Abstract
Purpose:
To identify the prevalence and microbial profile of infectious keratitis in a tertiary eye care hospital, and to test for the in vitro antimicrobial resistance of the bacterial isolates.
Methods:
A total of 312 patients presenting to a tertiary eye care hospital with infected corneal ulcer were enrolled in this study. Their socio-demographic data and risk factors were recorded. Corneal scrapings collected from the edge of the ulcer were processed for direct gram stain and KOH mount. Culture was recovered on blood agar, chocolate agar, MacConkey agar and Sabouraud's dextrose (SDA) agar in multiple C shaped streaks. After overnight incubation, bacterial culture was followed by standard biochemical tests and antimicrobial sensitivity according to the clinical and laboratory standards institute (CLSI) guidelines. Inoculated SDA was inspected daily for up to 10 days and the growth was identified by its colony morphology, pigment production and lacto-phenol cotton blue mount examination.
Results:
Of 312 patients, a microbial etiology was established in 117 cases (37.5%). Of these, 72 (61.5%) were male. The age range of 41-60 years was the most affected group. Of 117 positive cases, 52 (44.5%) were bacterial, 58 (49.5%) were fungal and 7 (6%) patients showed mixed bacterial and fungal infection. The most common isolated fungus was Fusarium which was detected in 36 (31%) cases, followed by Aspergillus spp in 13 (11%) subjects. Staphylococcus aureus was the most common isolated bacteria. All Gram positive cocci were susceptible to vancomycin followed by gatifloxacin, whereas all Gram negative bacilli were susceptible to gatifloxacin.
Conclusion:
Routine microbiological examination of patients with corneal ulcer is necessary in order to analyze and compare the changing trends of the etiology and their susceptibility patterns.
Keywords: Keratitis, Corneal Ulcer, Fungal, Bacterial, Antibiotic Susceptibility
INTRODUCTION
Infectious keratitis is a leading cause of corneal blindness in developing countries.[1] Corneal ulceration results in 1.5–2 million new cases of corneal blindness annually, posing a major public health problem according to the World Health Organization (WHO) reports.[2] Fungi are the most common etiological agents which account for 30–40% whereas bacteria account for 13–48% of all cases of suppurative keratitis; this varies by geographical area.[3] These pathogens lead to corneal damage directly or by release of toxins and enzymes or by activating the host immune system.[4] An intact corneal epithelium acts as a barrier for the majority of microorganisms. Microorganisms can penetrate through a breach in the epithelium either due to penetrating or perforating ocular trauma or due to surgery. Various risk factors have been implicated for increased incidence of fungal keratitis including widespread use of antibiotics and steroids, use of contact lenses, and postoperative infections.[5]
Ocular morbidity such as corneal scarring and subsequent visual loss can be significantly reduced by prompt institution of appropriate therapy guided by the knowledge of the causative agents. The present study is an attempt to identify the prevalence of microbial keratitis in this area and to test for the in vitro antimicrobial resistance.
METHODS
This study was conducted at a tertiary eye care hospital in Bangalore, India for two years from June 2012 to June 2014. Patients with suspected microbial corneal ulcers were included and their socio-demographic data and risk factors were recorded.
A corneal ulcer was defined as a corneal infiltrate associated with an overlying epithelial defect. Corneal scrapings were routinely collected from patients with corneal ulceration by an ophthalmologist viewing through a slit lamp. Scrapings were taken from the edge of the ulcer before administration of any antimicrobials using a sterile #15 blade after instillation of topical 4% xylocaine.
Direct microscopy was performed by taking the scrapings on two glass slides, one for Grams staining and the other for KOH mount. To prepare the culture, corneal scrapings or buttons were inoculated bed side on blood agar, chocolate agar, MacConkey agar and Sabouraud's dextrose agar (SDA) in multiple C shaped streaks. After overnight incubation, bacterial culture was confirmed by growth on blood agar, chocolate agar and MacConkey agar followed by standard biochemical tests according to the clinical and laboratory standards institute (CLSI) guidelines.
Inoculated SDA was inspected daily for up to ten days and declared as fungal culture negative thereafter. Fungal growth was grossly identified by its colony morphology on obverse, pigment production on reverse, and microscopically by lacto-phenol cotton blue stain. Diagnosis of fungal keratitis was done when any of the following criteria were met:
Correlation between direct KOH examination and growth on SDA
Growth on more than one C streak lines
Similar growth on more than one media.
RESULTS
Three hundred twelve eyes of 312 patients were included in this study. Of these, 117 (37.5%) were positive for smear and culture. Of 117 patients, 72 (61.5%) were male. The age range of 41–60 years was the most affected group. Microbial etiology was bacterial in 52 (44.5%) and fungal in 58 (49.5%) cases. Seven subjects showed mixed growth – both bacterial and fungal (6%). Risk factors were trauma in 54 (46%) followed by diabetes mellitus in 31 (26.5%), contact lens usage in 22 (19%) and corticosteroid therapy in 4 (3.5%) eyes.
The most common fungus isolated was Fusarium in 36 (31%) eyes followed by Aspergillus spp found in 13 (11%) cases. Common bacterial isolates were Staphylococcus aureus in 21 (18%) subjects followed by Streptococcus pneumoniae (9 eyes) from the Gram positive bacteria, and Pseudomonas aeruginosa in 10 (8.5%) cases followed by Klebsiella pneumoniae (4 eyes) from the Gram negative bacteria.
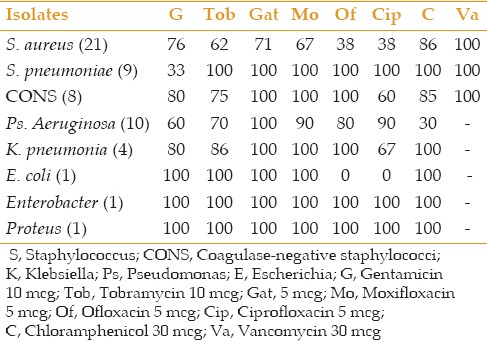
All Gram positive cocci were susceptible to vancomycin followed by gatifloxacin, chloramphenicol and moxifloxacin whereas all Gram negative bacilli were susceptible to gatifloxacin followed by moxifloxacin and ofloxacin. The highest resistance was seen to ciprofloxacin and gentamicin.
DISCUSSION
Suppurative keratitis and its complications constitute important causes of ocular morbidity often leading to blindness if early management is not instituted. A proper clinical history coupled with detailed clinical examination would be beneficial to identify the predisposing factors for corneal perforation in microbial keratitis.
In the present study, male subjects were more affected than female patients which is in agreement with the study done by Tityal et al.[6] The age range of 41–60 years was more affected consistent with the results of Cameron et al[7] in Sydney and Das et al[4] in Kolkata [Figure 1]. This could be attributed to the increased outdoor activity of men especially in the working age group. In contrast, in the study done in China, women were more affected and most of them were over the age of 60.[8] This could be due to higher employability of women particularly in the agricultural sector in China.
Figure 1.
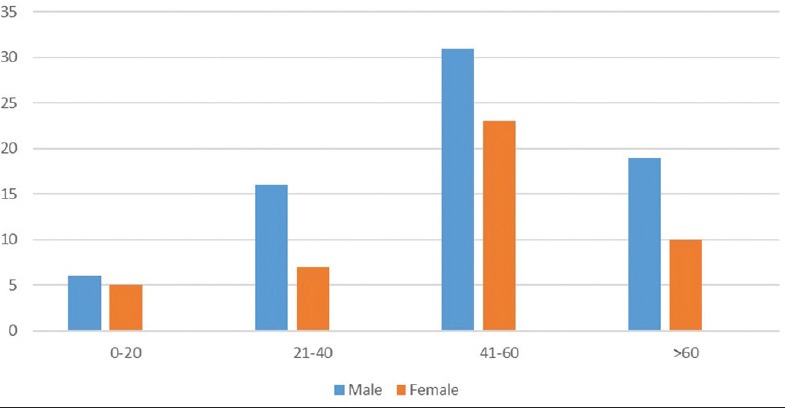
Age and gender wise distribution of corneal ulcer.
The most common associated risk factors in our study were trauma followed by diabetes mellitus and contact lens usage which is comparable with other studies.[1,9] While Yousuf et al[10] reported the use of contact lens as the major cause, Krishna et al[11] demonstrated injury to eye as the predominant risk factor followed by the foreign body induced microbial keratitis. The role of diabetes was negligible in the latter study (2%) [Figure 2].
Figure 2.
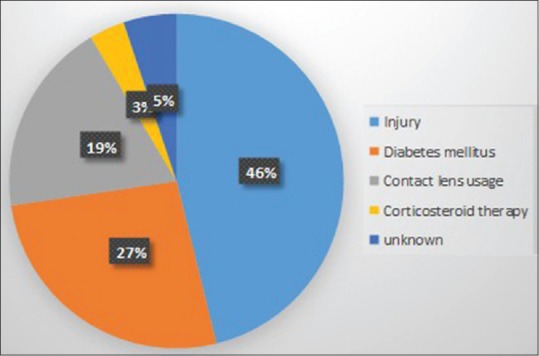
Risk factors of corneal ulceration.
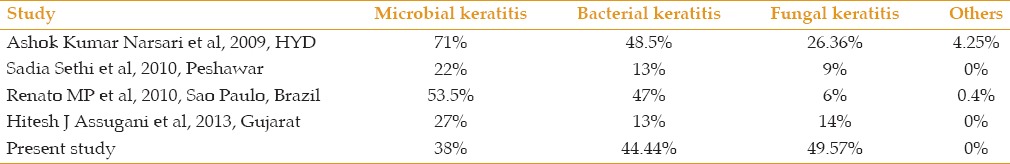
In our study, out of 312 cases, 117 (37.5%) were culture positive, and fungi were recovered slightly more frequently than bacteria (58 vs 52 eyes, respectively) [Tables 1 and 2]. Srinivasan et al[12] isolated equal numbers of bacterial (47.1%) and fungal (46.8%) agents causing infectious keratitis with 5.1% cases having mixed infections. Katara et al[13] also reported a culture positivity of 40%, of which 26% were fungal isolates and the remaining 14% of samples had bacterial etiology. A comparison of the prevalence rates of microbial keratitis due to bacterial and fungal agents is shown in Table 3.
Table 1.
Number and percentage of type of isolates

Table 2.
Pathogens isolated from patients with mixed infections
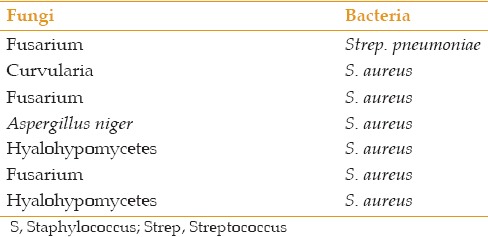
Table 3.
Prevalence rate of microbial keratitis in various studies
Among the fungal isolates, filamentous type was more common compared to yeast and Fusarium spp was the most isolated species followed by Aspergillus spp. [Table 4]. Comparable results were obtained in studies done by Alkatan[14] and Idiculla et al[15] In contrast, Lack et al[3] observed a higher incidence of Aspergillus spp in their series. In the current study, most of the fungal isolates (80%) were obtained during the months of July to September. In contrast, Krishna et al[11] reported maximum incidence of fungal keratitis in Bellary during the harvest months of January, February and June.
Table 4.
Etiological distribution of microbial keratitis
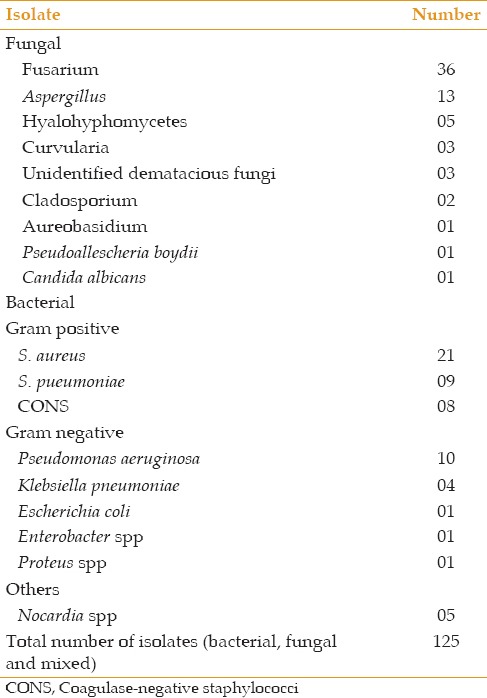
The difference in the isolation rates of these fungal pathogens can be explained by the differences in the climate and the natural environment of individual regions. Studies in the South Indian region have shown a higher incidence of Fusarium as compared to studies in the northern or western India. Fusarium keratitis has a more aggressive course and is less responsive to treatment than Aspergillus.[16,17] Katara et al in Gujarat showed Aspergillus as the dominant isolate.[13] The higher incidence of mycotic keratitis due to Aspergillus spp in their study may be due to the high tolerance of their spores to hot and dry weather conditions.[16] Furthermore, Aspergillus sppare more ubiquitous and can almost be found everywhere on every conceivable type of substrate including soil and decaying organic debris while Fusarium species are common plant pathogens and are mostly found in soil.[3]
Bacteria account for 65–90% of corneal infections with Staphylococcus aureus, S. pneumonia and Pseudomonas aeruginosa accounting for more than 80% of bacterial keratitis.[18] In our study, bacterial keratitis was predominated by Gram positive bacteria similar to other Indian studies by Gopinathan et al[19] and Das et al.[4] The most common bacterial isolate was S.aureus followed by Pseudomonas. A study conducted in Pakistan by Narsani et al[20] also showed higher isolation of Gram positive organisms with S. aureus being the most common (60%). This has been attributed to the climatic zone variation as Gram positive bacterial species are more frequently recovered in temperate zones and Gram negative species in tropical climates. While some studies have reported Pseudomonas as more common bacteria than S. aureus.[1,10] other studies showed coagulase-negative Staphylococci as the most common isolate [Table 4].[9,19,21]
The standard protocol for treatment of bacterial corneal ulcer in our patients was topical instillation of antibiotics.
As there are no standard CLSI guidelines yet for topical ocular antibiotics, proper interpretation of the drug sensitivity testing is not possible; antibiotic sensitivity pattern coupled with clinical improvement is needed to assess the efficacy of a particular antibiotic.
Regarding the antimicrobial susceptibility pattern, all Gram positive bacteria were sensitive to vancomycin. More than 70 percent sensitivity was seen in S.aureus isolates to gatifloxacin, gentamicin and chloramphenicol. All nine S.pneumoniae strains were sensitive to all antibiotics except gentamicin.
Among the Gram negative isolates, Pseudomonas aeruginosa exhibited good sensitivity to gatifloxacin (100%) followed by ciprofloxacin (90%) and moxifloxacin (90%) and was the least sensitive to chloramphenicol (30%). Enterobacter spp and Proteus spp were sensitive to all antimicrobials used [Table 5].
Table 5.
Antibiotic susceptibility pattern (%)
As we restricted our study to aerobic bacterial and fungal agents and did not include anaerobic bacterial, amoebic and viral agents causing keratitis, complete analysis regarding the microbial profile was not possible. Anaerobic organisms usually cause keratitis as mixed infection with aerobic organisms. Not many studies have been conducted in this regard due to the cost and no feasibility of maintaining anaerobic culture methods. Perry et al[22] reported a prevalence of 16.66% for anaerobic corneal infections. Further studies inclusive of all pathogens would give a comprehensive picture of infectious keratitis in our region.
In conclusion, routine microbiological examination of patients with corneal ulcer is necessary in order to analyze and compare the changing trends of the etiology and their susceptibility patterns which would be beneficial in applying an appropriate antimicrobial treatment.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Assudani HJ, Pandya JM, Sarvan R, Sapre AM, Gupta AR, Mehta SJ. Etiological diagnosis of microbial keratitis in a tertiary care hospital in Gujarat. Natl J Med Res. 2013;3:60. [Google Scholar]
- 2.Insan NG, Mane V, Chaudhary BL, Danu MS, Yadav A, Srivastava V. A review of fungal keratitis: Etiology and laboratory diagnosis. Int J Curr Microbiol App Sci. 2013;2:307–314. [Google Scholar]
- 3.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S, Konar J. Bacteriological profile of corneal ulcer with references to Antibiotic susceptibility in a tertiary care hospital in West Bengal. IOSR J Dent Med Sci. 2013;11:72–75. [Google Scholar]
- 5.Bakshi R, Rajagopal R, Sitalakshmi G, Sudhi R, Madhavan H, Bagayalakshmi R. Clinical and Microbiological Profile of Fungal Keratitis: A 7 Year Study at a Tertiary Hospital in South India. Cornea Session III; AIOC 2008 Proceedings. :207–209. [Google Scholar]
- 6.Titiyal JS, Negi S, Anand A, Tandon R, Sharma N, Vajpayee B. Risk factors for perforation in microbial corneal ulcers in north India. Br J Ophthalmol. 2006;90:686–689. doi: 10.1136/bjo.2005.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron NL, Pham JN, Paul BR, Sydney B, Glenn H, Diane RL, et al. Bacteria commonly isolated from Keratitis specimen retain antibiotic susceptibility to Fluoroquinolones and Gentamicin plus Cephalothin. Clin Exp Ophthalmol. 2006;34:44–50. doi: 10.1111/j.1442-9071.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Yang Y, Yang W, Wu R, Xio X, Yuan J, et al. Prevalence of infectious keratitis in Central China. BMC Ophthalmol. 2014;14:43. doi: 10.1186/1471-2415-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Sethi MJ, Iqba R. Causes of microbial keratitis in patients attending an eye clinic at Peshawar. Gomal J Med Sci. 2010;8:20–22. [Google Scholar]
- 10.Yusuf N. Microbial Keratitis in Kingdom of Bahrain: Clinical and Microbiology Study. Mid East Afr J Ophthalmol. 2009;16:3–7. doi: 10.4103/0974-9233.48855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishna S, Shafiyabi S, Sebastian L, Ramesha R, Pavitra D. Microbial keratitis in Bellary district, Karnataka, India: Influence of geographic, climatic, agricultural and occupational risk factors. Int J Pharm Biomed Res. 2013;4:189–193. [Google Scholar]
- 12.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katara RS, Patel ND, Sinha M. A Clinical Microbiological Study of Corneal Ulcer Patients at Western Gujarat, India. Acta Med Iran. 2013;51:399–403. [PubMed] [Google Scholar]
- 14.Alkatan H, Athmanathan S. Incidence and microbiological profile of mycotic keratitis in a tertiary care eye hospital. Saudi J Ophthalmol. 2012;26:217–221. doi: 10.1016/j.sjopt.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idiculla T, Zachariah G, Keshav B, Basu S. A retrospective study of fungal corneal ulcers in the south Sharqiyah region in Oman. Sultan Qaboos Univ Med J. 2009;9:59–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Amrutha KB, Venkatesha D. Microbiological profile of Ulcerative Keratitis in a tertiary care hospital. Int J Res Health Sci. 2014;2:599–603. [Google Scholar]
- 17.Thomas PA. Fungal infections of the cornea. Eye. 2003;17:852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 18.Forbes BA, Sahm DF, Weissfeld AS. In: Infections of the eyes, ears and sinuses. Bailey and Scott's Diagnostic Microbiology. 12th ed. Forbes BA, Sahm DF, Weissfeld AS, editors. St. Louis: Mosby; 2007. pp. 832–837. [Google Scholar]
- 19.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J Ophthalmol. 2009;57:273–279. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narsani AK, Jatoi SM, Lohana MK, Dabir SA, Gul S, Khanzada MA. Hospital-based epidemiology, risk factors and microbiological diagnosis of bacterial corneal ulcer. Int J Ophthalmol. 2009;2:362–366. [Google Scholar]
- 21.Bharati MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial Keratitis in South India: Influence of Risk Factors, Climate, and Geographical Variation. Ophthalmic Epidemiol. 2007;14:61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 22.Perry LD, Briner JH, Colander H. Anaerobic corneal ulcers. Ophthalmology. 1982;89:636–642. doi: 10.1016/s0161-6420(82)34741-7. [DOI] [PubMed] [Google Scholar]


