Abstract
Background:
Rapid increasing prevalence of diabetes mellitus is a serious health concern in the world. New data determined that the pathogenesis of diabetes mellitus is chronic low-grade inflammation, resulting insulin resistance. Pomegranate seed oil (PSO) has anti-inflammatory effects; though it may reduce insulin resistance and improve glycemia in diabetes mellitus. The present study has been designed to investigate the effects of PSO as a natural dietary component on metabolic state of patients with Type 2 diabetes mellitus.
Methods:
In a randomized double-blind clinical trial study, 80 patients (28 men) with Type 2 diabetes were randomly allocated to the intervention and control groups. The intervention group consumed PSO capsules, containing 1000 mg PSO twice daily (2000 mg PSO), whereas controls take placebo for 8 weeks. The participants followed their previous dietary patterns and medication use. Dietary factors and metabolic factors including lipid profile, fasting plasma sugar, and insulin and were assayed at the baseline and after 8 weeks.
Results:
Participants in two intervention and control group were similar regarding anthropometric and the dietary factors at baseline and after trial (P > 0.05). Mean level of total cholesterol, triglyceride, low-density lipoprotein-cholesterol, and high-density lipoprotein was not different significantly between groups after trial (P > 0.05). Consumption of PSO did not significantly affect the levels of parameters such as fasting blood sugar (FBS), insulin, HbA1c, alanine transferase, and homeostasis model assessment-insulin resistance.
Conclusions:
Consumption of 2000mg PSO per day for 8 weeks had no effect on FBS, insulin resistance and lipid profile in diabetic patients.
Keywords: Diabetes mellitus, insulin resistance, pomegranate seed oil
INTRODUCTION
Diabetes mellitus has now reached an epidemic level in both developing and developed countries. The prevalence of diabetes mellitus is estimated to be approximately 387 million people around the world.[1,2] As a result of pandemic level, macro- and micro-vascular complications caused by diabetes mellitus may emerge, which can be a major threat to general public health throughout the world with huge economic and social costs.[3,4] Recent evidences suggested the possible role of low-grade inflammation in the pathogenesis of diabetes mellitus. Insulin resistance and diabetes mellitus are usually characterized by an increase in oxidative stress and proinflammatory cytokines.[5,6,7] As a result, most therapies have been primarily focused on potential antioxidant or anti-inflammatory effects.[8,9,10] In this context, natural dietary components offer simple, yet effective alternative therapeutic strategy.
Among various natural dietary components, pomegranate juice has recently received much attention as a functional anti-inflammatory agent.[11,12] The high content of polyphenols in pomegranate juice could explain its anti-inflammatory effect. Over the years, numerous studies have been dedicated to pomegranate extract and its potential anti-inflammatory effects. However, pomegranate seed oil (PSO) can also offer an anti-inflammatory effects and few studies have examined its potentials.[13,14]
PSO consists of a high content of conjugated linolenic acid (9-cis, 11-trans, 13-cis) octadecatrienoic acid or punicic acid (main bioactive ingredient).[15,16]
Findings of different in vivo and in vitro studies regarding the effectiveness of PSO are controversial. Current investigations suggest that PSO may improve Type 2 diabetes by ameliorating insulin resistance and obesity in rats with high fat content diet.[17] In an in vitro study, punicic acid improved fasting plasma glucose (FPG) and lipid profiles.[18] Our goal in this study is to present a thorough investigation of PSO and its potentials as a possible natural dietary component for improving insulin resistance, plasma glucose, dyslipidemia, and blood pressure in diabetic patients.
METHODS
This study was a randomized, double-blind, placebo-controlled clinical trial. Eighty patients (28 men and 52 women), aging 52 ± 6.8 years, with Type 2 diabetes were enrolled by convenience sampling from the medical records of the patients in Isfahan Endocrine and Metabolism research center; all of the patients received oral hypoglycemic agents. Inclusion criteria were as follows: Willingness to entrance to the study; Type 2 diabetes mellitus (for 5–10 years); age between 35 and 65 years; body mass index between 20 and 30. Exclusion criteria were as follows: Smoking, pregnancy, any other chronic disease, taking estrogen, progesterone, and corticosteroid or antioxidant supplements or insulin as diabetes medication, any weight loss diets in the past month; patients with severe hyperglycemia (FPG >250); renal failure; heart disease; and liver failure.
Considering the suggested formula, given the Type 1 error of 5%, the study power of 80% and based on previous publications, we needed 33 people in each group. As we might have some missing, we added the number of each group to 40 people. At baseline, the participants were stratified by sex and randomly allocated in two: Intervention (PSO, n = 40) and control (placebo, n = 40) groups, using sequentially numbered containers. An assistant performed the randomization. Both the investigator and participants were blinded about the randomization.
Written informed consent was obtained from each of the selected patients. The Ethical Committee of National Nutrition and Food Technology Research Institute (Tehran, Iran) approved the protocol of this trial. The clinical trial has been registered in the Iranian Registry of Clinical Trials (IRCT201409031640N15).
All of the demographic data and medical history at baseline were documented.
The intervention group consumed PSO capsules, containing 1000 mg PSO twice daily (2000 mg PSO), while controls took placebo capsules for 8 weeks.
Subjects were advised to follow their previous dietary and physical activity patterns and medication use.
The dietary intakes of subjects were recorded using a 3-day dietary recall (2 weekdays and 1 weekend day) at baseline and at the end of the trial. Patients’ diets were analyzed using Nutritionist IV software (N-Squared Computing, San Bruno, CA, USA).
Measurements
Venous blood samples (10 mL) were obtained from each participant at baseline and the end of 8 weeks intervention after a 12–14 h overnight fasting. Blood samples were centrifuged at 4000 rpm for 10 min and their plasma samples were separated into aliquots. The samples were frozen at −70°C for further assessments.
FPG concentration determined by the colorimetry method using Pars Azmoon kits (Tehran-Iran). HbA1C levels were measured by ion-exchange chromatography (LDN Germany). Level of insulin was determined by ELISA (Dia Plus, USA) method. Plasma triglyceride (TG), total cholesterol, high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) were measured enzymatically by autoanalyzer using Pars Azmoon kits (Tehran-Iran). Homeostasis model assessment-insulin resistance (HOMA-IR) calculated as: (Glucose [mmol/L] × insulin [μU/mL]/22.5).
Body weight of the participants was measured at baseline and the end of 8 weeks intervention.
Compliance
To ascertain patient compliance, we provided each patient with a fixed number of PSO capsules and recommended to return the unused capsules at the end of the study.
Compliance to treatment was determined based on the number of returned capsules by each patient. The rate was 90% for our studied population.
Pomegranate seed oil and placebo capsules
PSO composition is shown in Table 1. PSO and placebo was prepared by Zahravi Inc., Tehran, Iran. Placebo capsules contained medium-chain triacylglycerol.
Table 1.
Composition of pomegranate seed oil
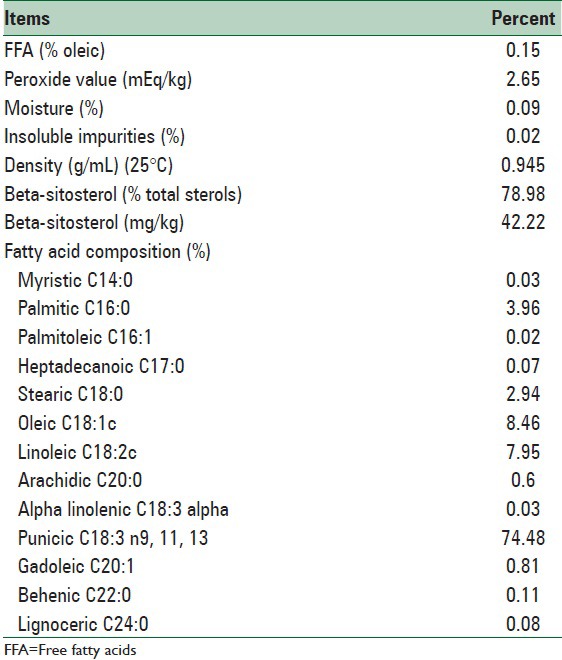
Based on the chemical analysis, the main fatty acid content of PSO were as follows; Punicic Acid (PA) (18:3) 74.48%; oleic acid (18:2) 8.46%; palmitic acid (16:0) 3.96%; stearic acid (18:0) 2.94%; and trace amounts (1%) of other fatty acids. The antioxidant Vitamin E content of crude PSO was 36·90 mg/100 g; which was kept at cold temperature to prevent oxidation until formulated in capsules.
Statistical analysis
This is an intention to treat clinical trial. The results are expressed as mean ± SD for quantitative variables and n (%) for qualitative variables, and differences were considered significant at P ≤ 0.05.
Statistical analysis of data was performed using SPSS software version 21.0 (Chicago, IL, USA). Qualitative variables compared between the two studied groups using Chi-square test. Normality of the quantitative parameters was determined by Kolmogorov–Smirnov test. To compare the quantitative parameters during pre- and post-intervention period, within and between groups paired t-test was used.
To compare the means of the variables after trial and determine the main effect of treatment, the general linear model (ANCOVA) was used with 8-week values (duration of trial) as dependent variables, baseline values as covariates, and treatment group as a fixed factor.
RESULTS
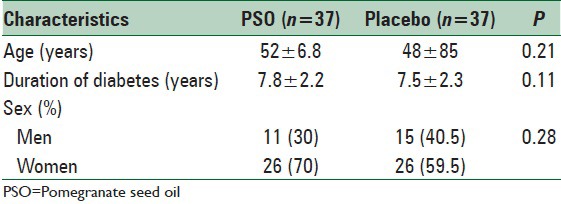
The baseline characteristics of patients did not differ significantly between the PSO treated and placebo groups mean age in PSO group was 52 ± 6.8 (years) and in placebo group was 48 ± 85 (years). In PSO group, 26% of people were women and 22% of people in placebo group were women. Duration of diabetes was 7.8 ± 2.2 (years) and 7.5 ± 2.3 (years) in PSO and placebo group, respectively [Table 2].
Table 2.
Baseline characteristics of patients in the pomegranate seed oil and placebo groups
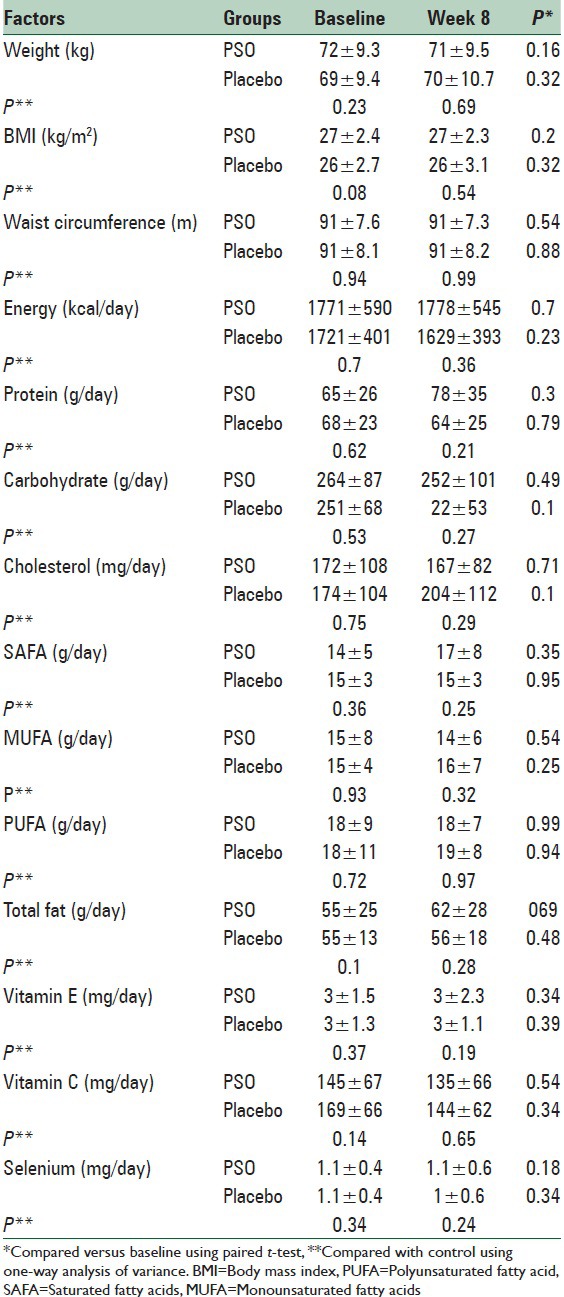
In between groups comparison, the anthropometric measurements and dietary factors were similar in two studied groups before and after trial. In within group comparison, studied variables had not significant changes after trial in the two studied groups [Table 3].
Table 3.
Anthropometric and dietary factors in the pomegranate seed oil and placebo groups
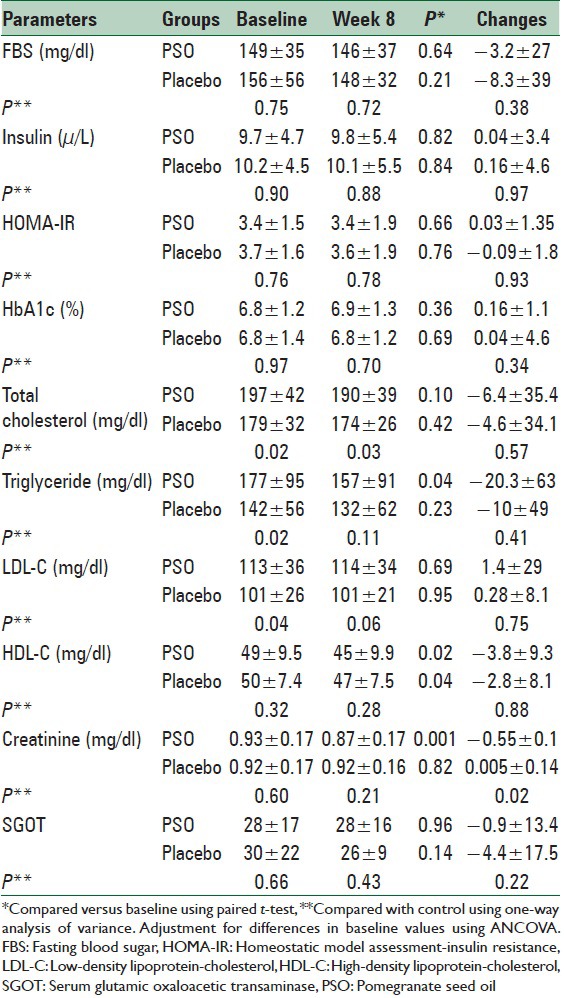
Mean concentration of total cholesterol was significantly different between two groups at the baseline and at the end; however, the mean changes were not different between two groups at the end of the study.
TG concentration was significantly different between two groups at the baseline, but there was not any significant difference between two groups at the 8 weeks. Also mean concentration of LDL-C was significantly different between two groups at the baseline; however there were not any significant differences between two groups at the 8th week and also in mean changes. HDL-C reduced significantly in both groups at the end of study compared baseline; however, there was not any significant differences between two groups at the end of the study. PSO consumption had not significant effect on the levels of FPG, insulin, HbA1c, alanine transferase, and HOMA-IR [Table 3].
Plasma creatinine concentration reduced significantly in the PSO group at the end of the 8th week compared with baseline (P < 0.01), whereas no significant change was observed in the placebo group. Decreases in plasma creatinine concentrations in the PSO group were significant in comparison with the placebo group (P < 0.05) [Table 4].
Table 4.
Plasma concentrations of measuring factors in the pomegranate seed oil and placebo groups
As it has been shown in consort form, four people in PSO group could not continue the study. Two women had rash and itching which disappeared after discontinuation of drug. A man had gastrointestinal (GI) upset. Another man had upper GI bleeding, which caused hospitalization of the patient. After treatment, he discharged. Three patients in placebo group discontinued the placebo because of headache or some mild abdominal upset [Figure 1].
Figure 1.
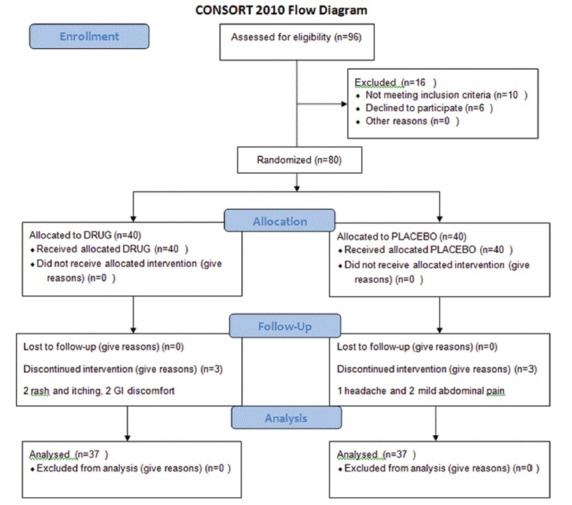
Flowchart of the study
DISCUSSION
In the present study, we found that the consumption of 2000 mg PSO/day for 8 weeks had no effect on fasting blood glucose, insulin resistance, and lipid profile in diabetic patients. To the best of our knowledge, this is the first trial which evaluated the outcomes of PSO consumption in diabetic patients. The previous studies have shown PSO effects in animal models.[19] In a study conducted on mice, Miranda et al. have shown PA did not decrease the fat accumulation in liver and other organs of rats fed an obesogenic diet and did not lead to improve glycemic control.[20] Nekooeian et al. investigated the impact of PSO Type 2 diabetic rats. They indicated that plasma insulin level increased without any reduction in plasma glucose level. The mechanism of this is unknown but it might be related to the upregulation of peroxisome proliferator-activated receptor-g (PPAR-g) genes.[21]
An in vitro study showed punicic acid ameliorate tumor necrosis factor-α (TNF-α) induced protein dysfunction therefore glucose uptake and insulin resistance may improve after administration of the punicic acid.[22] In another study, PSO consumption resulted in reduction of plasma TNF-α levels in mice. However, PSO treatment in dyslipidemic patients had no effect on the serum TNF-α in one study.[23]
Furthermore, in vitro studies have documented that punicic acid is an agonist for PPARs, the molecular target for thiazolidinediones, antidiabetic agents. However, the natural agonists of PPARs are punicic acid. PA may increases gene expression of PPARs and thus improves glucose homeostasis and inflammation-related insulin resistance.[24]
They also showed that PSO may reduce oxidative stress, but did not have an effect on serum lipid profile. The previous studies have documented ‘n-3 polyunsaturated fatty acid are hypolipidemic agent.[25,26] Results of recent studies on the effect of PSO on lipid profiles are controversial. In the present study, there was no significant difference in the serum total cholesterol, LDL, HDL-C levels, and TAG in two groups of diabetic patients. In an in vivo study on obese, hyperlipidemic rats, it was demonstrated that the diet supplemented with PSO did not have an effect on abdominal white adipose tissue and serum lipid profile but reduces hepatic triacylglycerol accumulation similar to the control group.[14] A probable mechanism confirmed in an in vitro study, that in HepG2 cells 9-cis, 11-trans, 13-cis CLN suppressed the synthesis of TAG.[27] Mirmiran et al. found that the consumption of 800 mg PSO did not change cholesterol and LDL-C but decreased TAG and TAG: HDL-C ratio during 4-week of study in hyperlipidemic subjects.[28]
It should be noted that there is no previous experience with PSO in diabetic patients, which limited us to administrate increase doses of PSO.
Limitations of this trial are small sample size of studied population and not evaluation of serum PSO level.
Further studies are needed to evaluate the effect of PSO supplementation in diabetic patients.
CONCLUSIONS
In the present study, we found that consumption of 2000 mg PSO/day for 8 weeks had no effect on fasting blood glucose, insulin resistance, and lipid profile in diabetic patients. It may be concerned to small sample size of our study. We need to investigate the effect of PSO in larger groups of people or we should increase the duration of the study. As we have shown the safety of 2000 mg/day of PSO, future studies can increase the dose of it.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dagogo-Jack S. 2015 presidential address: 75 years of battling diabetes – Our global challenge. Diabetes Care. 2016;39:3–9. doi: 10.2337/dc15-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Albers JW, Pop-Busui R DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–8. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the diabetes control and complications trial (DCCT) on peripheral neuropathy in type 1 diabetes during the epidemiology of diabetes interventions and complications (EDIC) study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, Ferreira I, van Greevenbroek MM, van der Kallen CJ, et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort Study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr. 2013;98:1533–42. doi: 10.3945/ajcn.112.056333. [DOI] [PubMed] [Google Scholar]
- 6.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–91. doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Puglisi MJ, Fernandez ML. Modulation of C-reactive protein, tumor necrosis factor-alpha, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–6. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 8.Daftardar S, Kaur G, Addepalli V. Nutraceutical approaches in the management of cardiovascular dysfunctions associated with diabetes mellitus. InDiabetic Cardiomyopathy. 2014:377–96. [Google Scholar]
- 9.Bialek A, Teryks M, Tokarz A. Conjugated linolenic acids (CLnA, super CLA) – Natural sources and biological activity. Postepy Hig Med Dosw (Online) 2014;68:1238–50. doi: 10.5604/17322693.1127881. [DOI] [PubMed] [Google Scholar]
- 10.Asgary S, Keshvari M, Sahebkar A, Hashemi M, Rafieian-Kopaei M. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler. 2013;9:326–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Sohrab G, Nasrollahzadeh J, Zand H, Amiri Z, Tohidi M, Kimiagar M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: A randomized, placebo-controlled trial. J Res Med Sci. 2014;19:215–20. [PMC free article] [PubMed] [Google Scholar]
- 12.Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–9. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki M, Kitagawa T, Koyanagi N, Chujo H, Maeda H, Kohno-Murase J, et al. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition. 2006;22:54–9. doi: 10.1016/j.nut.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Arao K, Wang YM, Inoue N, Hirata J, Cha JY, Nagao K, et al. Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis. 2004;3:24. doi: 10.1186/1476-511X-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem. 2007;55:10405–13. doi: 10.1021/jf072741q. [DOI] [PubMed] [Google Scholar]
- 16.Ahlers NH, Dennison AC, O’NeillL A. Spectroscopic examination of punicic acid. Nature. 1954;173:1045–6. [Google Scholar]
- 17.McFarlin BK, Strohacker KA, Kueht ML. Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risk in CD-1 mice. Br J Nutr. 2009;102:54–9. doi: 10.1017/S0007114508159001. [DOI] [PubMed] [Google Scholar]
- 18.Hontecillas R, O’shea M, Einerhand A, Diguardo M, Bassaganya-Riera J. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr. 2009;28:184–95. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- 19.Vroegrijk IO, van Diepen JA, van den Berg S, Westbroek I, Keizer H, Gambelli L, et al. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol. 2011;49:1426–30. doi: 10.1016/j.fct.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Miranda J, Aguirre L, Fernández-Quintela A, Macarulla MT, Martínez-Castaño MG, Ayo J, et al. Effects of pomegranate seed oil on glucose and lipid metabolism-related organs in rats fed an obesogenic diet. J Agric Food Chem. 2013;61:5089–96. doi: 10.1021/jf305076v. [DOI] [PubMed] [Google Scholar]
- 21.Nekooeian AA, Eftekhari MH, Adibi S, Rajaeifard A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran J Med Sci. 2014;39:130–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Anusree SS, Nisha VM, Priyanka A, Raghu KG. Insulin resistance by TNF-a is associated with mitochondrial dysfunction in 3T3-L1 adipocytes and is ameliorated by punicic acid, a PPARγ agonist. Mol Cell Endocrinol. 2015;413:120–8. doi: 10.1016/j.mce.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Asghari G, Sheikholeslami S, Mirmiran P, Chary A, Hedayati M, Shafiee A, et al. Effect of pomegranate seed oil on serum TNF-a level in dyslipidemic patients. Int J Food Sci Nutr. 2012;63:368–71. doi: 10.3109/09637486.2011.631521. [DOI] [PubMed] [Google Scholar]
- 24.Aruna P, Venkataramanamma D, Singh AK, Singh RP. Health Benefits of Punicic Acid: A Review. Comprehensive Reviews in Food Science and Food Safety. 2016:16–27. doi: 10.1111/1541-4337.12171. [DOI] [PubMed] [Google Scholar]
- 25.Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204:476–82. doi: 10.1016/j.atherosclerosis.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Salas-Salvadó J, Márquez-Sandoval F, Bulló M. Conjugated linoleic acid intake in humans: A systematic review focusing on its effect on body composition, glucose, and lipid metabolism. Crit Rev Food Sci Nutr. 2006;46:479–88. doi: 10.1080/10408390600723953. [DOI] [PubMed] [Google Scholar]
- 27.Arao K, Yotsumoto H, Han SY, Nagao K, Yanagita T. The 9cis, 11trans, 13cis isomer of conjugated linolenic acid reduces apolipoprotein B100 secretion and triacylglycerol synthesis in HepG2 cells. Biosci Biotechnol Biochem. 2004;68:2643–5. doi: 10.1271/bbb.68.2643. [DOI] [PubMed] [Google Scholar]
- 28.Mirmiran P, Fazeli MR, Asghari G, Shafiee A, Azizi F. Effect of pomegranate seed oil on hyperlipidaemic subjects: A double-blind placebo-controlled clinical trial. Br J Nutr. 2010;104:402–6. doi: 10.1017/S0007114510000504. [DOI] [PubMed] [Google Scholar]


