Dear Editor
Glutathione (GSH) is an essential tri-peptide important for several plant processes, including the control of cellular redox status, detoxification of xenobiotics, root development, heavy metal transport and resistance, and long-distance transport of organic sulfur. Cadmium and arsenic are detoxified by small GSH-derived peptides, called phytochelatins (PCs). Phytochelatins are rapidly synthesized in response to toxic metal exposure and have long been known to play an integral role in metal(loid) detoxification in plants. However, while phytochelatins are largely synthesized in roots, GSH is mainly synthesized in chloroplasts and transported in the phloem (Wachter et al., 2005; Mendoza-Cózatl et al., 2008). Thus, GSH transport from shoots to roots is critical for phytochelatin synthesis in plants. However, genes encoding plasma membrane GSH-specific transporters remain largely unknown (Zechmann, 2014; Pike et al., 2009; Cagnac et al., 2004).
In this study, a screening method to identify plant GSH transporters was pursued. We used a Saccharomyces cerevisiae sulfur amino acid auxotrophic mutant strain met15 opt1, which is defective in methionine (MET) synthesis and GSH uptake (Supplemental Figure 1A). This strain is unable to grow on media containing GSH as the sole sulfur source, but can be complemented using the galactose-inducible expression vector pYES2 carrying the native yeast high-affinity GSH transporter ScOPT1 (Supplemental Figure 1B). We hypothesized that an Arabidopsis homologue of ScOPT1 may be responsible for GSH transport in plants; thus, all nine Arabidopsis oligopeptide transporter genes (AtOPT 1–9) (Supplemental Figure 2) were cloned into the pYES2 expression vector along with ScOPT1 (Supplemental Table 1). These constructs were transformed individually into the yeast met15 opt1 mutant background along with the empty pYES2 vector (control). Transformant strains were then used for growth complementation assays on media containing GSH as the sole sulfur source (Figure 1A). While wild-type yeast grew well on GSH-containing media, the met15 opt1 mutant was unable to grow (Figure 1A). In positive controls, expression of ScOPT1 rescued the mutant phenotype as expected (Figure 1A). In addition, previous studies suggested that AtOPT6, BjGT1 and OsGT1 transport GSH in Arabidopsis, Brassica juncea and Oryza sativa, respectively, but none of them restored growth in the yeast strain using GSH as the sole sulfur source (Cagnac et al., 2004). Interestingly, AtOPT4 rescued the met15 opt1 mutant in the presence of GSH as the sole sulfur source. However, other members of the Arabidopsis oligopeptide transporter family were unable to rescue the auxotrophic phenotype of the met15 opt1 mutant (Figure 1A). AtOPT8 showed a weak growth phenotype that was not consistently observed under the imposed conditions in independent experiments. Furthermore, some AtOPTs (AtOPT1, 3, 5, 6, 7) inhibited yeast growth in control galactose-inducing media (Figure 1A, left) but not in glucose-containing media, indicating limitations in the functional analyses of these OPT transporters, consistent with a recent study on AtOPT3 expression in yeast (Mendoza-Cózatl et al., 2014).
Figure 1. The Arabidopsis Transporter AtOPT4 Mediates GSH Uptake in Yeast.
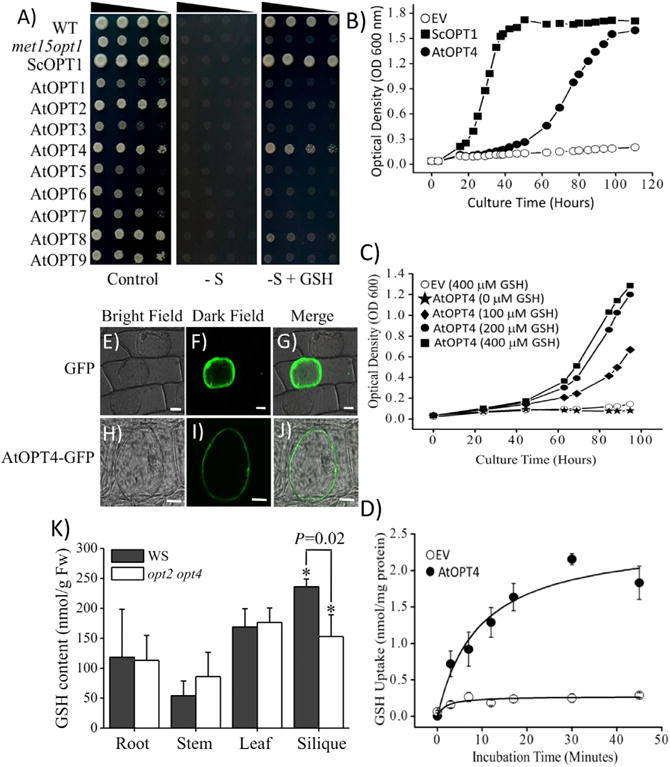
(A) Yeast growth complementation assays performed by expressing nine cDNAs (AtOPT1 to AtOPT9) in a sulfur amino acid auxotrophic yeast strain (met15 opt1) that cannot grow using GSH as the sole sulfur source (see Supplemental Figure 1). Transformed yeast strains carrying the pYES2 plasmid and the indicated constructs were inoculated and incubated at 28°C for 8 days on either control medium (control), medium without sulfur salts or sulfur-containing amino acids (−S), or medium without sulfur salts or sulfur-containing amino acids but supplemented with 400 μM GSH (−S + GSH).
(B) Rescue of met15 opt1 growth by expressing ScOPT1 or AtOPT4 in liquid medium supplemented with 400 μM GSH as the sole sulfur source.
(C) Growth of the auxotrophic yeast strain met15 opt1 expressing AtOPT4 is dependent on the concentration of GSH supplied to the medium as the sole sulfur source. GSH concentrations from 0–400 μM were evaluated.
(D) Radiolabeled [35S]GSH time course influx assays. The yeast met15 opt1 strain was transformed with AtOPT4 and the empty vector pYES2. The cells were incubated in 400 μM [35S]GSH solution and uptake was stopped by filtration. Radioactivity in yeast retained by filters was detected at the indicated times (n = 3). Empty vector (EV) symbols in (B–D) depict pYES2 empty vector controls.
(E–J) Subcellular localization of AtOPT4. Onion epidermal cells expressing GFP alone (E, F, and G) or an AtOPT4-GFP fusion protein (H, I, and J) were detected by confocal microscopy after plasmolysis with 0.8 M mannitol. Green regions show the fluorescence of GFP alone (F and G) or of the AtOPT4-GFP fusion protein (I and J). Bars represent 50 μm (E, F, and G) or 30 μm (H, I, and J).
(K) GSH content assays in various tissues of wild-type (WS) and opt2 opt4 double mutant. After growth in soil for 45 days, tissues were separated from plants of WS and opt2 opt4 double mutants for GSH measurements. GSH content was not significantly different in roots, stems, and leaves of the opt2 opt4 double mutants compared with WS, but was significantly lower in the siliques of the opt2 opt4 double mutant compared with WS. The asterisks on the bars indicate significant differences after t-test statistical analyses. Data represents means ± SE (n = 3).
Repeated experiments and additional complementation assays confirmed our original observation that expression of AtOPT4 was able to rescue the growth phenotype of met15 opt1 (Figure 1A and Supplemental Figure 3A). Radiolabeled [35S]GSH uptake assays provided additional direct evidence that AtOPT4 mediates GSH influx into yeast (Figure 1D).
To further characterize the ability of AtOPT4 to transport glutathione, we evaluated the growth rate of met15 opt1 expressing the empty pYES2 plasmid (EV), ScOPT1, or AtOPT4 in liquid media using GSH as the sole sulfur source. No growth was observed in cultures expressing the empty vector; however, cultures expressing ScOPT1 and AtOPT4 reached the stationary phase of growth (Figure 1B). We also determined that the growth of yeast expressing AtOPT4 was dependent on the GSH concentration in the culture media in the range of 0–400 μM GSH (Figure 1C). Notably, cultures expressing ScOPT1 reached stationary growth within 2 days, while cultures expressing AtOPT4 took more than 4 days (Figure 1B). These data suggested that the rate of GSH uptake may be lower for AtOPT4 than for ScOPT1. To test this hypothesis, we performed radiotracer experiments using media supplemented with [35S] GSH. Results from these experiments confirm that expression of AtOPT4 increases GSH influx above background. Furthermore, [35S]GSH uptake by AtOPT4 followed Michaelis–Menten kinetics with an apparent affinity constant (Km) of 1.4 ± 0.3 mM GSH and a maximum transport rate (Vmax) of 2.1 ± 0.3 nmol (mg protein)−1·min−1.(Supplemental Figure 3B). The yeast growth and [35S]GSH flux data suggest that AtOPT4 is a low-affinity GSH transporter.
To gain insight into the subcellular localization of AtOPT4 in plant cells, we fused green fluorescent protein (GFP) to the carboxyl terminus of AtOPT4 and expressed the construct under the control of the constitutive 35S promoter. The AtOPT4-GFP construct was transiently expressed in onion epidermal cells using untagged GFP as a positive control (Figure 1E–1J). Fluorescence from GFP alone localized in the cytoplasm and nucleus (Figure 1E–1G). In contrast, a ring of fluorescence was observed at the plasma membrane of osmotically plasmolyzed onion cells transfected with the AtOPT4-GFP construct (Figure 1H–1J).
To determine the function of AtOPT4 in GSH transport and metal(loid) detoxification in planta, we performed GSH measurements and root elongation assays in an Arabidopsis mutant carrying a T-DNA insertion in the AtOPT4 coding region and also in AtOPT2, which is the closest paralog of AtOPT4 (Supplemental Figure 2) and currently has no known function in planta. These experiments were also performed in opt2 opt4 double mutant plants. The GSH content was not significantly different in roots, stems, and leaves of either the opt4 mutant or the opt2 opt4 mutant compared with wild-type lines (WS) (Figure 1K and Supplemental Figure 4A). In addition, AtOPT2 loss-of-function did not affect GSH content in siliques compared with WS (Supplemental Figure 4B). However, the GSH content in siliques was consistently lower in opt4 and opt2 opt4 mutants compared with WS at a 93% (opt4) to 98% (opt2 opt4) CI (Figure 1K and Supplemental Figure 4A). Thus, GSH measurements showed decreased GSH content in the siliques of opt2 opt4 mutants suggesting a function in silique GSH transport. When opt4 and opt2 mutant seedlings were germinated and grown on media supplemented with 40 μM cadmium (Cd) or 5 μM arsenite (As (III)), no visible growth defects were observed (Supplemental Figure 5). Furthermore, we observed no significant differences in root growth between WS and the opt2 opt4 double mutant lines when germinated and grown on 40 μM Cd or 5 μM As(III) (Supplemental Figure 6).
A previous study did not observe complementation of GSH uptake by OPT4 in the met15 opt1 deletion strain (Osawa et al., 2006). This can likely be explained by relevant differences in methodology. Osawa et al. used 100 μM GSH for yeast growth experiments. In comparison, we used up to 400 μM GSH in our study. Furthermore, there may be temporal differences between the two studies. Our yeast complementation experiments were repeatedly performed over a period of 12 days (Supplemental Figure 3A) and complementation by AtOPT4 was clearly observed after 8 days (Figure 1A). Figure 1B shows that it took approximately 30 hours of growth on GSH for the AtOPT4 complemented yeast to reach an OD600 above background, and our kinetic studies show a low affinity for GSH transport by AtOPT4. It is not surprising that the relevant study of Osawa et al. (2006) did not observe GSH transport activities in 6-h yeast experiments. Our specific growth conditions allowed for the identification of AtOPT4 as a low-affinity GSH transporter.
Substantial progress has been made in elucidating the transport mechanisms of thiol-containing peptides and the processes that plants use to extract, detoxify, and store toxic heavy metal(loid)s. A recent advance was the identification and characterization of the vacuolar transporters AtABCC1, AtABCC2, and SpAbc2, which are responsible for vacuolar sequestration of phytochelatin/thiol-complexed As, Cd, and Hg in Arabidopsis and Schizosaccharomyces pombe (Mendoza-Cózatl et al., 2010; Song et al., 2010; Park et al., 2012). However, genes encoding plasma membrane GSH transporters remain largely elusive in plants. A previous study showed that AtOPT6 has a high affinity for penta- and dodecapeptides, and has a very low affinity for reduced GSH when expressed in Xenopus laevis oocytes (Pike et al., 2009). Our yeast complementation results coupled with our radiotracer transport analyses and in planta localization of AtOPT4 provide strong evidence that AtOPT4 functions as a low-affinity plasma membrane GSH transporter, with previous research showing transport of additional peptides (Osawa et al., 2006). AtOPT4 is preferentially expressed throughout the vasculature of rosette leaves, stems, and roots suggesting that OPT4 may function broadly in mediating the long-distance transport of GSH to various tissues in planta (Stacey et al., 2006). However, in the present study, GSH measurements in different tissues indicates that AtOPT4 loss-of-function mutants have decreased GSH content only in siliques compared with wild-type. Furthermore, this decrease in silique GSH content was exacerbated in the opt2 opt4 double mutant (Figure 1K and Supplemental Figure 4A). It has been previously shown that AtOPT4 expression is very high in the vascular tissues of funiculi and siliques during seed development (Stacey et al., 2006). Therefore, we propose that AtOPT4 functions in GSH loading/unloading in siliques. Consistent with this hypothesis, we found that AtOPT4 has no significant role in GSH transport in roots and leaves as shown by the lack of significant growth difference between opt4 mutant and WS seedlings with or without toxic metal(loid) treatments (Supplemental Figure 5). AtOPT2, the closest paralog of AtOPT4, showed no GSH transport activity in yeast or plant tissues under the imposed conditions. Because AtOPT4 is a low-affinity transporter, it is likely that no growth defect phenotypes were observed due to the presence of higher-affinity GSH transporters or additional low-affinity transporters compensating for the loss of AtOPT4 and AtOPT2 in the mutant lines. Creating higher-order mutants after additional GSH transporters have been identified will help to resolve this question.
The identification of substrates for AtOPT proteins has proven to be challenging. For instance, recent studies have demonstrated an important role for AtOPT3 in the regulation of iron nutrient status from leaves to roots (Mendoza-Cózatl et al., 2014; Zhai et al., 2014). However, the substrate of OPT3 remains controversial, because an iron transport function of OPT3 in yeast (Zhai et al., 2014) could not be confirmed (Mendoza-Cózatl et al., 2014 Further work will help to establish the precise functions of AtOPT3, AtOPT4, and other OPT proteins in the long-distance signaling and mobilization of peptides.
In summary, the present study demonstrates that AtOPT4 encodes a component of the long-sought plasma membrane GSH transport machinery. When expressed in a heterologous yeast system, AtOPT4 showed GSH uptake at levels sufficient to complement a sulfur auxotrophic yeast mutant. Further biochemical characterization showed that AtOPT4 functions as a low-affinity GSH transporter. Finally, GSH measurements in planta suggest that AtOPT4 may contribute to GSH loading/unloading in siliques. Because GSH plays such an important role in maintaining the cellular redox state, toxic metal(loid) resistance, and many other cellular processes, it is likely that screening for additional GSH/thiol peptide-related phenotypes may help identify specific roles of plant OPT transporters.
Supplementary Material
Acknowledgments
FUNDING
This research was supported by the National Institute of Environmental Health Sciences (grant no. ES010337; JIS). D.M.C. was supported by a US National Science Foundation CAREER grant (IOS-1252706). Z.Z. was supported by an international graduate fellowship from China Scholarship Council at UCSD and the National Natural Science Foundation of China (No. 31300211) and self-determined research funds of CCNU from the colleges’ basic research and operation of MOE. Q.Q.X. was supported by an international graduate fellowship from China Scholarship Council at UCSD. T.O.J. was supported by the UCSD-Salk NSF IGERT Plant Systems Biology Interdisciplinary Graduate Training Program (grant no. 0504645).
We thank Justin D. Nguyen for his support with the preparatory work for some of the experiments.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information is available at Molecular Plant Online.
No conflict of interest declared.
References
- Cagnac O, Bourbouloux A, Chakrabarty D, Zhang MY, Delrot S. AtOPT6 transports glutathione derivatives and is induced by primisulfuron. Plant Physiol. 2004;135:1378–1387. doi: 10.1104/pp.104.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Zhai Z, Jobe TO, Akmakjian GZ, Song WY, Limbo O, Russell MR, Kozlovskyy VI, Martinoia E, Vatamaniuk OK, et al. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. J Biol Chem. 2010;285:40416–40426. doi: 10.1074/jbc.M110.155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Xie Q, Akmakjian GZ, Jobe TO, Patel A, Stacey MG, Song L, Demoin DW, Jurisson SS, Stacey G, et al. OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Mol Plant. 2014;7:1455–1469. doi: 10.1093/mp/ssu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Stacey G, Gassmann W. ScOPT1 and AtOPT4 function as proton-coupled oligopeptide transporters with broad but distinct substrate specificities. Biochem J. 2006;393:267–275. doi: 10.1042/BJ20050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012;69:278–288. doi: 10.1111/j.1365-313X.2011.04789.x. [DOI] [PubMed] [Google Scholar]
- Pike S, Patel A, Stacey G, Gassmann W. Arabidopsis OPT6 is an oligopeptide transporter with exceptionally broad substrate specificity. Plant Cell Physiol. 2009;50:1923–1932. doi: 10.1093/pcp/pcp136. [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MG, Osawa H, Patel A, Gassmann W, Stacey G. Expression analyses of Arabidopsis oligopeptide transporters during seed germination, vegetative growth and reproduction. Planta. 2006;223:291–305. doi: 10.1007/s00425-005-0087-x. [DOI] [PubMed] [Google Scholar]
- Wachter A, Wolf S, Steininger H, Bogs J, Rausch T. Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 2005;41:15–30. doi: 10.1111/j.1365-313X.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- Zechmann B. Compartment-specific importance of glutathione during abiotic and biotic stress. Front Plant Sci. 2014;5:566. doi: 10.3389/fpls.2014.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Gayomba SR, Jung HI, Vimalakumari NK, Piñeros M, Craft E, Rutzke MA, Danku J, Lahner B, Punshon T, et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell. 2014;26:2249–2264. doi: 10.1105/tpc.114.123737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


