Abstract
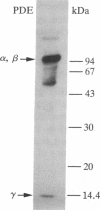
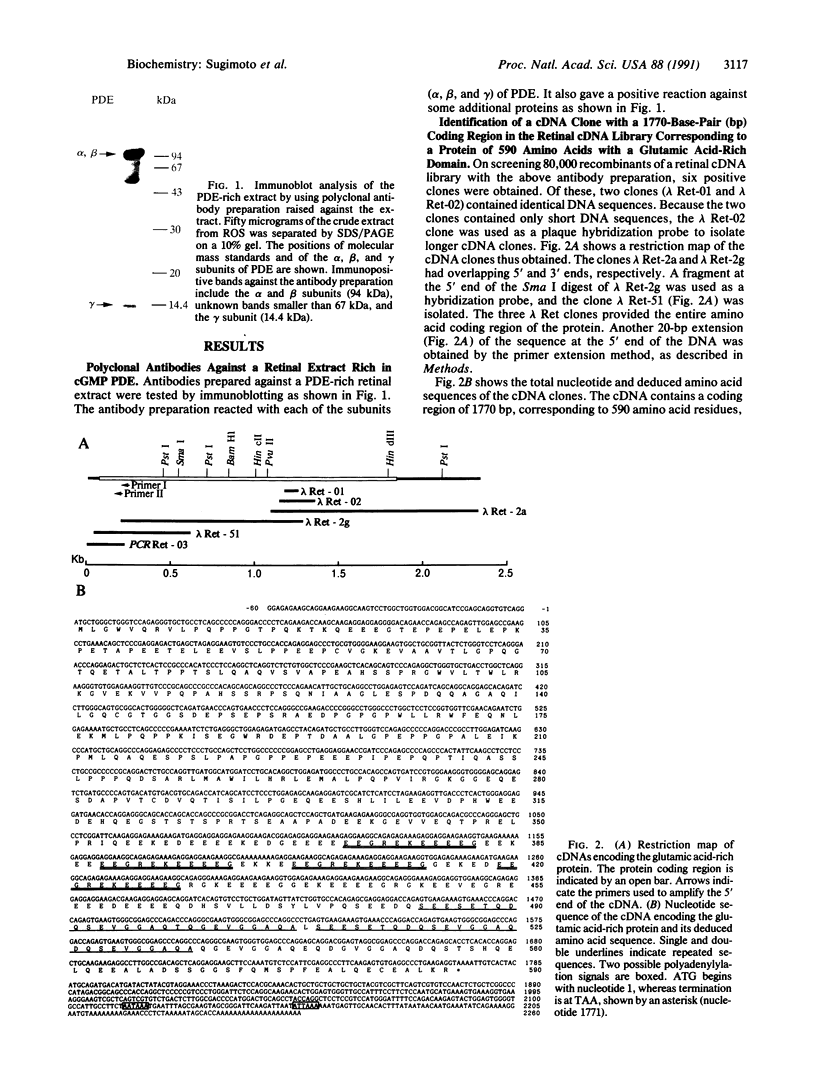
cDNA clones encoding a glutamic acid-rich protein were isolated from a bovine retina cDNA expression library. The cDNA sequence contained an open reading frame of 1770 base pairs encoding a protein of 590 amino acids (64,509 Da) and untranslated regions of 60 and 490 base pairs at the 5' and 3' ends, respectively. The cDNA hybridized to a 2.4-kilobase retinal mRNA. The amino acid sequence derived from the cDNA sequence contains a glutamic acid-rich domain in which 68 of 109 amino acids are glutamic acid. In addition, this domain contains four repeats of a peptide of 11 amino acids and two repeats of a peptide of 26 amino acids. A polyclonal antibody raised against a decapeptide corresponding to the undecapeptide repeat sequence reacted with a protein in an extract of bovine rod outer segments, whose molecular mass, 65 kDa, corresponded to that of the above glutamic acid-rich protein. The retinal glutamic acid-rich protein showed homology with glutamic acid-rich proteins from bovine brain and the C-terminal region of mammalian neurofilaments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Fischer S., Vandekerckhove J., Damme J. V., Plessmann U., Weber K. Protein-chemical characterization of NF-H, the largest mammalian neurofilament component; intermediate filament-type sequences followed by a unique carboxy-terminal extension. EMBO J. 1985 Jan;4(1):57–63. doi: 10.1002/j.1460-2075.1985.tb02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. The complete amino acid sequence of the major mammalian neurofilament protein (NF-L). FEBS Lett. 1985 Mar 25;182(2):475–478. doi: 10.1016/0014-5793(85)80357-4. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Isobe T., Ishioka N., Kadoya T., Okuyama T. Isolation of micro glutamic acid-rich protein from bovine brain. Biochem Biophys Res Commun. 1982 Apr 14;105(3):997–1004. doi: 10.1016/0006-291x(82)91069-5. [DOI] [PubMed] [Google Scholar]
- Isobe T., Okuyama T. Brain micro glutamic acid-rich protein is the C-terminal endpiece of the neurofilament 68-kDa protein as determined by the primary sequence. FEBS Lett. 1985 Mar 25;182(2):389–392. doi: 10.1016/0014-5793(85)80339-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. H., Taniura H., Watanabe Y., Fukada Y., Yoshizawa T., Miki N. Identification of a retina-specific MEKA protein as a 33 K protein. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1063–1068. doi: 10.1016/0006-291x(89)90781-x. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami K., Pandya B. V., Oprian D. D., Khorana H. G. cDNA-derived amino acid sequence of the gamma subunit of GTPase from bovine rod outer segments. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1936–1940. doi: 10.1073/pnas.82.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]