ppb Cd group spending 60% of their time in the odorant arm compared with 25% prior to exposure (P < .05, Fig. 1D). This trend continued following depuration; coho previously exposed to 0.3 and 2 ppb Cd exhibited a significant attraction towards L-cysteine compared with pre-exposure behaviors (P < .05, Fig. 1D). Coho in the control groups showed no significant changes in odorant response over time (Fig. 1B and D) whereas coho exposed to Cd for 48 h and 16 days exhibited transient alterations in their alarm responses towards the skin extract. However, these responses were highly variable, and thus did not significantly differ from to their pre-exposure behavior (Supplementary Fig. 1).
Bioaccumulation of Cd within the Peripheral Olfactory System
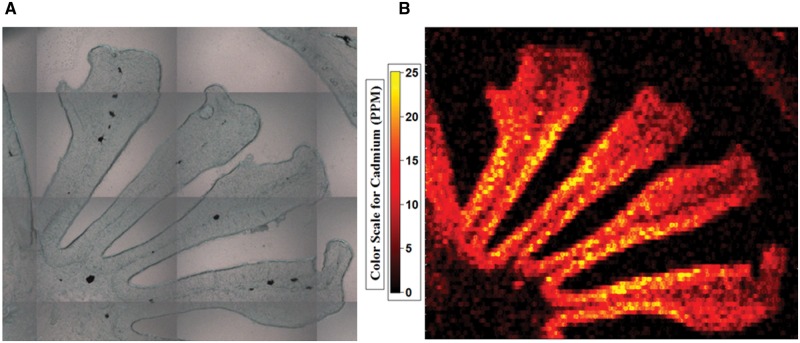
To investigate the link between persistent Cd induced behavioral alterations and the accumulation of Cd within the olfactory sensory epithelia, we conducted LA-ICP-MS analysis to measure Cd concentrations within the coho olfactory rosettes. LA-ICP-MS analysis found increased levels of Cd within the olfactory epithelium following all of the Cd exposures, compared with background tissue levels (Fig. 2). Cd accumulation within the olfactory sensory epithelium of coho in each of the exposure groups increased in a concentration- and time-dependent manner, and the Cd accumulation profile closely tracked the observed behavioral deficits (Table 1). The Cd concentrations within the olfactory rosettes of coho exposed to 2 and 30 ppb Cd for 48 h were 0.74 and 4.51 µg/g, respectively, compared with <0.5 µg/g (the quantitation limit) in control olfactory rosettes (Table 1). After depuration, rosette Cd concentrations remained elevated at 0.64 and 2.97 µg/g in coho previously exposed to 2 and 30 ppb Cd for 48 h, respectively (Table 1). For 16-day exposures, coho exposed to 0.3 and 2 ppb Cd for 16 days had significantly elevated Cd concentrations in the olfactory rosettes, with 1.29 and 5.91 µg/g, respectively, which was higher than the 48-h exposures (Table 1). Post-depuration rosette Cd concentrations remained significantly elevated (1.05 and 3.71 µg/g in coho that were previously exposed to 0.3 and 2 ppb Cd for 16 days, respectively) and higher than the 48-h post-depuration samples (Table 1).
FIG. 2.
LA-ICP-MS analysis of Cd accumulation within a representative coho salmon olfactory rosette following exposure to 30 ppb Cd for 48 h. (A) micrograph of the olfactory rosette prior to laser ablation. (B) LA-ICP-MS generated heat map of Cd bioaccumulation within the olfactory rosette.
TABLE 1.
Concentrations of Cd within the Olfactory Sensory Epithelium Following the 48 h and 16-Day Cd Exposures and Following the 16-Day Depuration
| Exposure time | Waterborne Cd Conc. (ppb) | Avg. Cd tissue conc. following exposure (ppm) | Avg. Cd tissue conc. following depuration (ppm) |
|---|---|---|---|
| 48 h | 0 | <LOQ | <LOQ |
| 48 h | 2 | 0.74 ± 0.06 | 0.64 ± 0.09 |
| 48 h | 30 | 4.51 ± 0.68* | 2.97 ± 0.31* |
| 16-day | 0 | <LOQ | <LOQ |
| 16-day | 0.3 | 1.29 ± 0.20 | 1.05 ± 0.09 |
| 16-day | 2 | 5.91 ± 1.22* | 3.71 ± 0.31* |
<LOQ: Concentration was less than the quantitation limit of 0.5 ppm. Data represents n = 3. Asterisks indicate statistically significant differences in Cd concentration relative to controls (*P ≤ .05).
Induction of mt1a, an Olfactory Metal Responsive Biomarker
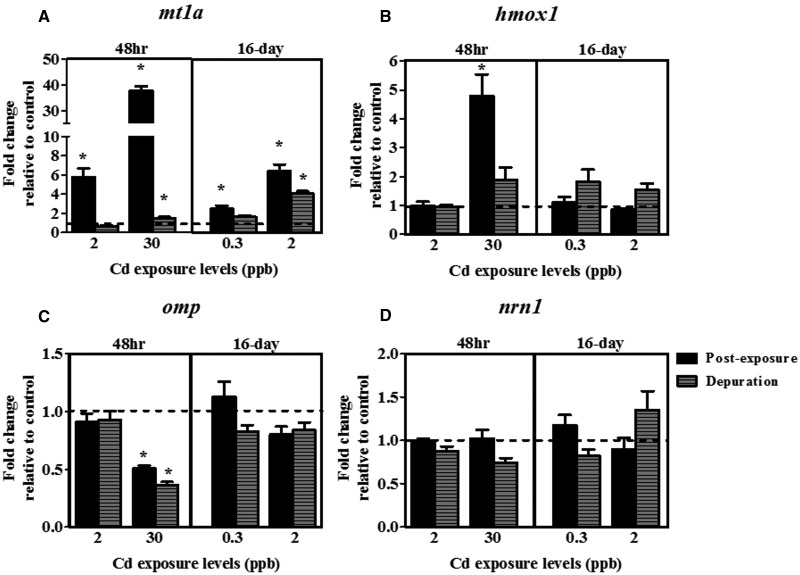
Metallothionein-1a is a critical metal response protein that can readily bind with free intracellular Cd, and represents a powerful and sensitive marker of Cd exposures (Coyle et al., 2002; Espinoza et al., 2012; Tallkvist et al., 2002). Our qPCR results showed that coho exposed to all levels of Cd showed a significant increase in expression of metallothionein-1a (mt1a) compared with pre-exposure controls, including a 38-fold induction in coho receiving 30 ppb Cd exposures over 48 h (Fig. 3A). Gene expression of mt1a mRNA remained elevated following depuration only in coho previously exposed to 30 ppb Cd for 48 h or 2 ppb Cd for 16 days (Fig. 3A) and showed a similar trend with the Cd tissue concentration profile by LA-ICP-MS.
FIG. 3.
Effects of Cd on expression of markers of olfactory cellular injury. Fold-change in mRNA expression of the olfactory (A) mt1a, (B) hmox1, (C) omp, and (D) nrn1 following acute (48 h) and sub-chronic (16-day) Cd exposures and a depuration. Data represent the mean ± SEM of n = 8 individuals normalized to the expression of β-actin mRNA, and expressed as fold-change relative to control levels. Asterisks indicates statistically significant differences in gene expression relative to controls (*P <.05).
Effects of Cd on Olfactory Sensory Epithelial Injury and Neuronal Stress Response
To investigate the effects of Cd exposures on olfactory sensory epithelial health, we analyzed gene expression of omp (a marker of all mature OSNs), nrn1 (a marker of neuronal regrowth and axonal growth following injury), and hmox1 (a sensitive marker of oxidative and cellular stress) (Kudo et al., 2009; Naeve et al., 1997; Ryter and Choi, 2002; Wang et al., 2008), and also the histology of the sensory epithelium for signs of injury. Analysis of the olfactory sensory epithelium following Cd exposures revealed evidence of decreased expression of omp and an induction of hmox1, whereas the general histology of the sensory epithelium remained largely unchanged. Coho exposed to Cd for 48 h and 16 days showed no histological differences in their olfactory sensory epithelium compared with coho from control exposures (Supplementary Fig. 2). Likewise, following Cd depuration, all coho had morphologically similar olfactory epithelia (Supplementary Fig. 2). Olfactory hmox1 gene expression was induced 4.5-fold in coho exposed to 30 ppb Cd for 48 h (Fig. 3B), but did not differ among the other exposure groups relative to controls. Expression of omp mRNA decreased by 50% in the olfactory rosettes of coho exposed to 30 ppb Cd for 48 h compared with pre-exposure controls and remained significantly depressed (60% below control levels) following Cd depuration (Fig. 3C). No significant effect on omp gene expression within the olfactory rosettes was observed in the other Cd exposures (Fig. 3C). Additionally, expression of nrn1 was not altered by Cd exposure or following depuration (Fig. 3D).
Olfactory sensory epithelial stem cells (OSCs) are critical for the maintenance and regeneration of olfactory cells following epithelial injury, and losses of these cells could be deleterious for the olfactory sensory epithelium (Huard et al., 1998; Jang et al., 2003). Immunostaining for sox2, a robust marker of OSCs, following the Cd exposures and depurations revealed no differences in the number of stem cells between control and Cd exposures (Supplementary Fig. 3).
Effects of Cd on ACIII and Gαo Protein Expression
Immunolabeling of ACIII, a major signal transduction molecule used only by ciliated OSNs; and G alpha subunit (Gαo), a major G-protein coupled receptor (GPCR) subunit used by microvillus OSNs showed a potential differential sensitivity among these two OSN populations to Cd (Ferrando et al., 2009; Hamdani el and Doving 2007). As shown in Figure 4A, ACIII immunostaining in the olfactory sensory epithelium was reduced by 50 and 70%, respectively, following 48-h exposure to 2 and 30 ppb Cd (Fig. 4A). However, the 50% decrease in ACIII staining at the 2 ppb exposure level was not statistically significant due to the variation in staining. Following depuration, analysis of ACIII immunostaining in these exposure groups showed no differences when compared with control coho (data not shown). ACIII levels in coho from the 16-day 0.3 and 2 ppb exposure groups did not differ from controls either after exposure or depuration (data not shown). Similarly, Gαo staining was not significantly altered following any of the Cd exposures or depurations (Fig. 4B).
FIG. 4.
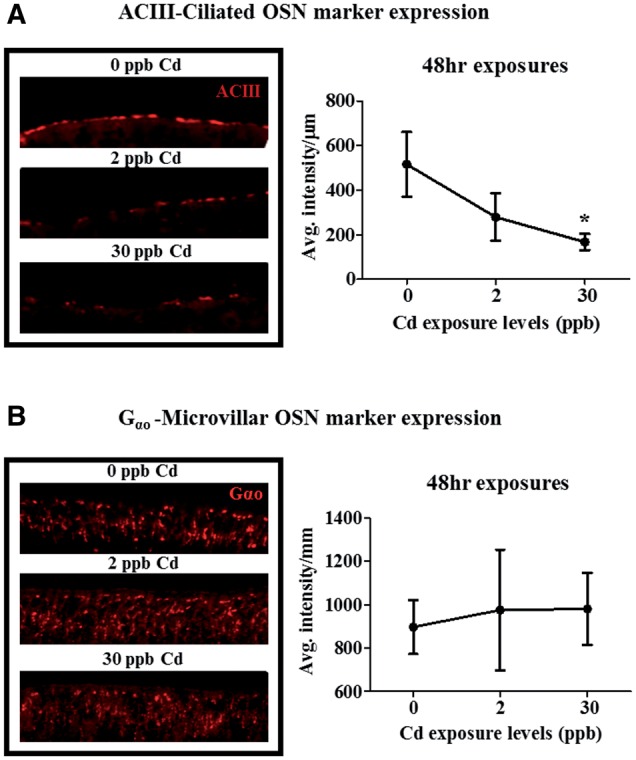
Effects of Cd on ACIII and Gαo protein expression in the olfactory epithelium. ACIII (A) and Gαo (B) immunolabeling as percent control levels following exposure to 2 and 30 ppb Cd for 48 h. Data represent the mean ± SEM of n = 6 individuals normalized to background fluorescence. Asterisks indicates statistically significant differences in signal relative to control (*P ≤ .05).
Effects of Cd on Gene Expression of Major ORs
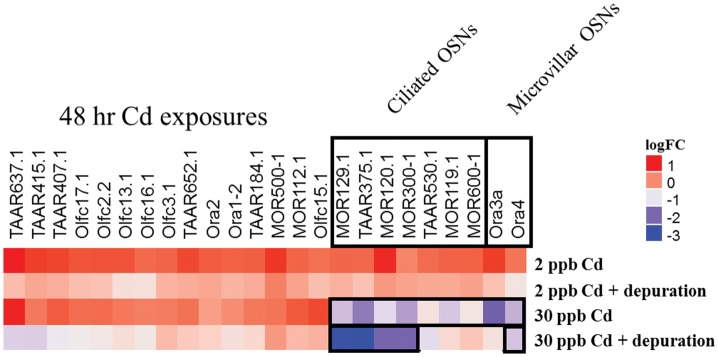
Using a custom Fluidigm array chip, we investigated the effects of different Cd exposure scenarios on a targeted group of major salmon ORs representing the four main classes of ORs critical for odorant identification. As shown by the heat map in Figure 5, exposure to 30 ppb Cd for 48 h resulted in a significant reduction of expression of 50% of the ciliated OSN-linked ORs analyzed (7 out of 14), and 20% of the microvillus OSN-linked ORs analyzed (2 out of 10). Following depuration, 28% (4 out of 14) of the ciliated OSN-linked ORs and 10% (1 out of 10) of the microvillus OSN-linked ORs remained significantly under-expressed compared with controls (Fig. 5). Coho in the other Cd exposure groups showed minor modulation of OR-encoding genes, however these effects were not statistically significant (data not shown).
FIG. 5.
Effects of Cd on expression of major olfactory receptors. Heat map of coho olfactory receptor gene expression changes in the olfactory epithelium following exposures to Cd for 48 h and following a 16-day depuration. Gene expression is represented as log2-fold change compared with controls. Data represents n = 8 individuals with OR gene expression normalized to the mean expression of elf1a and elf1b. Black boxes around genes indicate expression was significantly different from control levels (P ≤ .05).
DISCUSSION
In the present study, the effects of Cd on olfactory-mediated behaviors towards the different odorants tested revealed a greater disruption of behavioral responses towards the conspecific odorant relative to those for L-cysteine. These results may be due to the composition of the conspecific odor (likely a mixture of many individual odorants) as opposed to L-cysteine, a single compound. Conspecific odorant signaling may also be more complex as it likely activates many more types of ORs compared with L-cysteine, rendering conspecific odorant processing more susceptible to Cd disruption. The aforementioned phenomenon could have also led to the relatively weak effects of Cd on the behavioral responses towards the skin extract. A single class of compounds within the complex skin extract may be responsible for inducing alarm responses (Mathuru et al., 2012), however, the specific compound or compounds that elicit a fear response in salmon has yet to be identified. Our results indicate that Cd is differentially impacting odorant processing or perception to these three structurally and compositionally diverse odorants.
The accumulation of Cd within the olfactory system has been identified as a mechanism for olfactory deficits in fish (Scott et al., 2003). In the present study, we directly measured Cd concentrations within the sensory epithelium using LA-ICP-MS analysis, a unique method that allowed us to profile Cd accumulation spatially across the olfactory epithelium. We observed a rapid and persistent accumulation of Cd within the sensory epithelium at exposure levels as low as 0.3 ppb, which was correlated with persistent behavioral alterations. These findings demonstrate that the salmon peripheral olfactory system can rapidly accumulate waterborne Cd, consistent with studies of other fish (Harrison and Klaverkamp, 1989; Scott et al., 2003; Tjälve et al., 1986). One possible mechanism for the accumulation of Cd within olfactory neurons is the high concentration of calcium channels in the OSNs, a mechanism of Cd-mediated cell entry (Usai et al., 1999). Once inside the OSNs, Cd can induce cellular stress responses and cell death (dependent upon Cd concentration), all of which could be deleterious for olfactory function (Cuypers et al., 2010; Ercal et al., 2001). These effects may have been compounded by the persistence of the olfactory Cd accumulation following the 16-day depuration, indicating a potentially long depuration period for Cd within the peripheral olfactory system and susceptibility to long-term disruption of olfactory function. Although Cd readily accumulated within the olfactory sensory epithelium, we did not find evidence for any substantial morphological changes. These results are in agreement with our previous study which demonstrated that only very high Cd exposures significantly induced morphological injury in the olfactory sensory epithelium (Williams and Gallagher, 2013).
It should be noted that our findings do not exclude the potential for more subtle Cd-induced effects on specific olfactory cell populations that would not be reflected by histological analysis. For example, expression of omp mRNA, a marker of mature OSNs, was reduced at the 30 ppb Cd exposure and remained depressed following the depuration. These results indicate that mature OSNs are likely impacted at the higher Cd exposure levels. Previous studies have shown that metals can kill mature OSNs without impacting other olfactory cell populations (Julliard et al., 1996). This may be due to the fact that mature OSNs are in direct contact with the water, thus increasing the likelihood of Cd to compared with the more deeply-located immature OSNs and stem cell populations. Our immunohistochemistry analyses suggested that the Cd exposures had little impact on OSC populations. The OSCs (horizontal and globose basal cells), are robust as they must be able to fully regenerate the olfactory sensory epithelium following significant injury (Jang et al., 2003).
Collectively, our data indicate that cellular stress, as opposed to total cell loss, contributed to the observed Cd-induced behavioral deficits in coho. The induction of mt1a tracked with the increased Cd concentrations in the olfactory epithelium, confirming that Cd migrated into the olfactory cells and induced a rapid and persistent cellular protective response. Intracellular Cd causes oxidative stress and can disrupt cellular transporters and ion channels that maintain cellular metal homeostasis for calcium, zinc, and copper: metals for which Cd can interact (Choong et al., 2014; Cuypers et al., 2010; Hartwig, 2001; Jacobson and Turner, 1980). This potential destabilization of intracellular metal homeostasis and signaling can negatively affect normal OSN function. Furthermore, the elevated hmox1 gene expression indicates that oxidative stress is likely a factor in olfactory dysfunction at the higher acute Cd exposure levels. Collectively, these results suggest that the underlying mechanisms driving the behavioral alterations is not linked to morphological injury or OSN loss, but rather involves the interruption of OST due to an elevated and persistent intracellular OSN Cd burden.
As discussed, olfaction functions on a combinatorial principal in which a diverse and relatively small array of OSNs detect a structurally diverse and larger array of odorants (Malnic et al., 1999). Each odorant activates a unique combination of ORs, which initiates an odorant-specific signal (fingerprint) decoded in the brain which can result in a behavioral response for that specific odorant. Inhibition of distinct OSN populations can change an odorant’s OR activation “fingerprint” and alter the downstream behavioral response (Del Punta et al., 2002). Based on previous studies (Dew et al., 2014; Kolmakov et al., 2009), it is possible that Cd could induce a differential toxicity on distinct classes of OSNs. The decrease in ACIII, and not in Gαo staining, indicates that coho ciliated OSNs are more sensitive to Cd exposures compared with microvillus OSNs. Because Cd did not significantly change protein expression of ACIII or Gαo at lower exposure levels, it is possible the impaired olfactory behaviors may be explained by alterations in protein function instead of loss. For example, both ciliated (ACIII) and microvillus (IP3) OST pathways utilize calcium for signaling, and Cd has been shown to disrupt calcium signaling due to its affinity for calcium binding sites (Choong et al., 2014; Lansman et al., 1986). Deactivation of ACIII, following activation by ORs, may rely on calcium signaling via activation of CaMKII, which Cd has been shown to directly activate (Wei et al., 1998). However, this deactivation pathway for ACIII is still under debate (Cygnar et al., 2012).
In addition to the alteration of OST pathway machinery by Cd, alteration of OR expression patterns appeared to contribute to the observed behavioral alterations. Our OR gene expression array provided coverage of approximately 25% of the currently known putative salmon ORs (Johnstone et al., 2012; Tessarolo et al., 2014). Similar to our ACIII and Gαo immunostaining results, we found that mRNA expression of ORs encoding ciliated OSNs were predominantly impacted compared with those associated with microvillus OSNs. However, the mechanism by which differential loss of ciliated relative to microvillus OSN expression modulates specific behavioral changes remains unknown, as the relationships among their odorant binding specificities in salmon ORs and their associated olfactory-driven behaviors have not been extensively characterized. It has also been suggested that some olfactory ORs contain functional metal-binding sites (Wang et al., 2003). If so, it is possible that Cd could interact with these metal-binding sites and alter function independent of gene expression, as Cd has been shown to interact with zinc and copper binding sites on proteins (Hartwig, 2001). Furthermore, the persistent loss of expression of a subset of ORs following the Cd depuration period, a time frame in which ACIII staining recovered, suggest that there is a preferential sensitivity of certain ORs to Cd. Ongoing studies in our laboratory are addressing the differential sensitivity of salmon ORs to other metals found in urban waterways.
In conclusion, we provide evidence that exposure to low environmentally relevant Cd concentrations below those previously studied in salmonids causes a prolonged loss of olfaction in ecologically sensitive coho salmon. We have further demonstrated that the Cd-mediated olfactory injury involves bioaccumulation of Cd within the olfactory rosettes, poor Cd depuration, and a differential inhibition of ciliated and microvillus OSNs, associated with disruption of OST function. Under environmental conditions, such olfactory-mediated behavioral disruption could pose a risk to out-migrating juvenile salmon due to a failure to maintain normal behavioral functioning necessary for survivorship.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Andrew Yeh, Richard Ramsden, Dr Margaret Mills, and Dr Eva Ma. In addition, the assistance of Jon Wittouck at the University of Washington fisheries facility, and Dr Andrew Dittman at NOAA fisheries is greatly appreciated. Dr Brian Beckman and Abby Tillotson at NOAA fisheries, and also Heather Bartlett and John Kerwin (Washington Department of Fish and Wildlife) provided the juvenile coho salmon for our studies.
FUNDING
NIEHS Superfund Research Program (P42004696); the University of Washington Center for Ecogenetics and Environmental Health (P30-ES00703) and the University of Washington Environmental Toxicology and Pathology training grant (T32ES007032).
REFERENCES
- Atchison G. J., Henry M. G., Sandheinrich M. B. (1987). Effects of metals on fish behavior: a review. Environ. Biol. Fish Hague 18, 11–25. [Google Scholar]
- Baldwin D. H., Tatara C. P., Scholz N. L. (2011). Copper-induced olfactory toxicity in salmon and steelhead: extrapolation across species and rearing environments. Aquat. Toxicol. 101, 295–297. [DOI] [PubMed] [Google Scholar]
- Choong G., Liu Y., Templeton D. M. (2014). Interplay of calcium and cadmium in mediating cadmium toxicity. Chemico-Biol. Interact. 211, 54–65. [DOI] [PubMed] [Google Scholar]
- Cooper J. C., Scholz A. T., Horrall R. M., Hasler A. D., Madison D. M. (1976). Experimental confirmation of the olfactory hypothesis with homing, artificially imprinted coho salmon (Oncorhynchus kisutch). J. Fish. Res. Board Can. 33, 703–710. [Google Scholar]
- Coyle P., Philcox J. C., Carey L. C., Rofe A. M. (2002). Metallothionein: the multipurpose protein. Cell Mol. Life Sci. 59, 627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A., Plusquin M., Remans T., Jozefczak M., Keunen E., Gielen H., Opdenakker K., Nair A., Munters E., Artois T., et al. (2010). Cadmium stress: an oxidative challenge. BioMetals 23, 927–940. [DOI] [PubMed] [Google Scholar]
- Cygnar K. D., Collins S. E., Ferguson C. H., Bodkin-Clarke C., Zhao H. (2012). Phosphorylation of adenylyl cyclase III at serine(1076) does not attenuate olfactory response in mice. J. Neurosci. 32, 14557–14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino R., Pearson E. S. (1973). Tests for departure from normality. Empirical results for the distributions of b2 and √b1. Biometrika 60, 613–622. [Google Scholar]
- Del Punta K., Leinders-Zufall T., Rodriguez I., Jukam D., Wysocki C. J., Ogawa S., Zufall F., Mombaerts P. (2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419, 70–74. [DOI] [PubMed] [Google Scholar]
- DeMaria S., Ngai J. (2010). The cell biology of smell. J. Cell Biol. 191, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew W. A., Azizishirazi A., Pyle G. G. (2014). Contaminant-specific targeting of olfactory sensory neuron classes: connecting neuron class impairment with behavioural deficits. Chemosphere 112, 519–525. [DOI] [PubMed] [Google Scholar]
- Dittman A., Quinn T. (1996). Homing in Pacific salmon: mechanisms and ecological basis. J. Exp. Biol. 199, 83–91. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. (2016). Recommended Aquatic Life Ambient Water Quality Criteria for Cadmium. Code of Federal Regulations. Environmental Protection Agency, Washington, DC, 81 FR 19176, 19176–19178.
- Ercal N., Gurer-Orhan H., Aykin-Burns N. (2001). Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 1, 529–539. [DOI] [PubMed] [Google Scholar]
- Espinoza H. M., Williams C. R., Gallagher E. P. (2012). Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat. Toxicol. 110–111, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando S., Gambardella C., Ravera S., Bottero S., Ferrando T., Gallus L., Manno V., Salati A. P., Ramoino P., Tagliafierro G. (2009). Immunolocalization of G-protein alpha subunits in the olfactory system of the cartilaginous fish Scyliorhinus canicula. Anatom. Record Adv. Integr. Anat. Evol. Biol. 292, 1771–1779. [DOI] [PubMed] [Google Scholar]
- Green W. W., Mirza R. S., Wood C. M., Pyle G. G. (2010). Copper binding dynamics and olfactory impairment in fathead minnows (Pimephales promelas). Environ. Sci. Technol. 44, 1431–1437. [DOI] [PubMed] [Google Scholar]
- Hamdani el, H., Doving K. B. (2007). The functional organization of the fish olfactory system. Prog. Neurobiol. 82, 80–86. [DOI] [PubMed] [Google Scholar]
- Hansen A., Anderson K. T., Finger T. E. (2004). Differential distribution of olfactory receptor neurons in goldfish: structural and molecular correlates. J. Comp. Neurol. 477, 347–359. [DOI] [PubMed] [Google Scholar]
- Hansen A., Rolen S. H., Anderson K., Morita Y., Caprio J., Finger T. E. (2003). Correlation between olfactory receptor cell type and function in the channel catfish. J. Neurosci. 23, 9328–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T. J. (1992). Fish Chemoreception. Chapman & Hall, London. [Google Scholar]
- Harrison S. E., Klaverkamp J. F. (1989). Uptake, elimination and tissue distribution of dietary and aqueous cadmium by rainbow trout (salmo gairdneri richardson) and lake whitefish (coregonus clupeaformis mitchill). Environ. Toxicol. Chem. 8, 87–97. [Google Scholar]
- Hartwig A. (2001). Zinc finger proteins as potential targets for toxic metal ions: differential effects on structure and function. Antioxid. Redox Signal. 3, 625–634. [DOI] [PubMed] [Google Scholar]
- Huard J. M., Youngentob S. L., Goldstein B. J., Luskin M. B., Schwob J. E. (1998). Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J. Comp. Neurol. 400, 469–486. [PubMed] [Google Scholar]
- Jacobson K. B., Turner J. E. (1980). The interaction of cadmium and certain other metal ions with proteins and nucleic acids. Toxicology 16, 1–37. [DOI] [PubMed] [Google Scholar]
- Jang W., Youngentob S. L., Schwob J. E. (2003). Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J. Comp. Neurol. 460, 123–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. A., Banks M. A. (2011). Sequence conservation among orthologous vomeronasal type 1 receptor-like (ora) genes does not support the differential tuning hypothesis in Salmonidae. Gene 485, 16–21. [DOI] [PubMed] [Google Scholar]
- Johnstone K. A., Ciborowski K. L., Lubieniecki K., Chow P. W., Phillips R. B., Koop B. F., Jordan W. C., Davidson W. S. (2009). Genomic organization and evolution of the vomeronasal type 2 receptor-like (OlfC) gene clusters in Atlantic salmon, Salmo salar. Mol. Biol. Evol. 26, 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone K. A., Lubieniecki K. P., Chow W., Phillips R. B., Koop B. F., Davidson W. S. (2008). Genomic organization and characterization of two vomeronasal 1 receptor-like genes (ora1 and ora2) in Atlantic salmon Salmo salar. Mar. Genomics 1, 23–31. [DOI] [PubMed] [Google Scholar]
- Johnstone K. A., Lubieniecki K. P., Koop B. F., Davidson W. S. (2011). Expression of olfactory receptors in different life stages and life histories of wild Atlantic salmon (Salmo salar). Mol. Ecol. 20, 4059–4069. [DOI] [PubMed] [Google Scholar]
- Johnstone K. A., Lubieniecki K. P., Koop B. F., Davidson W. S. (2012). Identification of olfactory receptor genes in Atlantic salmon Salmo salar. J. Fish Biol. 81, 559–575. [DOI] [PubMed] [Google Scholar]
- Julliard A. K., Saucier D., Astic L. (1996). Time-course of apoptosis in the olfactory epithelium of rainbow trout exposed to a low copper level. Tissue Cell 28, 367–377. [DOI] [PubMed] [Google Scholar]
- Kolmakov N. N., Hubbard P. C., Lopes O., Canario A. V. (2009). Effect of acute copper sulfate exposure on olfactory responses to amino acids and pheromones in goldfish (Carassius auratus). Environ. Sci. Technol. 43, 8393–8399. [DOI] [PubMed] [Google Scholar]
- Kudo H., Doi Y., Ueda H., Kaeriyama M. (2009). Molecular characterization and histochemical demonstration of salmon olfactory marker protein in the olfactory epithelium of lacustrine sockeye salmon (Oncorhynchus nerka). Compar. Biochem. Physiol. A Mol. Integr. Physiol. 154, 142–150. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. (1986). Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 88, 321–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B., Hirono J., Sato T., Buck L. B. (1999). Combinatorial receptor codes for odors. Cell 96, 713–723. [DOI] [PubMed] [Google Scholar]
- Mathuru A. S., Kibat C., Cheong W. F., Shui G., Wenk M. R., Friedrich R. W., Jesuthasan S. (2012). Chondroitin fragments are odorants that trigger fear behavior in fish. Curr. Biol. 22, 538–544. [DOI] [PubMed] [Google Scholar]
- McIntyre J. K., Baldwin D. H., Beauchamp D. A., Scholz N. L. (2012). Low-level copper exposures increase visibility and vulnerability of juvenile coho salmon to cutthroat trout predators. Ecol. Appl. 22, 1460–1471. [DOI] [PubMed] [Google Scholar]
- McIntyre J. K., Baldwin D. H., Meador J. P., Scholz N. L. (2008). Chemosensory deprivation in juvenile coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ. Sci. Technol. 42, 1352–1358. [DOI] [PubMed] [Google Scholar]
- Naeve G. S., Ramakrishnan M., Kramer R., Hevroni D., Citri Y., Theill L. E. (1997). Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc. Natl. Acad. Sci. U.S.A. 94, 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. P. (2011). The Behavior and Ecology of Pacific Salmon and Trout. UBC Press, Vancouver. [Google Scholar]
- Ruggerone G. T., Volk E. C. (2003). Residence time and growth of natural and hatchery chinook salmon in the Duwamish Estuary and Elliott Bay, Washington, based on otolith chemical and structural attributes. Draft. Prepared for US Army Corps of Engineers, Seattle District and Port of Seattle. Natural Resources Consultants, Inc., Seattle, WA.
- Ryter S. W., Choi A. M. K. (2002). Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 4, 625–632. [DOI] [PubMed] [Google Scholar]
- Sandahl J. F., Baldwin D. H., Jenkins J. J., Scholz N. L. (2007). A sensory system at the interface between urban stormwater runoff and salmon survival. Environ. Sci. Technol. 41, 2998–3004. [DOI] [PubMed] [Google Scholar]
- Scott G. R., Sloman K. A., Rouleau C., Wood C. M. (2003). Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 206, 1779–1790. [DOI] [PubMed] [Google Scholar]
- Sloman K. A. (2007). Effects of trace metals on salmonid fish: the role of social hierarchies. Appl. Anim. Behav. Sci. 104, 326–345. [Google Scholar]
- Sloman K. A., Scott G. R., Diao Z., Rouleau C., Wood C. M., McDonald D. G. (2003). Cadmium affects the social behaviour of rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 65, 171–185. [DOI] [PubMed] [Google Scholar]
- Srinivasa Gowd S., Govil P. (2008). Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ. Monit. Assess. 136, 197–207. [DOI] [PubMed] [Google Scholar]
- Sutterlin A. M., Gray R. (1973). Chemical basis for homing of Atlantic Salmon (Salmo salar) to a Hatchery. J. Fish. Res. Board Can. 30, 985–989. [Google Scholar]
- Tallkvist J., Persson E., Henriksson J., Tjalve H. (2002). Cadmium-metallothionein interactions in the olfactory pathways of rats and pikes. Toxicol. Sci. 67, 108–113. [DOI] [PubMed] [Google Scholar]
- Tessarolo J. A., Tabesh M. J., Nesbitt M., Davidson W. S. (2014). Genomic Organization and Evolution of the Trace Amine-Associated Receptor (TAAR) Repertoire in Atlantic Salmon (Salmo salar). G3 4, 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenod F. (2009). Cadmium and cellular signaling cascades: To be or not to be?. Toxicol. Appl. Pharmacol. 238, 221–239. [DOI] [PubMed] [Google Scholar]
- Tierney K. B., Baldwin D. H., Hara T. J., Ross P. S., Scholz N. L., Kennedy C. J. (2010). Olfactory toxicity in fishes. Aquat. Toxicol. 96, 2–26. [DOI] [PubMed] [Google Scholar]
- Tierney K. B., Sampson J. L., Ross P. S., Sekela M. A., Kennedy C. J. (2008). Salmon olfaction is impaired by an environmentally realistic pesticide mixture. Environ. Sci. Technol. 42, 4996–5001. [DOI] [PubMed] [Google Scholar]
- Tjälve H., Gottofrey J., Björklund I. (1986). Tissue disposition of 109cd2+ in the brown trout (Salmo trutta) studied by autoradiography and impulse counting. Toxicol. Environ. Chem. 12, 31–45. [Google Scholar]
- Troemel E. R., Kimmel B. E., Bargmann C. I. (1997). Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169. [DOI] [PubMed] [Google Scholar]
- Usai C., Barberis A., Moccagatta L., Marchetti C. (1999). Pathways of cadmium influx in mammalian neurons. J. Neurochem. 72, 2154–2161. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhong X. P., Qiao Z. X., Gui J. F. (2008). Inductive transcription and protective role of fish heme oxygenase-1 under hypoxic stress. J. Exp. Biol. 211, 2700–2706. [DOI] [PubMed] [Google Scholar]
- Wang J., Luthey-Schulten Z. A., Suslick K. S. (2003). Is the olfactory receptor a metalloprotein? Proc. Natl. Acad. Sci. U.S.A. 100, 3035–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington Department of Ecology. (2008) Control of Toxic Chemicals in Puget Sound Phase 2: Improved Estimates of Loadings from Surface Runoff and Roadways. Technical report 1–162.
- Wei J., Zhao A. Z., Chan G. C. K., Baker L. P., Impey S., Beavo J. A., Storm D. R. (1998). Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron 21, 495–504. [DOI] [PubMed] [Google Scholar]
- Williams C. R., Gallagher E. P. (2013). Effects of cadmium on olfactory mediated behaviors and molecular biomarkers in coho salmon (Oncorhynchus kisutch). Aquat. Toxicol. 140, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippel H. P., Sorensen P. W., Hansen A. (1997). High correlation between microvillous olfactory receptor cell abundance and sensitivity to pheromones in olfactory nerve-sectioned goldfish. J. Comparat. Physiol. A 180, 39–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.