Abstract
Staphylococcus aureus produces several enterotoxins and superantigens, exposure to which can elicit profound toxic shock. A recombinant staphylococcal enterotoxin B (rSEB) containing 3 distinct mutations in the major histocompatibility complex class II binding site was combined with an alum adjuvant (Alhydrogel) and used as a potential parenteral vaccine named STEBVax. Consenting healthy adult volunteers (age range, 23 to 38 years) participated in a first-in-human open-label dose escalation study of parenteral doses of STEBVax ranging from 0.01 μg up to 20 μg. Safety was assessed by determination of the frequency of adverse events and reactogenicity. Immune responses to the vaccination were determined by measurement of anti-staphylococcal enterotoxin B (anti-SEB) IgG by enzyme-linked immunosorbent assay and a toxin neutralization assay (TNA). Twenty-eight participants were enrolled in 7 dosing cohorts. All doses were well tolerated. The participants exhibited heterogeneous baseline antibody titers. More seroconversions and a faster onset of serum anti-SEB IgG toxin-neutralizing antibodies were observed by TNA with increasing doses of STEBVax. There was a trend for a plateau in antibody responses with doses of STEBVax of between 2.5 and 20 μg. Among the participants vaccinated with 2.5 μg to 20 μg of STEBVax, ∼93% seroconverted for SEB toxin-neutralizing antibody. A strong correlation between individual SEB-specific serum IgG antibody titers and the neutralization of gamma interferon production was found in vitro. STEBvax appeared to be safe and immunogenic, inducing functional toxin-neutralizing antibodies. These data support its continued clinical development. (This study has been registered at ClinicalTrials.gov under registration no. NCT00974935.)
INTRODUCTION
Several staphylococcal enterotoxins (SEs) are bacterial superantigens (SAgs) produced by Staphylococcus aureus. The serologically and genetically distinct SEs (A, A1, A2, B, C, C1, C2, C3, D, E, G, H, and I) bind to major histocompatibility complex class II (MHC-II) proteins and to T-cell antigen receptors (TCRs), bypassing processing by antigen-presenting cells (1). Since T-cell engagement with SAgs is independent of clonal specificity, picomolar concentrations of a SAg can result in the polyclonal activation of large subsets of peripheral T cells (up to 20%) (2–4). The nonspecific activation of lymphocytes and the subsequent overwhelming production of cytokines make the clinical effects of SAgs potentially lethal. During the 1960s, staphylococcal enterotoxin B (SEB) became a focus of interest because aerosolization of minute amounts could be used to produce an incapacitating biological weapon (5).
Strategies to counteract the effects of SAgs are currently limited to treatment of the infection, which thereby limits toxin production. There are currently no approved vaccines for Staphylococcus aureus or SEB. There is evidence that anti-SEB antibodies capable of neutralizing the effect of the SAg can abrogate the superantigenic activation of the immune system and be protective for the host (6, 7). Nonetheless, efforts to develop SEB vaccines require caution because biological inactivation must be complete and the critical antigenic structural epitopes must be preserved.
An Escherichia coli-expressed recombinant SEB (rSEB) mutant containing mutations in the hydrophobic binding loop (L45R), the polar binding pocket (Y89A), and the disulfide loop (Y94A) was developed using a structure-based rational approach (8) wherein site-specific mutagenesis was used to direct mutations at the MHC-II binding site (9–11). In a limited number of animal challenge studies, parenteral immunization with a nonadjuvanted rSEB mutant elicited anti-SEB IgG antibodies in mice and rhesus monkeys that could be measured by enzyme-linked immunosorbent assay (ELISA) and that correlated with protection (9). Nasal and oral immunization with the rSEB mutant, given with cholera toxin (CT) as an adjuvant, also elicited anti-SEB antibodies and protected against lethal challenge (10). In expanded studies, mice and rhesus monkeys parenterally immunized with the rSEB mutant plus an alum adjuvant demonstrated anti-SEB antibodies that correlated with protection; animals that achieved anti-SEB titers of ≥104 were 100% protected, those with titers of ∼103 had partial protection, and those with titers of ≤103 were not protected (11). Furthermore, animals that received three doses of 20 μg the rSEB mutant consistently demonstrated anti-SEB endpoint titers of ≥104, whereas three doses of 5 μg the rSEB mutant or two doses of 20 μg the rSEB mutant failed to consistently produce anti-SEB titers of ≥104. A sublethal challenge model involving piglets demonstrated significant reductions in the amounts of cytokines elicited among animals immunized with a transgenic soybean seed-expressed mutant SEB (soy-mSEB) vaccine candidate (12).
To further explore the rSEB vaccine, we conducted a first-in-human phase 1 dose escalation study of rSEB administered with an alum adjuvant (here named the STEBVax vaccine). Because wild-type SEB at doses as low as 0.0004 μg/kg of body weight have been known to provoke significant toxicity (13, 14), vaccination of our initial cohort of subjects began with a single dose of 0.01 μg of STEBVax. Subsequent cohorts were used to evaluate increasingly higher doses up to a target dose of 20 μg of STEBVax. A final cohort (cohort 7) was used to evaluate two doses of 20 μg of STEBVax administered 21 days apart.
MATERIALS AND METHODS
Study design.
This single-site, dose escalation phase 1 study (ClinicalTrials registration no. NCT00974935) was designed to assess the safety and immunogenicity of the parenterally administered STEBVax vaccine. Subjects placed into six cohorts consisting of four subjects per cohort were enrolled to receive a single intramuscular (i.m.) dose of vaccine containing one of six escalating doses of 0.01, 0.1, 0.5, 2.5, 10, or 20 μg of STEBVax. Four subjects were enrolled in a seventh cohort, and the subjects received two i.m. STEBVax doses of 20 μg separated by 21 days. Eligible subjects were nonpregnant healthy adults who were 18 to 40 years of age, provided informed consent, and were screened for the absence of chronic medical conditions, immunodeficiencies, or any other conditions that could jeopardize the safety or welfare of the volunteer. The complete eligibility criteria are published at https://clinicaltrials.gov/show/NCT00974935.
The first two subjects in each cohort were vaccinated at least 24 h apart from each other. The next two subjects in each cohort were vaccinated after the 14-day safety data were reviewed and approved by the principal investigator, independent safety monitor, and Division of Microbiology and Infectious Diseases (DMID, National Institute of Allergy and Infectious Diseases) medical monitor. Prior to proceeding to evaluation of the next higher dose, an independent safety monitoring committee (SMC) reviewed and approved the cumulative safety data for all subjects enrolled in the contemporaneous cohort available through day 14 postvaccination. The study was approved by the University of Maryland, Baltimore, Institutional Review Board. The study began enrollment in January 2013 and completed enrollment in September 2014.
Vaccine.
The recombinant staphylococcal enterotoxin B (rSEB) recombinant purified protein mutant derivative was manufactured under current good manufacturing practices (cGMP) at the Pilot Bioproduction Facility of the Walter Reed Army Institute of Research (Silver Spring, MD), using the same procedures previously reported (8, 15). The study vaccine vials (lot no. 1752, May 2012) contained 1 mg/ml rSEB, 50 mM glycine, and 140 mM sodium chloride (pH 8.5). The predicted pH of the protein solution after dilution, even at the highest targeted dose, was near 7.0. The STEBVax vaccine was prepared by the Investigational Drug Service Pharmacy, University of Maryland Medical Center, by diluting rSEB to the desired concentration with sterile saline and then mixing the diluted rSEB with alum (Alhydrogel; 140 μg of AlOH3 per dose) as an adjuvant. Each dose of vaccine was delivered intramuscularly into the deltoid muscle in a 0.5-ml volume.
Safety and reactogenicity.
After vaccination, the subjects were observed in the clinic for at least 8 h for the development of any local or systemic reactions (including headache, malaise, myalgia, chills, nausea, vomiting, rash, diarrhea, or anorexia), and vital signs were recorded before and at 1, 2, 4, and 8 h after vaccination. For the next 14 days, the subjects recorded a daily oral temperature and the occurrence of any injection site reaction (pain, erythema, and induration) or systemic reactions (fever, headache, malaise, myalgia, chills, nausea, vomiting, rash, diarrhea, and anorexia). For the subjects receiving a single dose, a follow-up phone call on day 5 and clinic visits on days 1, 3, 7, 14, 21, and 28 were conducted for the reporting of adverse events (AEs). For the subjects receiving two doses, vaccination visits were on days 0 and 21, follow-up phone calls were on days 5 and 26, and clinic visits were completed on days 1, 3, 7, 14, 22, 24, 35, 42, and 49 for AE reporting. A clinic visit at day 56 (for subjects receiving a single dose) or 77 (for subjects receiving two doses) and a phone call contact at day 180 (for subjects receiving a single dose) or 201 (for subjects receiving two doses) were completed for the reporting of serious adverse events (SAEs) and new-onset medical conditions. Serum was collected on days 0 (prevaccination), 7, 14, 21, 28, and 56 from subjects receiving a single dose; serum was collected on days 0 (predosing of dose one), 7, 14, 21 (predosing of dose two), 28, 35, 42, 49, and 77 from subjects receiving two doses.
Laboratory analyses for determination of the clinical safety of the vaccine were performed on days 3, 7, and 28 (relative to the time of administration of each dose of vaccine) and included hematology analyses (complete blood count with differential and platelets), analysis of a coagulation panel (prothrombin time, partial thromboplastin time), and biochemistry analyses (sodium, potassium, creatinine, glucose, aspartate transaminase, alanine transaminase, alkaline phosphatase, total bilirubin, and creatine phosphokinase [CPK] concentrations). Subjects graded the severity of their symptoms as mild (no interference with normal activities), moderate (some interference with normal activities), or severe (prevention of normal activities). The clinical signs and abnormal laboratory values were graded using predefined criteria.
Anti-SEB IgG ELISA.
Serum anti-SEB IgG antibodies were measured by ELISA. Briefly, 96-well U-bottom microtiter plates (Immulon 2HB; Thermo Scientific, Rochester, NY) were coated with 0.5 μg/ml SEB (catalog number NR-10049; BEI Resources) that had been diluted in carbonate buffer (pH 9.6) and incubated for 3 h at 37°C. After washing with phosphate-buffered saline (PBS)–Tween 20 (0.05%), the plates were blocked with PBS containing 10% nonfat dry milk overnight at 4°C. The plates were washed again, serially diluted serum samples were added, and the plates were incubated for 1 h at 37°C. Bound antibodies were detected by adding horseradish peroxidase (HRP)-labeled goat anti-human IgG (Southern Biotech, Birmingham, AL) to the plates and incubating the plates for 1 h at 37°C, followed by addition of tetramethylbenzidine microwell peroxidase substrate (Invitrogen, Carlsbad, CA) and incubation for 15 min at room temperature in the dark. The colorimetric reaction was stopped by adding 1 M phosphoric acid, and the values of the absorbance at 450 nm were read on a Multiskan Ascent microplate reader. All samples were tested in duplicate, starting at a dilution of 1:50, and 2-fold dilutions were performed on the plates until the absorbance values (at least 4 data points) were within the linear range. Endpoint titers for individual samples were calculated through linear regression, using the inverse of the serum dilution which produced an absorbance of 0.2 above the mean for the blanks. Titers are reported in ELISA units (EU) per milliliter. Positive and negative controls were included in each assay. A positive response to the vaccine was defined as a 4-fold or greater rise in titer after vaccination.
TNA.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood from healthy human donors (obtained under a separate Chesapeake Institutional Review Board-approved protocol) by Ficoll gradient centrifugation as previously described (16). PBMCs were washed twice in PBS, frozen in fetal bovine serum (FBS) containing 10% dimethyl sulfoxide overnight at −80°C, and stored in liquid nitrogen until further use. For the toxin neutralization assay (TNA), PBMCs were quickly thawed and resuspended in RPMI 1640 with 10% FBS. The cells were washed in RPMI 1640 with 10% FBS, enumerated by trypan blue exclusion, and adjusted to 2 × 106 cells/ml. Seventy-five microliters of this suspension of cells (1.5 × 105 cells), which had a viability of >95%, was added to duplicate wells of 96-well flat-bottom plates containing 37.5 μl of semilogarithmically diluted (0.02 to 20 μg/ml) serum samples and 0.1 ng of SEB (Toxin Technology Inc., Sarasota, FL). Wells containing medium with toxin only were used as negative controls. The cultures were incubated at 37°C in 5% CO2 for 48 h. The cells were centrifuged at 1,600 × g for 10 min, the culture supernatants were harvested, and the level of gamma interferon (IFN-γ) production was assessed by ELISA (R&D Systems, Minneapolis, MN) following the manufacturer's protocol. The plates were read at 450 nm using a VersaMax plate reader (Molecular Devices, Sunnyvale, CA). Cells stimulated with toxin in the absence of a neutralizing agent served as a positive control, and the level of IFN-γ inhibition with these cells was considered 0%. Accordingly, inhibition of IFN-γ production in the presence of neutralizing serum was calculated as the difference between the positive control and the test sample. The median (50%) inhibitory concentration (IC50) values for the neutralizing serum were determined using a 4-parameter logistic model (equation 205, XLFit software, version 5.4; IDBS, Alameda, CA).
Statistical analysis.
Although no formal sample size calculation was performed, the number of subjects was selected to be appropriate for a first-in-human study. Local and systemic reactogenicity symptoms are summarized using the number of subjects who experienced each event overall. The primary immunogenicity objective was based on the results of the anti-SEB IgG ELISA, whereas the TNA was performed on specimens that had been stored for future use, and evaluation of the toxin-neutralizing antibody titer was an exploratory objective. Geometric mean titers were computed by transforming the results to a logarithmic scale to satisfy asymptotic normality conditions, computing the mean, and then converting the mean value back to the original scale. Due to the small sample sizes within the cohorts, no formal group comparisons were made. All data analyses and statistical computations were conducted with SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Participants.
A total of 28 eligible volunteers were enrolled in this study, with 4 participants being distributed into each of 7 cohorts. The mean age of all participants was 30.9 years (age range, 23 to 38 years), and the distribution of the participants by gender was 17 (61%) males and 11 (39%) females. The distribution of the participants by race and ethnicity was 12 (43%) white, 12 (43%) black, 2 (7%) Asian, and 2 (7%) multirace; among the 28 participants, 2 were Hispanic and 26 were non-Hispanic (see Table S1 in the supplemental material, Demographics).
Vaccine safety.
No subjects reported severe solicited local or systemic reactions in the 14 days after the first vaccination. Seven (25.0%) subjects reported mild headache, and 3 (10.7%) reported moderate headache. Following the second vaccination, no subjects reported moderate or severe solicited systemic reactions; 1 (25%) subject reported a mild event of diarrhea, 1 subject had mild pain, and 1 subject had mild injection site erythema (Table 1, solicited reactogenicity).
TABLE 1.
Incidence of vaccine reactogenicity within 14 days of receipt of vaccine
| Symptom | No. of subjects with symptom/total no. of subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (0.01 μg) | Cohort 2 (0.1 μg) | Cohort 3 (0.5 μg) | Cohort 4 (2.5 μg) | Cohort 5 (10 μg) | Cohort 6 (20 μg) | Cohort 7 |
Totala | ||
| First 20-μg dose | Second 20-μg dose | ||||||||
| Local symptoms | |||||||||
| Pain | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 1/4 | 2/4 | 3/28 |
| Erythema | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 1/28 |
| Induration | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/28 |
| Systemic symptoms | |||||||||
| Fever | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/28 |
| Headache | 0/4 | 2/4 | 0/4 | 1/4 | 0/4 | 0/4 | 1/4 | 0/4 | 4/28 |
| Fatigue/malaise | 1/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 2/28 |
| Myalgia | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 1/4 | 0/4 | 0/4 | 2/28 |
| Chills | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/28 |
| Nausea/vomiting | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 | 1/4 | 0/4 | 2/28 |
| Rash | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/28 |
| Diarrhea | 0/4 | 1/4 | 0/4 | 0/4 | 1/4 | 0/4 | 0/4 | 1/4 | 3/28 |
| Anorexia | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/28 |
Reactogenicity in the two-dose cohort was not counted twice if a person experienced the same symptom after both vaccinations.
There were a total of 7 unsolicited nonserious adverse events, which were experienced by 6 subjects (21.4%), and 2 serious adverse events, which were experienced by 2 subjects (7.1%). None of these events, including the 2 SAEs, were determined by the investigator to be related to vaccination. The most frequent abnormal laboratory measure was the creatine phosphokinase (CPK) concentration, which was detected in 11 subjects (39.3%); these laboratory abnormalities self-resolved, and there was no trend for a dose-response relationship with any abnormal laboratory measure. There was no trend for additional reactogenicity, abnormalities on laboratory analyses for clinical safety, or AEs with the administration of the second dose of vaccine. There were no new-onset chronic medical conditions reported.
Vaccine immunogenicity.
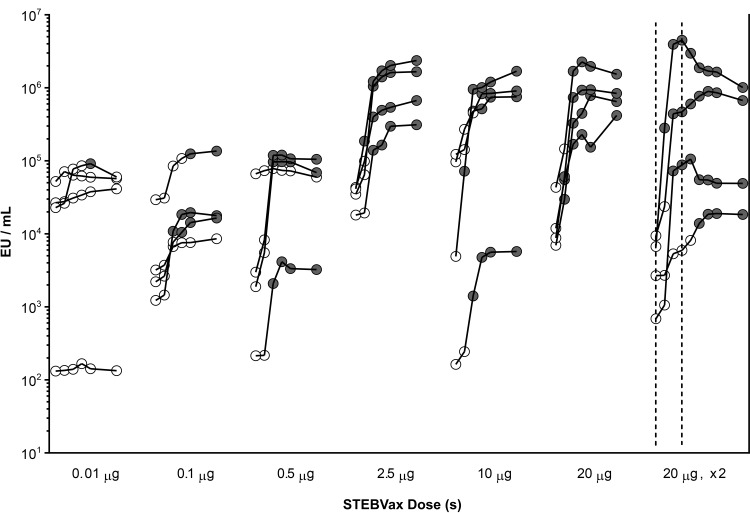
The anti-SEB IgG levels were measured by ELISA (Fig. 1; see also Table S2 in the supplemental material). There was wide heterogeneity in the levels of preexisting antibody at the baseline, with a range of 2 to 5 log EU/ml. Nonetheless, there appeared to be a dose-response relationship for the elicitation of SEB-specific IgG subsequent to the administration of STEBVax at increasing doses. Only 1 of 4 subjects experienced seroconversion with the lowest STEBVax dose evaluated, 0.01 μg. The next STEBVax dose evaluated, 0.1 μg (which had 10-fold more antigen than the 0.01-μg STEBVax dose), elicited seroconversions among 3 of 4 subjects: at as early as 14 days in 1 subject, at 21 days in another subject, and at 28 days postvaccination in the third subject. All 3 subjects maintained antibody levels ≥4-fold above those at the baseline through day 56 postvaccination. The next STEBVax dose evaluated, 0.5 μg, also elicited seroconversions in 3 of 4 subjects; the seroconversion was observed at 14 days postvaccination in all 3 subjects and antibody levels were maintained at levels ≥4-fold above those at the baseline through day 56 postvaccination. The one subject in that dosing group which did not demonstrate a significant increase in anti-SEB IgG was found to have a high baseline titer (4.8 logs). For each of the subsequent single-dose cohorts (in which the subjects received doses of 2.5, 10, and 20 μg), there was a 100% seroconversion rate (seroconversion in 4 of 4 subjects) and there was possibly a trend for earlier seroconversion, such that the single STEBVax dose of 20 μg elicited seroconversions at day 7 postvaccination in 3 subjects; the fourth subject seroconverted at day 14. All subjects receiving 2.5, 10, or 20 μg of STEBVax maintained anti-SEB IgG levels of ≥4-fold above those at the baseline through day 56 postvaccination (Fig. 1).
FIG 1.
Serum anti-SEB IgG antibody detection by ELISA. Individual serum anti-SEB IgG titers were measured by ELISA subsequent to administration of a single dose of 0.01 μg, 0.1 μg, 0.5 μg, 2.5 μg, 10 μg, or 20 μg or two doses of 20 μg of the STEBVax vaccine and are expressed as the number of ELISA units (EU) per milliliter. For the recipients of single doses, the circles denote (from left to right) data from the following six time points: the baseline and 7, 14, 21, 28, and 56 days postvaccination. For the recipients of two doses, the vertical dotted lines indicate the times of administration of the first and second doses, which were day 0 (baseline) and day 21, respectively, and the circles denote (from left to right) data from the following time points: the baseline and 7, 14, 21, 28, 35, 42, 49, and 77 days after administration of the first dose of vaccine. The baseline antibody level and any postvaccination antibody level which failed to achieve a 4-fold increase from the baseline are indicated by open circles. Positive antibody responses, defined by achievement of a ≥4-fold increase in titer compared to that at the baseline, are indicated by shaded circles.
For the two-dose cohort, 3 of 4 subjects demonstrated seroconversions in response to the first dose, and the remaining subject seroconverted after the second dose. Because this study involved small sample sizes (its primary endpoint was safety), it is difficult to make definitive conclusions regarding any differences in the antibody responses between the single doses and two doses of STEBVax.
The serum toxin-neutralizing antibody titers were also measured by TNA (Fig. 2; see also Table S3 in the supplemental material). As was observed with the SEB-specific IgG levels, there was significant heterogeneity in the levels of preexisting serum toxin-neutralizing antibody determined by TNA prior to vaccination. There also appeared to be a dose-response relationship for the antibody responses observed by TNA with increasing doses of STEBVax. Among the subjects in the cohort receiving the lowest STEBVax dose, 0.01 μg, only one short-lived seroconversion to positivity for toxin-neutralizing antibody was found by TNA. In the cohort receiving the next higher STEBVax dose, 0.1 μg, seroconversions to positivity for toxin-neutralizing antibody were elicited in 3 of 4 participants, as observed by TNA. Higher doses of STEBVax (2.5 to 20 μg) achieved seroconversions to positivity for toxin-neutralizing antibody as early as 7 days after vaccination in some participants, but these responses were heterogeneous, as detected by TNA. One subject in the cohort receiving the 10-μg dose did not consent to storage of that subject's research specimens for future use, and thus, the specimen was not available for the TNA. Another subject in the cohort receiving the 10-μg dose demonstrated a 4-fold increase in ELISA antibody titer but failed to demonstrate a response by TNA. The second dose of vaccine in the two-dose cohort appeared to be successful in boosting the toxin-neutralizing response, as detected by TNA (Fig. 2).
FIG 2.
Serum toxin-neutralizing antibody responses. Individual toxin-neutralizing antibody titer subsequent to administration of a single dose of 0.01 μg, 0.1 μg, 0.5 μg, 1 μg, 5 μg, 10 μg, or 20 μg or two doses of 20 μg of vaccine. The neutralization of IFN-γ production, expressed as the median (50%) inhibitory concentration (IC50), in the supernatant of human PBMCs stimulated with 0.1 ng of SEB and blocked with serially diluted serum was measured by ELISA. For the recipients of single doses, the circles denote (from left to right) data from the following six time points: the baseline and 7, 14, 21, 28, and 56 days postvaccination. For the recipients of two doses, the vertical dotted lines indicate the times of administration of the first and second doses, which were day 0 (baseline) and day 21, respectively, and the circles denote (from left to right) data from the following time points: the baseline and 7, 14, 21, 28, 35, 42, 49, and 77 days after administration of the first dose of vaccine. The baseline antibody level and any postvaccination antibody level which failed to achieve a 4-fold increase from that at the baseline are indicated by open circles. Positive responses, defined by ≥4-fold increases in titer compared to that at the baseline, are indicated by shaded circles. One subject in the 10-μg-dose cohort did not consent to storage of that subject's research specimens for future use, and thus, specimens from that subject were not available for assessment of toxin-neutralizing activity.
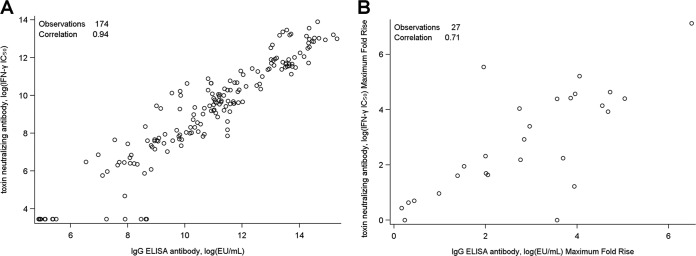
In order to further examine whether antigen-specific binding was associated with the functional capacity to neutralize toxin, we analyzed the serum anti-SEB IgG titer determined by ELISA versus the antibody titer determined by TNA for all participants and all time points (Fig. 3A). There was a high level of congruence with a nearly linear correlation between the antigen specificity and the ability to neutralize IFN-γ production in vitro (correlation coefficient, 0.94; 95% confidence interval [CI], 0.92 to 0.95). The correlation analysis was also performed on the maximum fold rise in titer from the baseline for each subject (correlation coefficient, 0.71; 95% CI, 0.44 to 0.86) (Fig. 3B).
FIG 3.
Correlation of antibody titers by ELISA and TNA. A scatter plot of individual serum anti-SEB ELISA IgG titers (x axis) versus the SEB toxin-neutralizing antibody titer (y axis) is shown. (A) Representative results for all subjects and time points (n = 174; r = 0.94, 95% CI, 0.92 to 0.95); (B) representative results for all subjects at the baseline and at the times of the postvaccination peaks (n = 27; r = 0.71, 95% CI, 0.44 to 0.86).
DISCUSSION
The clinical syndromes which might be precipitated by a Staphylococcus aureus infection and exposure to SAgs can range from acute food poisoning (17, 18) to highly lethal toxic shock syndrome (menstruation associated [19, 20] and nonmenstruation associated [21, 22]). A role for staphylococcal SAgs has also been implicated in the pathogenesis of atopic dermatitis (23) and psoriasis (24). Furthermore, staphylococcal enterotoxin B (SEB) is a category B select agent because of the recognition that ingestion of a product created by the intentional aerosolization of minute amounts of SEB can result in marked incapacitation and death (14, 25).
This is the first-in-human evaluation of a vaccine based on a recombinantly expressed, genetically modified SEB protein, STEBVax. Due to concerns for wide-scale T-cell activation and an overwhelming cytokine storm, as was seen with the infusion of a CD28 superagonist (26), we carefully selected the starting dose (0.01 μg) to be near the lower end of the known biologically relevant dose (equivalent) of wild-type SEB (0.0004 μg/kg and >1,000 times lower than the final target dose of 20 μg). Despite this potential for toxicity, an overall lack of reactogenicity, laboratory abnormalities, and unsolicited adverse events was recorded. There was no trend for a dose-response relationship with any reactogenicity symptom or sign; no serious adverse events associated with vaccination were observed; and all other AEs were of mild or moderate severity, were of short duration, and self-resolved. Therefore, we appear to confirm the elimination of the superantigenic toxicity of STEBVax through the three site-directed mutations incorporated in the MHC-II binding site of rSEB.
There was a trend for more seroconversions and a faster onset of serum anti-SEB IgG and toxin-neutralizing antibodies with increasing doses of STEBVax. The anti-SEB IgG antibody response appeared to reach a plateau (leveling off) following single doses of between 2.5 and 20 μg of STEBVax. Although the sample size for each dose cohort was very small, we may conclude that the lowest doses of STEBVax, 0.01 μg to 0.5 μg, are too low to ensure consistent elicitation of anti-SEB IgG antibody. It is difficult to make conclusions regarding the necessity of a multidose regimen, given the limited sample size. A comparison of a single-dose regimen and multidose regimens evaluating different intervals between doses should be performed in a follow-on study. Furthermore, we did not evaluate the duration over which the antibodies elicited by the vaccine remained. Nonetheless, documentation of the production of protective antibodies which are of a long duration would be another important objective in future studies of STEBVax.
The serum anti-SEB IgG antibody titers determined by ELISA correlated closely with functional (toxin-neutralizing) antibody activity, i.e., the in vitro suppression of IFN-γ production. Although the vaccine antigen, rSEB, was expressed from E. coli, it was reassuring that the antibodies produced by vaccination recognized native SEB and were functionally able to neutralize cell toxicity in vitro. The fact that some participants had relatively high baseline levels of SEB-specific IgG antibody, which correlated with high baseline toxin neutralization, implies that either colonization or prior infection events may have occurred in those individuals. The eligibility criteria for study participants were designed to evaluate the vaccination of healthy volunteers, and there was no reporting of recent or frequent skin infections or other elements in the medical histories which might explain the presence of high titers of preexisting antibodies. Alternatively, these antibodies may have been induced by other organisms and represent cross-reactive antibodies.
Toxin neutralization is considered the primary functional mechanism of protection against SEB intoxication. Prior animal studies indicated a correlation between survival from lethal SEB challenge and the serum anti-SEB IgG antibody titers determined by ELISA (9, 11). Among the participants in our study vaccinated with doses of STEBVax of 2.5 μg to 20 μg, we observed a positive response rate, consisting of 4-fold increases in SEB neutralizing antibody titers, of ∼93% (14 of 15 subjects; 1 subject did not consent to storage of that subject's samples for future use). The SEB-neutralizing antibody levels were sustained in all 14 participants through 8 weeks after vaccination. For the one subject (in the 10-μg-dose cohort) that failed to demonstrate an SEB-neutralizing antibody response, we cannot speculate why this occurred. Currently, the ELISA antibody or toxin-neutralizing antibody level (threshold) necessary for protection against SEB intoxication in humans is unknown. The protective threshold may be dependent on the dose of SEB that an individual is exposed to. Efficacy trials in humans are not feasible due to the rare occurrence of SEB intoxication, so a correlate of immunity remains to be determined in future animal challenge studies.
The clinical development of STEBVax could serve multiple purposes. The most likely application of STEBVax is for prevention of SEB intoxication resulting from a biowarfare or bioterror attack. Like other biodefense vaccines, such as anthrax vaccine absorbed or the smallpox vaccine, the product can be stockpiled to be used for immunization of troops or first responders in the event of an imminent threat. Furthermore, STEBVax could be used as an experimental vaccine (under an investigational new drug application) to generate hyperimmune globulin for postexposure treatment of SEB-induced toxic shock for both civilian and military applications.
Another use for STEBVax would be as a component of a multivalent immunotherapeutic vaccine for treating or preventing disease caused by S. aureus and its many superantigens. Varshney et al. recently reported that a neutralizing monoclonal antibody against SEB provides partial protection against S. aureus sepsis and deep tissue infection (27). Toxic shock syndrome toxin (TSST) represents another superantigen produced by S. aureus. The results of a phase 1 trial of a recombinant detoxified toxic shock syndrome toxin 1 variant (rTSST-1v) vaccine were recently published and demonstrated that vaccine showed tolerability and immunogenicity (28). A long-range goal would include the design of a vaccine candidate that will protect against the broad range of staphylococcal superantigens and other toxins. We have previously demonstrated that the presence of higher titers of antiexotoxin antibodies at the time of sepsis is associated with improved outcomes in patients with invasive S. aureus infection (7). We have previously shown that a toxoid vaccine for S. aureus pore-forming toxins, such as alpha-hemolysin (Hla) and leukocidins, can provide protection against S. aureus infection in mice (29, 30). A multivalent toxoid vaccine can also be combined with surface antigens to induce both toxin neutralization and opsonophagocytic activity. A recent report indicates that treatment of mice with a combination of polyclonal antibodies against SEB, Hla, and the manganese transport protein C (MntC) reduced the levels of bacteremia (31). A multivalent toxoid-based vaccine would likely provide protection by limitation of the severity of disease rather than prevention of infection with S. aureus.
In summary, the positive safety profile of STEBVax at doses of up to 20 μg and the strong correlation between antigen-specific binding and the elicitation of functional antibodies by STEBVax are reassuring for the continued clinical development of STEBVax.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the guidance and oversight of the safety monitoring committee: Robert S. Daum (chair), Mary-Claire Roghmann (independent safety monitor), and Michael W. Ellis. We are grateful for the contributions of Carol Tacket toward the planning and preparation for this study; Myounghee Lee and the assistance of the Investigational Drug Service Pharmacy at the University of Maryland Medical Center; and Xiaolin Wang, Nancy Greenberg, Lisa Chrisley, and all the research nurses of the Outpatient Research Clinic at the Center for Vaccine Development and at the GCRC. We also thank Shahida Baqar, Eric Zhou, Mohamed Elsafy (DMID medical monitor), Stephanie Zafonte, Gabriele Feolo, and the Enteric and Hepatic Diseases Branch of NIAID for their support on the project. We extend a special thanks to Jill Barrett, Carey Petrie, and The Emmes Corporation (Rockville, MD) for data management and analysis. We further acknowledge Richard Millward and Kenneth Eckels of the Walter Reed Army Institute of Research for overseeing the manufacture of STEBVax according to cGMP. We also thank Robert Ulrich, U.S. Army Medical Research Institute of Infectious Diseases, for scientific advice.
M.J.A., R.D., G.C.L., R.P.A., and F.W.H. are employed by Integrated BioTherapeutics; K.L.W. was formerly employed by Integrated BioTherapeutics. The remaining authors have no commercial association or conflict of interest.
This work was supported by a National Institute of Allergy and Infectious Diseases (NIAID) Food & Waterborne Diseases Integrated Research Network Clinical Research Unit (FWD IRN CRU) contract (N01-AI-40014; principal investigator, W.H.C.), an NIAID Vaccine and Treatment Evaluation Unit (VTEU) contract (N01-AI-80001), and the University of Maryland General Clinical Research Center grant (M01-RR-16500). The manufacture of STEBVax was supported by a U.S. Army phase II SBIR grant to M.J.A. (W81WH-08-C-0004).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00399-16.
REFERENCES
- 1.McCormick JK, Yarwood JM, Schlievert PM. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Llera A, Malchiodi EL, Mariuzza RA. 1999. The structural basis of T cell activation by superantigens. Annu Rev Immunol 17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Schlievert PM. 1993. Role of superantigens in human disease. J Infect Dis 167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 4.Proft T, Fraser JD. 2003. Bacterial superantigens. Clin Exp Immunol 133:299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, Pavlin JA, Christopher GW, Eitzen EM Jr. 1997. Clinical recognition and management of patients exposed to biological warfare agents. JAMA 278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 6.LeClaire RD, Bavari S. 2001. Human antibodies to bacterial superantigens and their ability to inhibit T-cell activation and lethality. Antimicrob Agents Chemother 45:460–463. doi: 10.1128/AAC.45.2.460-463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. 2012. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 206:915–923. doi: 10.1093/infdis/jis462. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich RG, Bavari S, Olson MA. 1995. Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat Struct Biol 2:554–560. doi: 10.1038/nsb0795-554. [DOI] [PubMed] [Google Scholar]
- 9.Ulrich RG, Olson MA, Bavari S. 1998. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine 16:1857–1864. doi: 10.1016/S0264-410X(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 10.Stiles BG, Garza AR, Ulrich RG, Boles JW. 2001. Mucosal vaccination with recombinantly attenuated staphylococcal enterotoxin B and protection in a murine model. Infect Immun 69:2031–2036. doi: 10.1128/IAI.69.4.2031-2036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boles JW, Pitt ML, LeClaire RD, Gibbs PH, Torres E, Dyas B, Ulrich RG, Bavari S. 2003. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin Immunol 108:51–59. doi: 10.1016/S1521-6616(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 12.Hudson LC, Seabolt BS, Odle J, Bost KL, Stahl CH, Piller KJ. 2013. Sublethal staphylococcal enterotoxin B challenge model in pigs to evaluate protection following immunization with a soybean-derived vaccine. Clin Vaccine Immunol 20:24–32. doi: 10.1128/CVI.00526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahanotu E, Alvelo-Ceron D, Ravita T, Gaunt E. 2006. Staphylococcal enterotoxin B as a biological weapon: recognition, management, and surveillance of staphylococcal enterotoxin. Appl Biosafety 11:120–126. doi: 10.1177/153567600601100303. [DOI] [Google Scholar]
- 14.Rusnak JM, Kortepeter M, Ulrich R, Poli M, Boudreau E. 2004. Laboratory exposures to staphylococcal enterotoxin B. Emerg Infect Dis 10:1544–1549. doi: 10.3201/eid1009.040250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffman JD, Zhu J, Roach JM, Bavari S, Ulrich RG, Giardina SL. 2002. Production and purification of a recombinant staphylococcal enterotoxin B vaccine candidate expressed in Escherichia coli. Protein Expr Purif 24:302–312. doi: 10.1006/prep.2001.1556. [DOI] [PubMed] [Google Scholar]
- 16.Berthold F. 1981. Isolation of human monocytes by Ficoll density gradient centrifugation. Blut 43:367–371. doi: 10.1007/BF00320315. [DOI] [PubMed] [Google Scholar]
- 17.Le Loir Y, Baron F, Gautier M. 2003. Staphylococcus aureus and food poisoning. Genet Mol Res 2:63–76. [PubMed] [Google Scholar]
- 18.Tranter HS. 1990. Foodborne staphylococcal illness. Lancet 336:1044–1046. doi: 10.1016/0140-6736(90)92500-H. [DOI] [PubMed] [Google Scholar]
- 19.Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Anderson RL, Hill DL, Broome CV, Band JD, Fraser DW. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med 303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 20.Davis JP, Chesney PJ, Wand PJ, LaVenture M. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med 303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 21.Andrews MM, Parent EM, Barry M, Parsonnet J. 2001. Recurrent nonmenstrual toxic shock syndrome: clinical manifestations, diagnosis, and treatment. Clin Infect Dis 32:1470–1479. doi: 10.1086/320170. [DOI] [PubMed] [Google Scholar]
- 22.Gaventa S, Reingold AL, Hightower AW, Broome CV, Schwartz B, Hoppe C, Harwell J, Lefkowitz LK, Makintubee S, Cundiff DR, Sitze S. 1989. Active surveillance for toxic shock syndrome in the United States, 1986. Rev Infect Dis 11(Suppl 1):S28–S34. [DOI] [PubMed] [Google Scholar]
- 23.Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, Wahn U, Renz H. 2000. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol 105:814–819. doi: 10.1067/mai.2000.105528. [DOI] [PubMed] [Google Scholar]
- 24.Balci DD, Duran N, Ozer B, Gunesacar R, Onlen Y, Yenin JZ. 2009. High prevalence of Staphylococcus aureus cultivation and superantigen production in patients with psoriasis. Eur J Dermatol 19:238–242. doi: 10.1684/ejd.2009.0663. [DOI] [PubMed] [Google Scholar]
- 25.Gill DM. 1982. Bacterial toxins: a table of lethal amounts. Microbiol Rev 46:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 27.Varshney AK, Wang X, MacIntyre J, Zollner RS, Kelleher K, Kovalenko OV, Pechuan X, Byrne FR, Fries BC. 2014. Humanized staphylococcal enterotoxin B (SEB)-specific monoclonal antibodies protect from SEB intoxication and Staphylococcus aureus infections alone or as adjunctive therapy with vancomycin. J Infect Dis 210:973–981. doi: 10.1093/infdis/jiu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwameis M, Roppenser B, Firbas C, Gruener CS, Model N, Stich N, Roetzer A, Buchtele N, Jilma B, Eibl MM. 2016. Safety, tolerability, and immunogenicity of a recombinant toxic shock syndrome toxin (rTSST)-1 variant vaccine: a randomised, double-blind, adjuvant-controlled, dose escalation first-in-man trial. Lancet Infect Dis 16:1036–1044. doi: 10.1016/S1473-3099(16)30115-3. [DOI] [PubMed] [Google Scholar]
- 29.Karauzum H, Adhikari RP, Sarwar J, Devi VS, Abaandou L, Haudenschild C, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Warfield KL, Shulenin S, Aman MJ. 2013. Structurally designed attenuated subunit vaccines for S. aureus LukS-PV and LukF-PV confer protection in a mouse bacteremia model. PLoS One 8:e65384. doi: 10.1371/journal.pone.0065384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ. 2012. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 7:e38567. doi: 10.1371/journal.pone.0038567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Yang F, Zhang X, Jing H, Ren C, Cai C, Dong Y, Zhang Y, Zou Q, Zeng H. 2015. Protective efficacy and mechanism of passive immunization with polyclonal antibodies in a sepsis model of Staphylococcus aureus infection. Sci Rep 5:15553. doi: 10.1038/srep15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.