Abstract
Background
In-hospital worsening heart failure (WHF) is an important event that has inconsistent definitions used across trials. We used data from two acute heart failure (HF) trials from the NIH HF Network, DOSE and ROSE, to understand event rates associated with different WHF definitions.
Methods and Results
We pooled data from 668 patients in DOSE and ROSE and assessed the relationship between WHF and the composite endpoint of re-hospitalization, emergency room visits for HF, and mortality through 60 days. We also assessed for a differential relationship between the timing of WHF development and outcomes. The overall incidence of WHF was 14.6% (24.1% in DOSE, 6.3% in ROSE, and 5.0% in DOSE using the ROSE definition). WHF was associated with an increase in the composite endpoint (hazard ratio [HR] 1.64; 95% confidence interval [CI] 1.11–2.42; p= 0.01). However, the association between WHF and outcomes was significantly stronger in ROSE compared to DOSE (HR 2.67, 95% 1.45–4.91, p<0.01 vs. HR 1.28, 95% CI 0.79–2.08, p=0.31, respectively). Development of WHF between baseline to 24 hours compared with 24–48 hours or 48–72 hours demonstrated a trend towards improved outcomes (HR 0.49, 95% CI 0.21 – 1.17, p= 0.11 and HR 0.45, 95% CI 0.20 – 1.04, p =0.06, respectively).
Conclusions
A WHF definition that excluded the intensification of diuretics resulted in a lower event rate but a stronger association with outcomes. These data support the need for continued efforts to standardize WHF definitions in clinical trials.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifiers: NCT00577135 (DOSE) and NCT01132846 (ROSE).
Keywords: heart failure, acute heart failure, outcomes research, worsening heart failure, definitions
Acute heart failure (AHF) is associated with significant morbidity and mortality.1 A subset of patients hospitalized with AHF experience worsening heart failure (WHF) during hospitalization, which is associated with increased risk of worse clinical outcomes.2 WHF is generally considered to require both persistent or worsening signs or symptoms and some escalation of therapy directed at those symptoms. WHF has been an endpoint in previous and ongoing AHF trials.3–4 Outcomes data from registries and secondary analyses from clinical trials suggest that patients who experience WHF are at increased risk for subsequent events.2, 5–7
There have been several small studies and an analysis of the ADHERE registry that have defined the prevalence and assessed the association between WHF and clinical outcomes.2, 8–10 The definitions used in these previous analyses have varied in two ways (1) by whether “worsening” is a subjective assessment (e.g., treating physician making a categorical assessment of WHF on loosely defined scoring system) by the clinician or an objective measure (e.g., at least 1 sign, symptom, or radiologic evidence of new, persistent, or worsening acute HF requiring addition of a new intravenous therapy or mechanical support during a patient’s index hospitalization targeted specifically at HF symptoms), and (2) by the variables that constitute “escalation of therapy” ranging from additional open-label loop or thiazide diuretic to intravenous inotrope/vasodilator therapy or mechanical circulatory support.3, 8, 9, 11–13
Butler et al. have recently suggested that the clinical implications of inpatient WHF are clear, but there is a need to better understand the underlying pathophysiology of WHF, and the implications of the lack of a consensus definition of WHF that currently exists.14 We used data from two acute HF trials conducted by the National Heart, Lung, and Blood Institute (NHLBI)-sponsored Heart Failure Network to understand how different WHF definitions impact clinical event rates and outcomes.11,12 The influence of the time of development of WHF clinical events was also evaluated.
Methods
Data Source
We performed a pooled analysis from non-overlapping patients from two acute HF trials conducted by the HF Network: Renal Optimization Strategies in Acute Heart Failure (ROSE-AHF) and Diuretic Optimization Strategies Evaluation in Acute Heart Failure (DOSE-AHF) totaling 668 patients. 646 patients were available for analysis given the inability to evaluate 4 patients enrolled in both DOSE and ROSE and 18 patients without ascertainment of WHF from randomization to 72 hours (Figure 1). ROSE-AHF was a multicenter, double-blind, placebo-controlled clinical trial that enrolled 360 hospitalized patients with acute HF and renal dysfunction, randomized within 24 hours of admission. Participants were randomized in an open, 1:1 allocation ratio to dopamine or nesiritide.12 Within each group, participants were randomized in a double-blind, 2:1 ratio to active treatment or placebo leading to participants on dopamine (n=122), nesiritide (n=119) and placebo (n=119) with independent comparisons with the pooled placebo group.12,15 The ROSE co-primary end points included 72-hour cumulative urine volume (decongestion end point) and the change in serum cystatin C from enrollment to 72 hours (renal function end point). DOSE was a prospective, double-blind, randomized trial that enrolled 308 patients with acute HF.11 Participants were similarly randomized as in ROSE to receive furosemide administered intravenously by means of either a bolus every 12 hours or continuous infusion and at either a low dose (numerically equivalent to the patient’s previous oral dose given intravenously) or a high dose (2.5 times the previous oral dose given intravenously). The protocol allowed specified dose adjustments after 48 hours.
Figure 1.
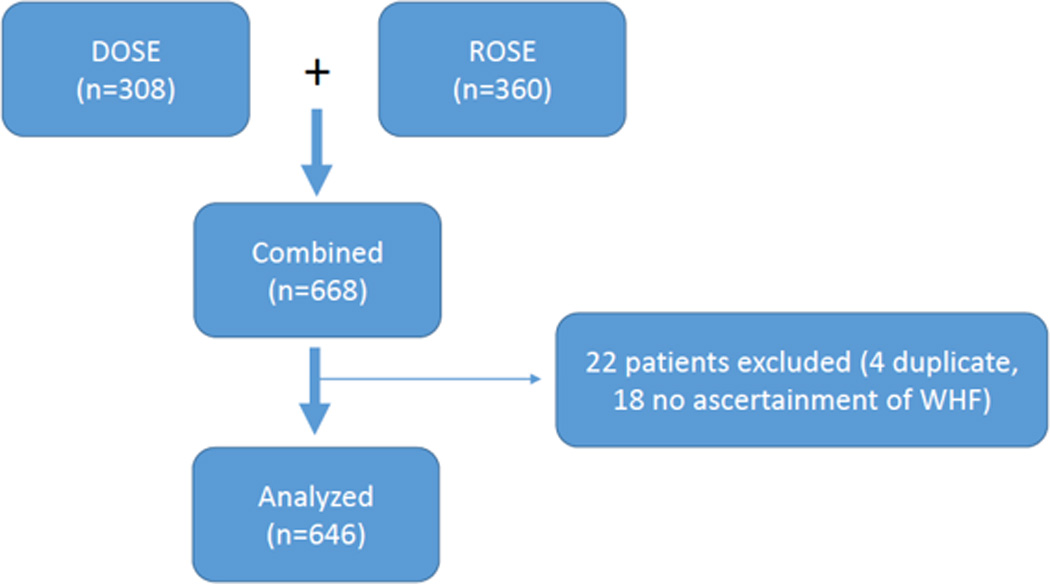
Flow chart of patients in ROSE and DOSE who were analyzed in this analysis. 668 patients were available for analysis with 22 total patients excluded. 4 patients were excluded because of duplicate participation and 18 patients were excluded because there was no ascertainment of WHF.
Patients were eligible for enrollment in DOSE if they presented to the hospital within the previous 24 hours with acute HF. Acute HF was diagnosed on the basis of the presence of at least one symptom (dyspnea, orthopnea, or edema) and one sign (rales, peripheral edema, ascites, or pulmonary vascular congestion on chest radiography) of HF regarding of ejection fraction. Additional eligibility criteria for DOSE were a history of chronic HF and receipt of an oral loop diuretic for at least 1 month before hospitalization, at a dose between 80 mg and 240 mg daily in the case of furosemide and an equivalent dose in the case of a different loop diuretic. Similarly, patients were eligible for ROSE if they had a prior clinical diagnosis of HF, were hospitalized for acute HF and had renal dysfunction (glomerular filtration rate of 15–60 mL/min/1.73 m2 as estimated by the Modification of diet Renal Disease equation) at admission and were enrolled within 24 hours of admission. Patients were excluded from DOSE and ROSE with a systolic blood pressure less than 90 mm Hg or receiving or anticipated to need intravenous vasodilators or inotropic agents (other than digoxin) or ultra-filtration therapy for HF. Patients were excluded from DOSE with a serum creatinine level that was greater than 3.0 mg per deciliter (265.2 umol per liter). Thiazide diuretics were permitted if the patient had been taking them on a long-term basis.
Each participating center’s ethics committee or institutional review board approved the study and all patients gave written informed consent. The present analysis compares patients enrolled in ROSE and DOSE who experienced WHF during their index hospitalization compared with patients who did not experience WHF. The authors are solely responsible for the design, conduct of this study, all study analyses, and the drafting of the manuscript and final contents.
Definitions
In both trials, WHF was defined as persistent or worsening HF requiring rescue therapy including IV vasoactive agents, ultrafiltration, or mechanical support over 72 hours after randomization. In addition, in DOSE, the WHF definition also included additional open label loop or thiazide diuretic. Specifically, WHF had to be treated with open label loop or thiazide diuretic doses greater than what the patient was taking at their randomized treatment assignment. Because the ROSE case report form did not record the additional DOSE qualifying definition for WHF, a sensitivity analysis was unable to be performed using each WHF definition in each trial. However, we were able to assess the event rate in DOSE using the stricter ROSE definition. WHF status was documented post-randomization, at 0–24 hours, 24–48 hours, and 48–72 hours post-randomization based on protocol defined daily assessments. HF re-hospitalization, all-cause mortality, and ER visits for HF were documented by study coordinators and clinicians on a standardized case report form. HF re-hospitalization and assessment of adverse events, including death, were evaluated within 60 days after randomization. All clinical endpoints were investigator reported. Rehospitalization was defined as a hospital stay ≥ 24 hours. ER visits were defined as a visit to an emergency department related to HF with signs, symptoms or IV treatment for HF.
Outcomes of interest
The primary outcome for the present analysis was the composite endpoint of HF rehospitalization, ER visits for HF, and mortality through 60 days.
Statistical analysis and clinical endpoints
Demographics, physical and laboratory findings, medical history, and therapies were summarized as frequencies and percentages for categorical variables and by medians and 25th and 75th percentiles for continuous variables. Baseline characteristics were compared using Wilcoxon rank sum test for continuous variables, and Pearson chi-square tests for categorical variables. We assessed the relationship between development of WHF within 72 hours of randomization as well as timing of development of WHF (early vs. late) and the composite endpoint of HF re-hospitalization, emergency room (ER) visits for HF, and mortality through 60 days. Cox proportional hazards models using Landmark analysis were used to evaluate time to first event in the composite endpoint from 72 hours to 60 days. Subjects experiencing the composite endpoint prior to 72 hours were eliminated from the analyses and the anchor for time to event was 72 hours. Analyses in which both trials are combined, the models were adjusted for trial; otherwise, all models were unadjusted for any potential confounders. Statistical significance was assessed using 2-sided p-values, with values <0.05 considered statistically significant. All statistical computations were generated using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) or higher.
Results
WHF occurred in 14.6% of patients in the pooled cohort--24.1% in DOSE and 6.3% in ROSE (Table 1). Using the ROSE definition in the DOSE trial cohort decreased the WHF event rate to 5.0% in the DOSE cohort of patients (Table 2). Patients who developed WHF within 72 hours post-randomization were generally similar to those that did not with regard to age, race, gender, comorbidities, and hospitalization for HF in the previous year. Patients who developed WHF had higher baseline daily diuretic use compared to patients who did not develop WHF (p=0.003). Vital signs, physical examination findings, and laboratory values were similar for patients with and without WHF, with the exception of median systolic blood pressure, which was lower in patients with WHF (p= 0.03). A larger percentage of patients who did not develop WHF had history of peripheral edema when compared to subjects with WHF (p<0.01).
Table 1.
Baseline Characteristics by Worsening Heart Failure from Randomization to 72 Hours in ROSE and DOSE
| No WHF† | WHF† | p-value | |
|---|---|---|---|
| N* | 552 | 94 | |
| Ages, yrs | 69.0 (60.0, 79.0) | 68.5 (58.0, 76.0) | 0.33 |
| Male sex | 406/552 (73.6%) | 68/94 (72.3%) | 0.81 |
| White | 406/552 (73.6%) | 74/94 (78.7%) | 0.29 |
| EF | 32.0 (20.0, 52.0) | 28.0 (20.0, 50.0) | 0.15 |
| Hospitalization for HF in last year |
380/548 (69.3%) | 70/92 (76.1%) | 0.19 |
| Ischemic etiology of HF | 320/552 (58.0%) | 54/94 (57.4%) | 0.92 |
| Atrial fibrillation/flutter | 308/552 (55.8%) | 55/94 (58.5%) | 0.62 |
| Diabetes | 298/552 (54.0%) | 53/94 (56.4%) | 0.67 |
| Beta Blockers | 461/552 (83.5%) | 78/94 (83.0%) | 0.90 |
| Aldosterone antagonist | 152/552 (27.5%) | 34/94 (36.2%) | 0.09 |
| Furosemide equivalent dose, mg/day |
0.003 | ||
| 80.0 (80.0, 160.0) | 120.0 (80.0, 160.0) | ||
| Weight, lbs | 200.5 (170.4, 245.6) | 203.0 (180.5, 235.9) | 0.64 |
| Systolic blood pressure, mmHg |
115.0 (104.0, 130.0) | 110.5 (100.0, 125.0) | 0.03 |
| Heart rate, beats/min | 75.0 (67.0, 84.0) | 76.0 (68.0, 90.0) | 0.22 |
| JVP >= 8 cm | 490/524 (93.5%) | 86/91 (94.5%) | 0.72 |
| Orthopnea | 476/525 (90.7%) | 81/92 (88.0%) | 0.43 |
| Rales | 248/548 (45.3%) | 31/91 (34.1%) | 0.06 |
| Peripheral Edema b | 106/550 (19.3%) | 15/94 (16.0%) | < 0.0001 |
| COPD | 137/552 (24.8%) | 28/94 (29.8%) | 0.31 |
| NYHA Class | 0.22 | ||
| I | 1/519 (0.2%) | 0/82 (0.0%) | |
| II | 24/519 (4.6%) | 3/82 (3.7%) | |
| III | 344/519 (66.3%) | 46/82 (56.1%) | |
| IV | 150/519 (28.9%) | 33/82 (40.2%) | |
| Sodium, mg/L | 139.0 (136.0, 141.0) | 138.0 (135.0, 141.0) | 0.18 |
| BUN, mg/dl | 33.5 (24.0, 49.0) | 38.7 (26.2, 58.0) | 0.048 |
| Mean (sd) | 39.5 (22.2) | 43.7 (22.1) | |
| Creatinine, mg/dl | 1.6 (1.3, 2.0) | 1.7 (1.4, 2.0) | 0.33 |
| NT-pro BNP, pg/ml | 4725 (2353, 10386) | 4357 (2587, 9833) | 0.99 |
WHF indicates worsening heart failure; sd, standard deviation; lbs, pounds; JVP, jugular venous pressure; COPD, chronic obstructive pulmonary disorder; NYHA, New York Heart Association; NT-proBNP, N-terminal-pro brain natriuretic peptide; pg, pictogram; ml, milliliter; number
data presented as median (25th, 75th) or n (%) unless otherwise reported.
Model has been adjusted for trial participant was enrolled in.
Table 2.
WHF rates according to Trial Definition
| Therapy | DOSE trial | ROSE trial |
|---|---|---|
| DOSE WHF definition | 72/299 (24.1%) | N/A |
| ROSE WHF definition | 15/299 (5.0%) | 22/347 (6.3%) |
WHF indicates worsening heart failure; ROSE, Renal Optimization Strategies; DOSE, Diuretic Optimization Strategies Evaluation; N/A, Not applicable.
In ROSE, WHF events were driven by treatment with IV vasoactive agents (90.9%) and ultrafitration in one patient (4.5%) (Table 3). WHF events in DOSE were largely driven by additional loop diuretic, thiazide or metolazone (90.3%) compared with IV vasoactive agents (12.5%).
Table 3.
Qualifying therapies among subjects who developed WHF*.
| Therapy | DOSE N=72 |
ROSE N=22 |
|---|---|---|
| IV vasoactive agent | 9 (12.5%) | 20 (90.9%) |
| Mechanical or circulatory support |
0 (0%) | 0 (0%) |
| Ultra-filtration | 0 (0%) | 1 (4.5%) |
| Loop diuretic, thiazide or metolazone |
65 (90.3%) | N/A |
WHF indicates worsening heart failure; ROSE, Renal Optimization Strategies; DOSE, Diuretic Optimization Strategies Evaluation; n, number; IV, intravenous
WHF was reported on the case report form with qualifying therapies listed below. The specific qualifying therapy was listed below with the option to select (or not) one or more qualifying therapies.
An overall test was performed before subgroups were analyzed utilizing a 3 df test because there were 4 groups (no whf, 0–24 hour WHF, 24–48 hour WHF, and 48–72 hour WHF). We report the following: p-value for DOSE (3 df) = 0.27, ROSE (3 df) p-value =0.0026, and all patients (3 df) p-value = 0.0089.The unadjusted hazard ratios of the composite endpoint of 60-day death, HF re-hospitalization, and ER visits for HF for those who developed WHF through 72 hours vs. those that did not are presented in Table 4. Patients who develop WHF within 72 hours of admission had a significantly higher risk of death, HF re-hospitalization or HF ER visit through 60 days (combined dataset hazard ratio [HR] 1.64; 95% confidence interval [CI] 1.11–2.42; p= 0.01). When the components of the combined dataset are evaluated using the individual trial definitions, the association between WHF and worse clinical outcomes remain significant in ROSE (unadjusted [HR] 2.67; 95% CI 1.45–4.91,p=0.002) but not in DOSE (unadjusted [HR] 1.28; 95% CI 0.79–2.08, p=0.31). The unadjusted hazard ratios of the composite endpoint for DOSE patients whose WHF was only treated with additional diuretics and DOSE patients who met the ROSE definition of WHF compared to those DOSE patients who did not develop WHF are presented in Table 5. The hazard for the composite endpoint did not differ between those DOSE patients whose WHF was only treated with additional diuretics vs. those DOSE patients who did not develop WHF (HR of 0.97, 95% CI 0.55 – 1.7, p=0.90), whereas the hazard of the composite endpoint was 3.4 times larger in those DOSE patients who met the ROSE definition vs. DOSE patients with no WHF (HR of 3.4, 95% CI 1.62–7.14, p=0.001).
Table 4.
Unadjusted Association of WHF through 72 hours with 60-Day Death, HF Re-hospitalization, and HF ER Visits (WHF vs. no WHF).†
| n | Events n (%) |
Hazard Ratio (95% CI) |
p-value | |
|---|---|---|---|---|
| All patients* | 638 | 166(26.0%) | 1.64 (1.11, 2.42) | 0.01 |
| DOSE | 291 | 81(27.8%) | 1.28 (0.79, 2.08) | 0.31 |
| ROSE | 347 | 85 (24.5%) | 2.67 (1.45, 4.91) | 0.002 |
N indicates number; CI, confidence interval
Model has been adjusted for trial participant was enrolled in.
Patients who died, had a HF re-hospitalization, or HF ER visit within the first 72 hours were excluded from the analysis.
Table 5.
Unadjusted Association of WHF through 72 hours with 60-Day Death, HF Re-hospitalization, and HF ER Visits in DOSE patients (DOSE, diuretic only vs ROSE definition vs No WHF).†
| n | Events n (%) |
Hazard Ratio (95% CI) |
p- value |
|
|---|---|---|---|---|
| DOSE patients* | 291 | 81 (28.0%) |
||
| DOSE (diuretic only WHF) vs No WHF | 0.97 (0.55,1.70) |
0.90 | ||
| ROSE def. vs No WHF | 3.40 (1.62,7.14) |
0.001 |
N indicates number; CI, confidence interval
Model is not adjusted for trial participant as our definition includes adjustment for trial
Patients who died, had a HF re-hospitalization, or HF ER visit within the first 72 hours were excluded from the analysis
Patients who developed WHF from baseline to 24 hours compared to those who did not develop WHF had no difference in outcomes in the pooled or individual cohorts (pooled cohort [HR] 0.99, 95% CI 0.50 – 1.97, p= 0.99; DOSE [HR] 0.80 95% CI 0.34 – 1.85, p=0.60; and ROSE [HR] 1.50, 95% CI 0.47–4.76, p=0.49) (Figure 2). Patients who developed WHF from 24–48 and 48–72 hours in the pooled cohorts tended to have worse outcomes compared to patients who did not develop WHF ([HR] 2.02, 95% CI 1.10–3.70, p=0.02 and [HR] 2.20, 95% CI 1.25–3.87, p=0.006, respectively). However, patients who developed WHF from 48–72 hours tended to have worse outcomes than patients who did not develop WHF in ROSE ([HR] 4.32, 95% CI 1.88–9.95, p=0.001) but not in DOSE ([HR] 1.53, 95% CI 0.73–3.20, p=0.26).
Figure 2.
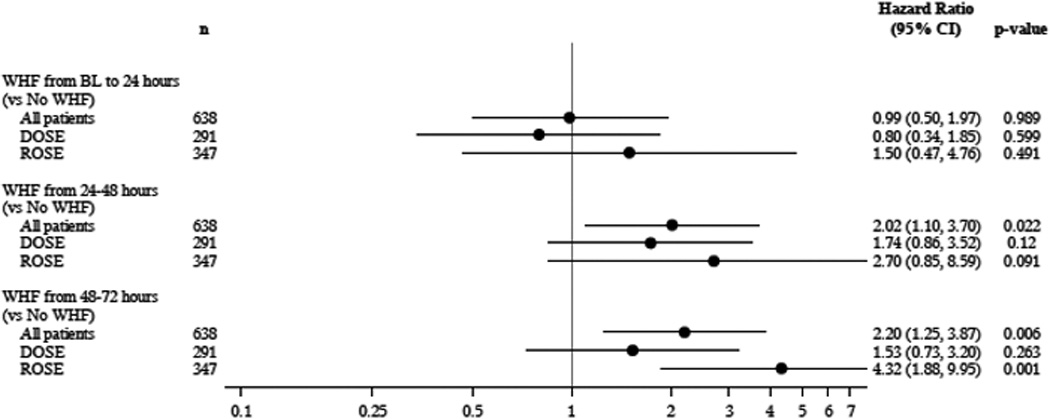
Forrest Plot of the pooled and individual ROSE and DOSE clinic trials demonstrating the Hazard Ratio of developing WHF compared with no development of WHF with outcomes of 60 Day death, HF Rehospitalization and ER visit for HF.
The hazard ratios and 95% CI of death, HF re-hospitalization or ER visit for HF based on timing of the development of WHF is presented in Figure 3. There was a trend towards worse outcome in patients who had later development of WHF (24–48 hours and 48–72 hours) compared with earlier development of WHF (0–24 hours).
Figure 3.
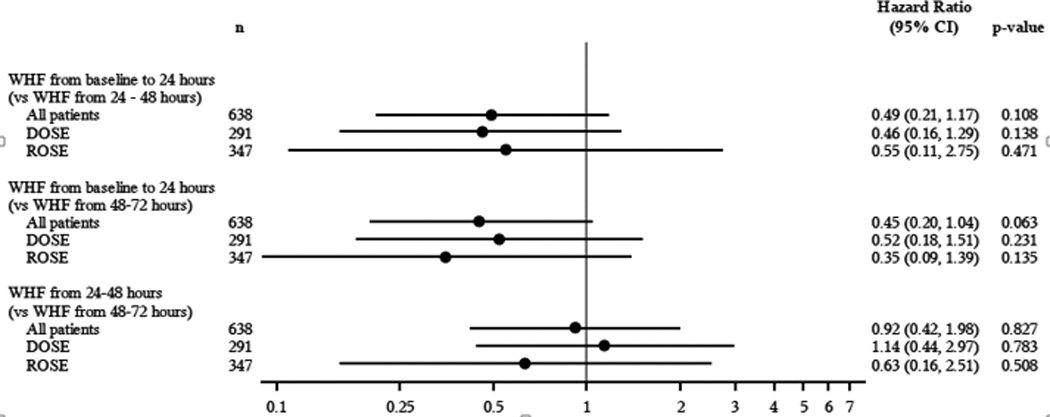
Forrest Plot of the pooled and individual ROSE and DOSE clinic trials demonstrating the Hazard Ratio of developing WHF between baseline and 24–72 hours with outcomes of 60 Day death, HF Rehospitalization and ER visit for HF.
Discussion
Using two clinical trials evaluating different treatment strategies in acute decompensated heart failure, we found that use of different definitions of WHF yielded distinct outcome rates with differential associations with clinical outcomes. Specifically, the use of intensification of diuretics in the definition of WHF led to modestly higher clinical event rates. However, a much stronger association with clinical outcomes was observed when additional diuretic use was not included. Furthermore, when using the more stringent definition from ROSE in patients from DOSE, there was a 3.4 fold worse hazard of experiencing 60-day death, HF re-hospitalization or HF ER visit when compared to DOSE patients with no WHF. There was a trend towards worse outcomes in patients who developed WHF later compared with earlier in the pooled cohorts. Thus, continued efforts to standardize WHF definitions in clinical trials are needed to optimize the utility of WHF as an endpoint in future studies.
Development of WHF during a patient’s clinical course has emerged as a key indicator in clinical practice and trials that predicts future clinical events. Clinicians and clinical trialists are using WHF to identify high-risk patients and increase event rates in clinical trials given its association with clinical outcomes. The efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF (RELAX-AHF2) clinical trial is evaluating the efficacy of serelaxin in an ongoing clinical trial enrolling 6,800 patients using WHF as a co-primary endpoint. The Phase III, multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of ularitide (urodilatin) intravenous infusion in patients suffering from acute decompensated heart failure (TRUE-AHF) trial is evaluating Ularitide intravenous infusion in patients with acute decompensated HF with WHF as a co-primary endpoint in 2,100 patients contributing to more than 9,000 total patients in current trials utilizing in-hospital WHF as a component of the primary outcome.14 WHF is a valuable clinical endpoint that merits continued and even increased use because it predicts and closely aligns with mortality and hospital readmission.
The prevalence of WHF ranges from 5–42%.2, 3, 8, 9, 11, 12, 16–19 Most studies have defined WHF based on signs and symptoms of worsening HF requiring some escalation in therapy. In the largest AHF trial, the Acute Study of Clinical Effectiveness of Neseritide in Decompensated Heart Failure study (ASCEND-HF), the incidence of WHF was 5% with an associated mortality of 41.5% through 180 days with WHF defined as intravenous or mechanical therapy in addition to typical clinical signs and symptoms of worsening HF.7 A secondary analysis in the Acute decompensated heart failure national registry (ADHERE), revealed a WHF prevalence of 11%, which was also defined as the need for an escalation of therapy (specifically inotropic medications or intravenous vasodilator therapy) resulting in mortality at 30 days and 1 year of 19% and 50%, respectively.2 The largest clinical trial and registry of AHF reveal that despite variations in definitions, the strong association with mortality remains. Use of a universal definition for WHF will improve clarity in communication between clinicians, investigators and regulatory approval officials while allowing future studies to unravel the mechanistic and clinical nuances behind WHF.
If a specific point in time is associated with worse outcomes, then a targeted intervention can be deployed. However, the pooled analysis of ROSE and DOSE demonstrated a trend towards worse outcomes with later development of WHF suggesting future studies should focus on earlier use of vasoactive or mechanical therapies. These findings are contrary to previous evidence from secondary analyses from the ASCEND-HF and PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) clinical trials. Although there are complexities when comparing patient characteristics from different trial datasets, data across multiple studies suggest that patients who develop WHF appear similar with regard to certain baseline characteristics including age and number of comorbidities. A post-hoc analysis from the PROTECT trial found no difference in early (days 2–3 after randomization) vs. late (4–7 days after randomization) development of WHF.20 Similarly, no differential relationship was found between clinical outcomes and early versus late WHF in ASCEND-HF, where early WHF occurred in the first 3 days of index hospitalization and late WHF started at 4 days and continued through index hospital discharge or day 30.7 However, these are post-hoc analyses and the use of different definitions for early and late development of WHF precludes direct comparison. However, using a similar definition for WHF with the same time points to record important clinical events in trials evaluating WHF will allow clear communication and pooled analyses across trials that are able tease out important clues into the underlying WHF.
The pooled analysis of ROSE and DOSE demonstrate that using a different definition for WHF may potentially yield different incidences and associated clinical event rates. A secondary analysis of the PROTECT trial that incorporated a low versus high intensity definition of WHF partially supports this finding. The PROTECT analysis revealed incidence rates of 11.7% and 2.9%, respectively for low and high intensity WHF definitions, without an increased association with worse outcomes in the higher intensity definition.20 The pooled analysis of the PROTECT and RELAX-AHF trials used a similar WHF definition to our definition, which required signs and symptoms of WHF with intensification of therapy with intravenous or mechanical support and yielded tighter 12.4% pooled incidence (range 9.5–14.5%) compared with 6.3–24.1% in ROSE and DOSE.21 Using a similar, reproducible definition for WHF in distinct clinical trials may facilitate improved comparison and communication between associated WHF findings among clinicians, scientists, and regulators. Our analysis supports a recent editorial highlighting that the use of a clear, universal definition of WHF will allow investigators and regulators to use WHF as an important clinical endpoint for clinical trials and drug approval process.22
The findings of our analysis should be considered in light of several limitations. This was a retrospective analysis pooling results from two separate clinical trials. ROSE and DOSE were clinical trials with specific but similar inclusion and exclusion criteria that may differ from other populations. The definition for WHF in ROSE and DOSE were similar but different and the available data from the case report forms did not allow evaluation of the DOSE WHF definition in the ROSE trial patients. Multiple statistical tests involving the composite endpoint were evaluated but no methods were utilized to adjust for multiple comparisons; however, an overall test was performed before further subgroup analyses were analyzed.
Conclusions
The results from this pooled analysis of two acute heart failure clinical trials emphasize the importance of using a standardized definition for WHF. Various definitions lead to different incidence rates of WHF and limit the precision of predicting event rates and outcomes. There was trend towards worse outcomes in patients who developed later versus earlier WHF. A WHF definition that incorporates intensification of diuretics may increase event rates without strengthening the association with worse clinical outcomes. Using a standardized WHF definition in clinical trials may facilitate more effective assessments of future pharmaceutical or device therapies in the war on acute heart failure.
Clinical Perspective.
In the context of clinical trials, worsening heart failure (WHF) is an emerging endpoint that is increasingly being used as a component of a composite endpoint. However, the definitions used in previous analyses and clinical trials have varied with regard to the subjectivity of their assessment and how intense an escalation of therapy is required to qualify as a WHF event. Using two acute HF trials with different intensity WHF definitions, we found that use of different definitions of WHF yielded distinct event rates and differential associations with clinical outcomes. Intensification of diuretics led to higher clinical event rates whereas use of a more stringent definition of WHF that required more than additional diuretic led to a 3.4 fold worse hazard of experiencing 60-day death, HF rehospitalization or HF Emergency Room visit when compared to patients with no WHF. Our study highlights the importance of standardizing WHF definitions in clinical trials to optimize the utility of WHF as an important clinical endpoint in future studies.
Acknowledgments
Sources of Funding
DOSE-AHF and ROSE-AHF were funded by the National Heart, Lung, and Blood Institute. Dr. Kelly is supported by grant T32HL7101-39 from the National Institute of Health. Dr. Cooper is supported by grant T32HL069749-11A1 from the National Institute of Health.
Dr. Felker receives research support from NIH, Amgen, Novartis, Roche Diagnostics and Otsuka, and consulting fees from Amgen, Novartis, Roche Diagnostics, Otsuka, Trevena, Singulex, Medtronic and Celladon. Dr. Hernandez receives research support from Amgen, Astrazeneca, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Janssen; and consulting fees from Amgen, Novartis, and Janssen.
Dr.Mentz receives research support from the NIH (U10HL110312), Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Novartis, Otsuka, and ResMed; honoraria from Novartis, Thoratec and HeartWare; and has served on an advisory board for Luitpold Pharmaceuticals, Inc.
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
All other authors report no relevant disclosures.
References
- 1.O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: An analysis from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 2.DeVore AD, Hammill BG, Sharma PP, Qualls LG, Mentz RJ, Waltman Johnson K, Fonarow GC, Curtis LH, Hernandez AF. In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014;3:1–11. doi: 10.1161/JAHA.114.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Soergel DG, Teerlink JR, Violin JD, Voors AA, Pang PS. Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the blast-ahf study (biased ligand of the angiotensin receptor study in acute heart failure) JACC Heart Fail. 2015;3:193–201. doi: 10.1016/j.jchf.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, Massie BM. The protect pilot study: A randomized, placebo-controlled, dose-finding study of the adenosine a1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. 2008;14:631–640. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, Givertz MM, Mansoor GA, Ponikowski P, Teerlink JR, Voors AA, Fiuzat M, Wojdyla D, Chiswell K, Massie BM. The protect in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. 2012;14:605–612. doi: 10.1093/eurjhf/hfs029. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JP, Mentz RJ, Hasselblad V, Ezekowitz JA, Armstrong PW, Zannad F, Felker GM, Califf RM, O’Connor CM, Hernandez AF. Worsening heart failure during acute heart failure hospitalization: Insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) Am Heart J. 2015;170:298–305. doi: 10.1016/j.ahj.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torre-Amione G, Milo-Cotter O, Kaluski E, Perchenet L, Kobrin I, Frey A, Rund MM, Weatherley BD, Cotter G. Early worsening heart failure in patients admitted for acute heart failure: Time course, hemodynamic predictors, and outcome. J Card Fail. 2009;15:639–644. doi: 10.1016/j.cardfail.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Weatherley BD, Milo-Cotter O, Felker GM, Uriel N, Kaluski E, Vered Z, O'Connor CM, Adams KF, Cotter G. Early worsening heart failure in patients admitted with acute heart failure--a new outcome measure associated with long-term prognosis? Fundam Clin Pharmacol. 2009;23:633–639. doi: 10.1111/j.1472-8206.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 10.Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G. Dyspnoea and worsening heart failure in patients with acute heart failure: Results from the pre-relax-ahf study. Eur J Heart Fail. 2010;12:1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The rose acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: The veritas randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Gheorghiade M, Kelkar A, Fonarow GC, Anker S, Greene SJ, Papadimitriou L, Collins S, Ruschitzka F, Yancy CW, Teerlink JR, Adams K, Cotter G, Ponikowski P, Felker GM, Metra M, Filippatos G. In-hospital worsening heart failure. Eur J Heart Fail. 2015;17:1104–1113. doi: 10.1002/ejhf.333. [DOI] [PubMed] [Google Scholar]
- 15.Chen HH, AbouEzzeddine OF, Anstrom KJ, Givertz MM, Bart BA, Felker GM, Hernandez AF, Lee KL, Braunwald E, Redfield MM. Targeting the kidney in acute heart failure: Can old drugs provide new benefit? Renal optimization strategies evaluation in acute heart failure (ROSE-AHF) trial. Circ Heart Fail. 2013;6:1087–1094. doi: 10.1161/CIRCHEARTFAILURE.113.000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, Rovithis D, Economou D, Savvatis K, Kirlidis T, Tsaknakis T, Skoularigis J, Westermann D, Tschope C, Triposkiadis F. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 17.Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine a1-receptor antagonist, in acute heart failure. N Engl J. Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 18.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J. Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, Padley RJ, Thakkar R, Delgado-Herrera L, Salon J, Garratt C, Huang B, Sarapohja T. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Mentz RJ, Metra M, Cotter G, Milo O, McKendry C, Chiswell K, Davison BA, Cleland JG, Bloomfield DM, Dittrich HC, Fiuzat M, Ponikowski P, Givertz MM, Voors AA, Teerlink JR, O'Connor CM. Early vs. Late worsening heart failure during acute heart failure hospitalization: Insights from the protect trial. Eur J Heart Fail. 2015;17:697–706. doi: 10.1002/ejhf.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davison BA, Metra M, Cotter G, Massie BM, Cleland JG, Dittrich HC, Edwards C, Filippatos G, Givertz MM, Greenberg B, Ponikowski P, Voors AA, O'Connor CM, Teerlink JR. Worsening heart failure following admission for acute heart failure: A pooled analysis of the protect and relax-ahf studies. JACC Heart Fail. 2015;3:395–403. doi: 10.1016/j.jchf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Tang WH, Grodin JL. Worsening heart failure: Challenges as a therapeutic target. JACC Heart Fail. 2015;3:404–407. doi: 10.1016/j.jchf.2015.02.003. [DOI] [PubMed] [Google Scholar]


