Abstract
Innate immune memory, or trained immunity, has recently been described to be an important property of cells of the innate immune system. Due to the increased interest in this important new field of immunological investigation, we sought to determine the optimal conditions for an in vitro experimental protocol of monocyte training using three of the most commonly used training stimuli from the literature: β-glucan, the bacillus Calmette-Guérin (BCG) vaccine, and oxidized low-density lipoprotein (oxLDL). We investigated and optimized a protocol of monocyte trained immunity induced by an initial training period with β-glucan, BCG, or oxLDL, followed by washing and resting of the cells and, thereafter, restimulation with secondary bacterial stimuli. The training and resting time intervals were varied to identify the optimal setting for the long-term induction of trained immunity. Trained immunity was assessed in terms of the secondary cytokine response, the production of reactive oxygen species, cell morphology, and induction of glycolysis. Monocytes primed with β-glucan, BCG, and oxLDL showed increased pro- and anti-inflammatory cytokine responses upon restimulation with nonrelated stimuli. Also, all three stimuli induced a switch to glycolysis (the Warburg effect). These effects were most pronounced when the training interval was 24 h and the resting time interval was 6 days. Training with BCG and oxLDL also led to the increased production of reactive oxygen species, whereas training with β-glucan led to the decreased production of reactive oxygen species. We describe the optimal conditions for an in vitro experimental model with human primary monocytes for study of the induction of trained innate immunity by microbial and metabolic stimuli.
INTRODUCTION
The immune response is a complex system of cellular and humoral components which have the ability to detect non-self-structures and confer protection against invading pathogens, playing a crucial role in the survival of multicellular organisms. The immune response has traditionally been divided into the innate immune system and the adaptive immune system. Adaptive immunity, with T and B cells as cellular effectors, develops over several weeks after birth, is highly specific, and builds a specific immunological memory, leading to protection against reinfection. The innate immune system, on the other hand, is classically thought to act rapidly in a nonspecific and identical manner every time that it encounters a pathogen, without having the ability to build an immunological memory.
Recently, the concept that innate immunity is nonspecific and completely lacks immunological memory has been challenged. First, pattern recognition receptors (PRRs) confer some specificity to innate immunity, and second, a growing body of literature shows that innate immunity can adapt its function after a first insult (1, 2). Several studies have shown that plants and invertebrates, organisms which lack an adaptive immune system, show enhanced immune responses upon reinfection (3). This phenomenon has recently been confirmed in higher vertebrates and humans and has been named “trained immunity” or “innate immune memory.” Trained immunity is dependent on epigenetic remodeling and on rewiring of intracellular metabolic pathways, which in turn lead to a long-term proinflammatory phenotype that is characterized by increased cytokine responses (4, 5).
A series of recent in vitro and in vivo experiments has revealed that the phenomenon of trained immunity is induced by both pathogen-associated molecular patterns (PAMPs) and a number of danger-associated molecular patterns (DAMPs). First, in mice, a single dose of Candida albicans confers protection against reinfection with Staphylococcus aureus. In vitro studies showed that the C. albicans cell wall component β-glucan induces epigenetic remodeling and functional reprogramming through a dectin-1/Raf1-dependent pathway (6, 7). Second, bacillus Calmette-Guérin (BCG) vaccination of healthy human volunteers resulted in the nonspecific upregulation of ex vivo cytokine production by isolated monocytes that persisted for at least 3 months after vaccination. This effect is dependent on NOD2 signaling, autophagy, and epigenetic changes in histone methylation leading to increased transcription and likely explains, at least in part, the observed nonspecific effects of BCG on overall mortality (8–10). Third, the atherogenic lipid oxidized low-density lipoprotein (oxLDL) induces long-term proatherogenic changes in monocytes in vitro leading to increased proatherogenic cytokine expression, increased scavenger receptor expression, decreased cholesterol efflux transporter expression, and, ultimately, increased foam cell formation. This effect is dependent on Toll-like receptor 4 (TLR4)/TLR2 and subsequent phosphatidylinositol 3-kinase and extracellular signal-regulated kinase activation and epigenetic changes in histone methylation (e.g., H3K4me3, H3K9me3, and H3K27me3), resulting in increased transcription (11, 12).
In recent years, there has been an increased interest in immunological research in the study of trained immunity, with studies assessing different organisms and diseases (13–17) Therefore, we sought to determine the optimal conditions for an in vitro experimental protocol of monocyte training using three of the most commonly used training stimuli from the literature: β-glucan, BCG vaccine, and oxLDL.
MATERIALS AND METHODS
Reagents.
β-1,3-(d)-Glucan was kindly provided by David Williams (TN, USA), BCG (vaccine Statens Serum Institut) was obtained from The Netherlands Vaccine Institute. oxLDL was prepared as described before (18). Escherichia coli lipopolysaccharide (LPS; serotype O55:B5; Sigma-Aldrich, St. Louis, MO, USA) was further purified as described previously (19), and Pam3Cys was obtained from EMC Microcollections (catalog number L2000). Luminol and zymosan (from Saccharomyces cerevisiae) were purchased from Sigma-Aldrich.
PBMC and monocyte isolation.
Buffy coats from healthy donors were obtained after they provided written informed consent (Sanquin Blood Bank, Nijmegen, The Netherlands). Isolation of peripheral blood mononuclear cells (PBMCs) was performed by dilution of blood in sterile phosphate-buffered saline (PBS) and density centrifugation over Ficoll-Paque (GE Healthcare, UK). Cells were washed three times in cold PBS. Percoll isolation of monocytes was performed as described previously (20). Briefly, 150 × 106 to 200 × 106 PBMCs were layered on top of a hyperosmotic Percoll solution (48.5% Percoll [Sigma-Aldrich, St. Louis, MO, USA], 41.5% sterile H2O, 0.16 M filter-sterilized NaCl) and centrifuged for 15 min at 580 × g. The interphase layer was isolated, and the cells were washed with cold PBS. Cells were resuspended in Dutch modified RPMI culture medium (Invitrogen, CA, USA) supplemented with 10 μg/ml gentamicin, 10 mM GlutaMAX, and 10 mM pyruvate and counted. An extra purification step was added by allowing Percoll-isolated monocytes to adhere to polystyrene flat-bottom plates (Corning, NY, USA) for 1 h at 37°C; the cells were washed with warm PBS to obtain maximal purity. At this point, the level of T-cell contamination, which was measured by fluorescence-activated cell sorting, was 5% (see Fig. S1 in the supplemental material).
Trained immunity model in human monocytes.
Cells (100,000 cells/well) were added to flat-bottom 96-well plates. After incubation for 1 h at 37°C and washing with warm PBS, monocytes were incubated with culture medium only as a negative control or 5 μg/ml β-glucan, 10 μg/ml BCG, or 10 μg/ml oxLDL for 2, 4, or 24 h (in 200 μl/well RPMI plus 10% pooled human serum) (Fig. 1). After the incubation time indicated above, the cells were washed once with 200 μl warm PBS and incubated for 24 h, 3 days, or 6 days in culture medium with 10% serum, and the medium was changed once at day 3 for cells resting for 6 days. Cells were restimulated with 200 μl RPMI, 10 ng/ml LPS, or 10 μg/ml Pam3Cys. After 24 h, supernatants were collected and stored at −20°C until cytokine measurement. Before restimulation, the supernatants were stored at −20°C for lactate measurement.
FIG 1.
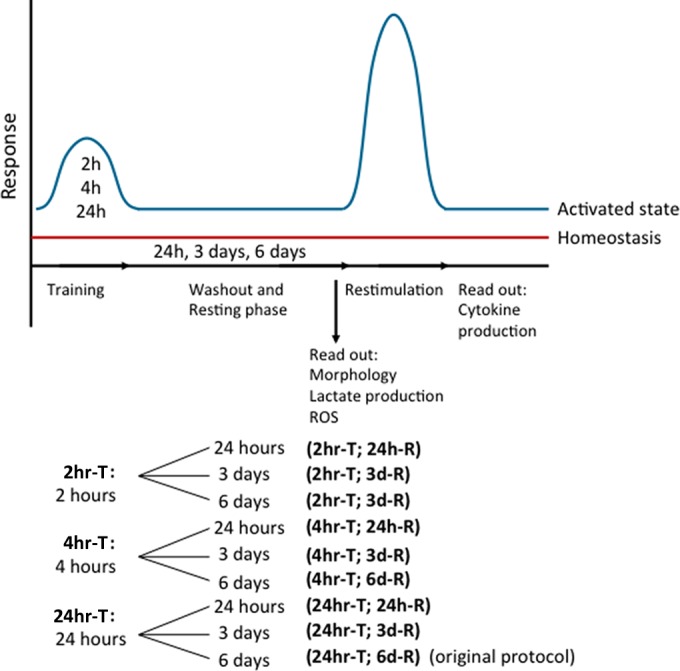
Schematic overview of trained immunity methodology. Monocytes were trained for 2 h (2 hr-T), 4 h (4 hr-T), or 24 h (24 hr-T). After the training stimulus was washed away, the cells were rested for 24 h (24 h-R), 3 days (3d-R), or 6 days (6d-R), after which the cells were restimulated with RPMI, LPS, or Pam3Cys for 24 h.
For production of reactive oxygen species (ROS) and for cell counting experiments, 5 × 106 monocytes were trained in vitro in 10-cm petri dishes (Greiner) in 5-ml medium volumes for 24 h as described above followed by a 6-day resting period. At day 6, cells were detached from the plate with EDTA (Life Technologies) and counted. Cells (25,000 cells/well) were restimulated as described above, and 100,000 cells/well were used for ROS measurement, as described below.
Microscopy.
Cell morphology was studied by conventional light microscopy during incubation each day. Before restimulation, pictures were taken at ×4 and ×20 magnifications. Cell size was quantified using Leica LAS EZ software (Leica Microsystems).
Reactive oxygen species measurements.
For measurement of reactive oxygen species production, a luminol-enhanced luminescence assay was used. After detachment and counting of trained monocytes, a total of 1 × 105 cells were added per well of a white 96-well assay plate (Corning) in a volume of 200 μl. Cells were left either unstimulated or stimulated with serum-opsonized zymosan at a concentration of 1 mg/ml. Luminol (145 μg/ml) was added, and chemiluminescence was measured every 142 s for 1 h. All measurements were performed at least in duplicate, depending on the number of cells available. Opsonized zymosan particles were prepared by incubation of zymosan derived from Saccharomyces cerevisiae (Sigma-Aldrich, St. Louis, MO, USA) in pooled human serum for 30 min at 37°C, after which the particles were washed twice in PBS and suspended in PBS.
Cytokine and lactate measurements.
Cytokine production in supernatants was determined using commercial enzyme-linked immunosorbent assay kits for tumor necrosis factor alpha (TNF-α; R&D Systems, MN, USA), interleukin-1 receptor antagonist (IL-1Ra; R&D Systems, MN, USA), and IL-6 and IL-10 (Sanquin, Amsterdam, The Netherlands) following the instructions of the manufacturers. The lactate concentration was measured using a lactate fluorometric assay kit (BioVision, CA, USA).
Statistics.
In vitro monocyte experiments were performed at least 6 times, and the results were analyzed using a Wilcoxon matched-pairs signed-rank test comparing the fold change or raw cytokine values to the values for the RPMI control. A two-sided P value below 0.05 was considered statistically significant. All data were analyzed using GraphPad Prism software version 5.0 (La Jolla, CA, USA). Data are shown as means ± standard errors of the means.
RESULTS
Training induces an increase in proinflammatory cytokine production, which is dependent on both training and resting time.
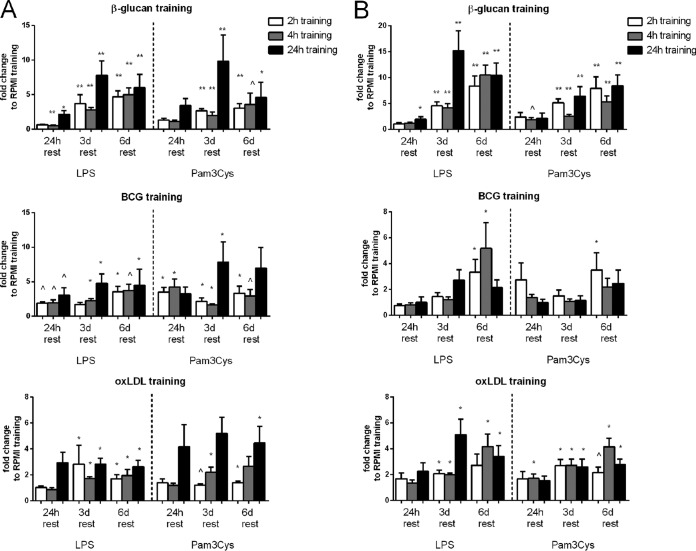
As was shown previously, 24 h of incubation with β-glucan, BCG, or oxLDL induces a trained immune phenotype characterized by the increased production of proinflammatory cytokines upon restimulation at day 7 with heterologous stimuli. Here we aimed to investigate how temporal changes in the duration of the first stimulus and the timing of the second stimulus impact the development of the trained immunity phenotype (Fig. 2A and B; see also Fig. S2 in the supplemental material). Training with β-glucan induced the strongest increase in cytokine production upon restimulation, whereas the effects of BCG and oxLDL were less pronounced, although they were still associated with an approximately 4- to 5-fold higher level of production of cytokines. For β-glucan and oxLDL training, the fold increases in the levels of IL-6 and TNF-α production were comparable, while priming with BCG led to a greater amplification of IL-6 (Fig. 2A) than TNF-α (Fig. 2B) production. For all three stimuli, priming for either 2 h, 4 h, or 24 h induced training, when the cells were rested for 3 or 6 days. However, the fold increase in cytokine production was highest with a 24-h training time interval. When comparing the different time periods between stimulation and restimulation, it follows that the trained phenotype developed only after at least 3 days, with a maximum response occurring after 6 days.
FIG 2.
Increased proinflammatory cytokine production is dependent on both the training interval and the resting time. (A) IL-6 production after restimulation. Cells were trained for 2 h, 4 h, or 24 h with β-glucan, BCG, or oxLDL and rested for 24 h, 3 days (3d), or 6 days (6d). (B) TNF-α production after restimulation. Cells were trained for 2 h, 4 h, or 24 h with β-glucan, BCG, or oxLDL and rested for 24 h, 3 days, or 6 days. Shown are the fold changes compared to the results for the RPMI control (n = 6; ^, P = 0.06 compared to the RPMI control; *, P < 0.05 compared to the RPMI control; **, P < 0.01 compared to the RPMI control).
Training-associated morphological changes.
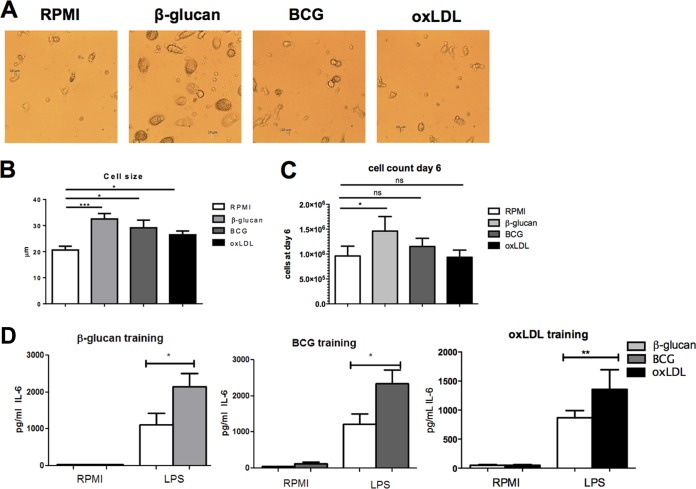
Upon training, the monocytes not only changed their response to secondary stimuli and, subsequently, their cytokine production but also changed their morphology. Just before restimulation, we analyzed cell morphology and cell size by using light microscopy at two different magnifications (Fig. 3A and B; see also Fig. S3 in the supplemental material). When cells were trained for 24 h and rested for 6 days, β-glucan induced the most remarkable changes in cell morphology: the cells were bigger than nontrained cells (32.5 μm for β-glucan-trained cells versus 20.6 μm for RPMI-trained cells). BCG and oxLDL training induced only minor changes in cell morphology, with a slight increase in cell size compared to that for the untrained control cells being seen (29.2 μm for BCG-trained cells and 26.5 μm for oxLDL-trained cells). These morphological changes persisted upon restimulation with LPS and Pam3Cys (see Fig. S5).
FIG 3.
Trained immunity effects on cell morphology and numbers. (A) Morphology of cells after 24 h of training and 6 days of rest when the cells were trained with RPMI (negative control), β-glucan, BCG, or oxLDL. Pictures were taken before restimulation at day 6. Magnification, ×20. (B) Size of cells after 24 h of training and 6 days of rest before restimulation at day 6 (n = 3, *, P < 0.05; compared to the RPMI control; ***, P < 0.001 compared to the RPMI control). (C) Relative cell counts before restimulation at day 6 (n = 6; *, P < 0.05, compared to the RPMI control; ns, not significant). (D) IL-6 and TNF-α production after restimulation for corrected cell counts (n = 6; *, P < 0.05 compared to the RPMI control; **, P < 0.01 compared to the RPMI control).
We also analyzed cell numbers at the end of the most optimal protocol of trained immunity induction (24 h of training, 6 days of rest) by culturing cells in petri dishes, detaching them on day 6 before restimulation, and reseeding corrected cell numbers for each condition. Counting experiments showed that β-glucan training induced a small increase in cell numbers on day 6 compared to the cell numbers for the RPMI control (Fig. 3C), whereas BCG or oxLDL training did not induce this increase in cell numbers. However, after correction for cell numbers, the level of cytokine production upon restimulation was still significantly increased, indicating that the increase in cytokine levels was not due to an increased cell number but was mainly dependent on the amount of cytokines produced by each cell (Fig. 3D).
The production of anti-inflammatory cytokines IL-10 and IL-1Ra is increased in trained cells.
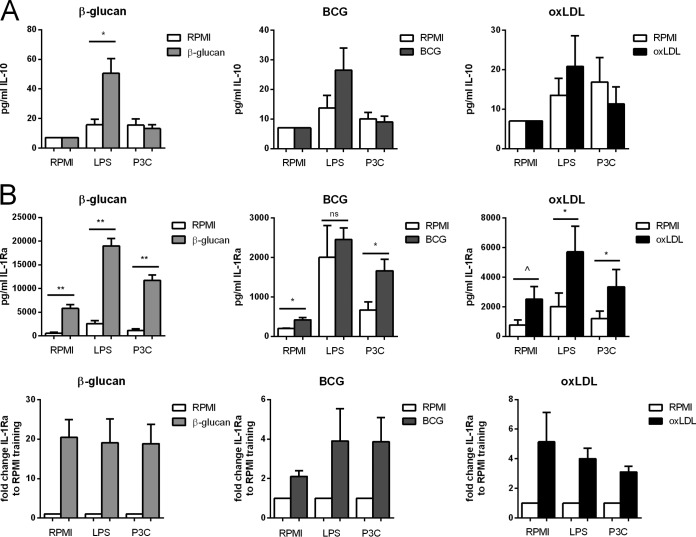
Previously we have shown that β-glucan training increases not only proinflammatory cytokine production but also anti-inflammatory cytokine production (6). Therefore, we measured the levels of production of two anti-inflammatory cytokines, IL-10 and IL-Ra, upon restimulation for all three training stimuli and all training protocols. Training was associated with increased LPS-induced IL-10 production, depending on the training time interval and the resting time (Fig. 4A; see also Fig. S4A in the supplemental material), but this reached statistical significance only for training with β-glucan. The production of IL-1Ra was increased for all three training stimuli even without restimulation (Fig. 4B), and this was dependent on the training duration and the resting time (see Fig. S4B).
FIG 4.
Anti-inflammatory cytokine production is increased in trained monocytes. (A) IL-10 production after restimulation. Cells were trained for 24 h with β-glucan, BCG, or oxLDL and rested for 6 days. The level of production of the anti-inflammatory cytokine IL-10 increased upon training. (B) IL-1Ra production. Cells were trained for 24 h with β-glucan, BCG, or oxLDL and rested for 6 days. The level of production of the anti-inflammatory cytokine IL-1Ra increased upon training not only after restimulation but also at the baseline (n = 6; ^, P = 0.06 compared to the RPMI control; *, P < 0.05 compared to the RPMI control; **, P < 0.01 compared to the RPMI control; ns, not significant). P3C, Pam3Cys.
ROS production is increased upon BCG and oxLDL training but not upon β-glucan training.
To further characterize the functional phenotype of trained monocytes, we measured another innate immune effector mechanism, the oxidative burst, in the most optimal protocol (24 h of training, 6 days of rest). Trained monocytes were stimulated with opsonized zymosan since LPS was a very poor inducer of the oxidative burst, and reactive oxygen species induction with Pam3Cys was very poor compared to that with zymosan (21) (data not shown).
Cells trained with both BCG and oxLDL showed increased production of ROS compared to the RPMI-treated control cells when cells were stimulated with zymosan, although for oxLDL this effect was not statistically significant (Fig. 5). Interestingly, cells trained with β-glucan showed a very different pattern with a slight but statistically significant downregulation of ROS production (Fig. 5).
FIG 5.
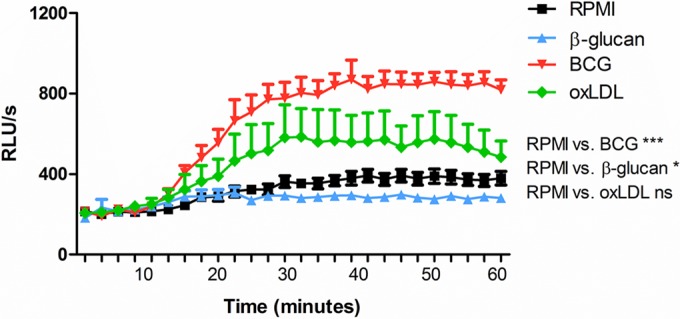
ROS production is a component of trained immunity for some stimuli. The level of ROS production was significantly increased for monocytes trained with BCG and oxLDL; training with β-glucan decreased the level of ROS production (n = 6; *, P < 0.05 compared to the RPMI control; ***, P < 0.001 compared to the RPMI control; ns, not significant). Cells were trained for 24 h using β-glucan, BCG, or oxLDL, and normalized cell numbers were restimulated using zymosan. Luminol was added, and luminescence was measured for 1 h. RLU/s, relative light units per second.
The metabolic switch toward the increased glycolysis induced upon training is dependent on both training and resting time.
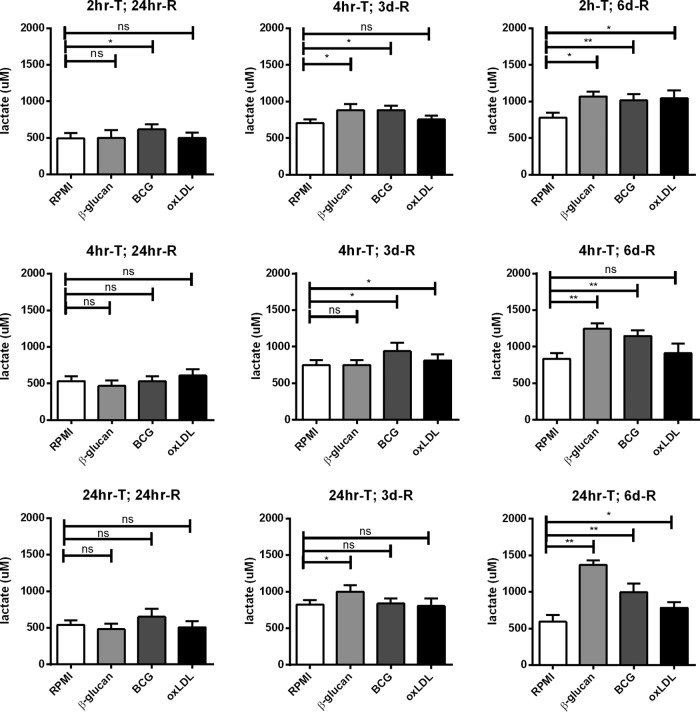
Previously, we have shown that trained immunity depends on a metabolic shift from oxidative phosphorylation toward aerobic glycolysis (the Warburg effect) (22). Increased glycolysis results in increased lactate production by the cell, which can easily be measured by analyzing the accumulation of lactate in the culture medium. Before the cells were restimulated, the culture medium was removed and stored and the lactate concentration was measured (Fig. 6). A 24-h resting time, independent of the training time, did not increase glycolysis or lactate production. When cells were rested for 3 days, lactate production was slightly increased for cells trained with β-glucan for 2 h and 24 h; for cells trained with BCG, 2 h and 4 h of training induced a slight increase in glycolysis, while for cells trained with oxLDL, only a 4-h training period was sufficient to induce a significant increase in lactate production. When cells were rested for 6 days, however, all three training durations induced a significant increase in lactate production for all three stimuli. Interestingly, the level of lactate production and, thus, glycolysis was increased even more with an increase in the training duration.
FIG 6.
Trained immunity induction of glycolysis. Cells were trained for 2 h (2hr-T), 4 h (4hr-T), or 24 h (24hr-T) with RPMI (negative control), β-glucan, BCG, or oxLDL and rested for 24 h (24hr-R), 3 days (3d-R), or 6 days (6d-R). The level of lactate production in the supernatants was measured before restimulation (n = 6; *, P < 0.05 compared to the RPMI control; **, P < 0.01 compared to the RPMI control; ns, not significant).
DISCUSSION
In the present study, we describe the optimal conditions for the induction of trained immunity in an in vitro model in primary human monocytes. This model has previously been used to investigate several different stimuli, but the optimal parameters necessary to induce trained immunity have not yet been characterized. In this model, microbial or endogenous stimuli, such as β-glucan, BCG, and oxidized LDL, were added to monocytes for a short period of time (training period), after which the cells were washed and allowed to rest in culture medium, followed by restimulation with unrelated antigens (6, 8, 11, 23, 24).
This study provides several new insights into this basic model of trained innate immunity that will aid with the design of future studies. First, induction of trained immunity depends on the duration of the first stimulation time interval, the training period, with an increase in the training time leading to the increased induction of trained immunity, an effect which was observed for all three stimuli. Second, the magnitude of the induction of nonrelated cytokine responses increases with the time that trained cells are left to rest, most notably for β-glucan-primed cells. The importance of resting time for the establishment of trained immunity has also recently been demonstrated in a study which described the role of the transcription factor ATF7 in mediating the epigenetic changes involved in innate immune memory, most notably, those after LPS stimulation. At 3 days after the first LPS challenge, the expression of TNF-α and Cxcl2 was decreased upon rechallenge with LPS, whereas the opposite was true 3 weeks after LPS challenge. Multiple proinflammatory genes were upregulated at the later time point, and this upregulation was accompanied by reduced amounts of epigenetic marks associated with gene silencing and the incomplete recruitment of ATF7, which was associated with the formation of heterochromatin. This study also found that treatment with β-glucan induced the phosphorylation of ATF7, a finding analogous to that observed after treatment with LPS (25). Similarly, our study showed the induction of tolerance, most notably, with β-glucan and BCG, when cells were only briefly left to rest before rechallenge (24 h), whereas a training effect was seen if cells were left to rest for 3 or 6 days. This could suggest the intriguing possibility that ATF7 or analogous mechanisms may be at play here. The importance of resting time in the induction of trained immunity was also shown by the remarkable increases in glycolysis in trained cells after 6 days of rest compared to that after 1 day or 3 days of rest.
Another insight from this study is that trained immunity does not only translate into an increased proinflammatory response of monocytes but likely involves a more general increase in the responsiveness of the cells, as witnessed by the amplification of both pro- and anti-inflammatory cytokine production, ROS production (in the case of training with BCG and oxLDL), and metabolic activation. Of course, we should be cautious in the extrapolation of all the details of the in vitro model to the in vivo situation: for example, vaccination trials performed both in infants and in adults showed increased proinflammatory cytokine responses after BCG vaccination, whereas no effects on anti-inflammatory cytokine responses were noted (8, 24, 26). Furthermore, in patients suffering from elevated levels of lipoprotein(a), which induces trained innate immunity in vitro, the anti-inflammatory cytokine response was even decreased (17). In addition, it would be most interesting to also assess the influence of trained immunity on other important immunological effector mechanisms, such as phagocytosis and microbial killing capacity. While this was beyond the aims of the present study, such an investigation is warranted for the future.
It is also important to note that different inducers of trained immunity led to different phenotypic and functional changes, as witnessed by light microscopy, cytokine production, and induction of ROS. Although a common denominator for the three priming stimuli studied here was the upregulation of both pro- and anti-inflammatory cytokine responses and the stimulation of glycolysis, other physiological processes, such as induction of ROS or cell proliferation, differed between different priming stimuli. When cytokine responses were corrected for cell counts, the cytokine responses were still strongly upregulated nevertheless. All in all, however, these data suggest that each of these training stimuli induces different trained immunity functional programs, and this is not a surprise, considering their diverse nature (5).
In conclusion, although the effects of these three stimuli on trained immunity have been described before, this is the first time that the kinetics of this in vitro model have been studied. This study describes the characteristics of an in vitro model for the study of the influence of different stimuli on the induction of trained innate immunity. This is a practical and useful methodological tool that will assist the research community with the investigation of this emerging field of immunology.
Supplementary Material
ACKNOWLEDGMENTS
N.P.R. was supported by a grant from the Netherlands Heart Foundation (2012T051). N.P.R. and M.G.N. are supported by a Horizon2020 grant of the European Union (667837). M.G.N. is supported by an ERC consolidator grant (310372) and a Spinoza grant from the Netherlands Organization for Scientific Research (NOW). R.V.C. is supported by the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305279. L.A.B.J. is supported by the Dutch Arthritis Foundation (NR 12-2-303).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00349-16.
REFERENCES
- 1.Medzhitov R, Janeway C Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol Rev 173:89–97. doi: 10.1034/j.1600-065X.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 2.Bowdish DME, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. 2007. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes Infect 9:1680–1687. doi: 10.1016/j.micinf.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz J. 2005. Specific memory within innate immune systems. Trends Immunol 26:186–192. doi: 10.1016/j.it.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Quintin J, Cheng S-C, van der Meer JWM, Netea MG. 2014. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol 29:1–7. doi: 10.1016/j.coi.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, ONeill LAJ, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quintin J, Saeed S, Martens JHA, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LAB, Xavier RJ, Van Der Meer JWM, Stunnenberg HG, Netea MG. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, Sharifi N, Janssen-Megens EM, Ter Huurne M, Mandoli A, van Schaik T, Ng A, Burden F, Downes K, Frontini M, Kumar V, Giamarellos-Bourboulis EJ, Ouwehand WH, van der Meer JWM, Joosten LAB, Wijmenga C, Martens JHA, Xavier RJ, Logie C, Netea MG, Stunnenberg HG. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JWM, van Crevel R, Netea MG. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LAB, Jacobs C, Van Loenhout J, Xavier RJ, Aaby P, Van Der Meer JWM, Van Crevel R, Netea MG. 2014. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun 6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blok BA, Arts RJW, van Crevel R, Benn CS, Netea MG. 2015. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol 98:347–356. doi: 10.1189/jlb.5RI0315-096R. [DOI] [PubMed] [Google Scholar]
- 11.Bekkering S, Quintin J, Joosten LAB, Van Der Meer JWM, Netea MG, Riksen NP. 2014. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol 34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- 12.Bekkering S, Joosten LAB, van der Meer JWM, Netea MG, Riksen NP. 2013. Trained innate immunity and atherosclerosis. Curr Opin Lipidol 24:487–492. doi: 10.1097/MOL.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 13.Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, Urbani S, Chong Y-S, Guccione E, Bertoletti A. 2015. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun 6:6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petit J, Wiegertjes GF. 2016. Long-lived effects of administering β-glucans: indications for trained immunity in fish. Dev Comp Immunol 64:93–102. doi: 10.1016/j.dci.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Christ A, Bekkering S, Latz E, Riksen NP. 2016. Long-term activation of the innate immune system in atherosclerosis. Semin Immunol 28:384–393. doi: 10.1016/j.smim.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner CM, Mills KHGG. 2016. The cells that mediate innate immune memory and their functional significance in inflammatory and infectious diseases. Semin Immunol 28:343–350. doi: 10.1016/j.smim.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 17.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LAB, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ESG. 2016. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Tits LJH, Stienstra R, van Lent PL, Netea MG, Joosten LAB, Stalenhoef AFH. 2011. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis 214:345–349. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol 165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 20.Repnik U, Knezevic M, Jeras M. 2003. Simple and cost-effective isolation of monocytes from buffy coats. J Immunol Methods 278:283–292. doi: 10.1016/S0022-1759(03)00231-X. [DOI] [PubMed] [Google Scholar]
- 21.Santos SS, Carmo AM, Brunialti MKC, Machado FR, Azevedo LC, Assunção M, Trevelin SC, Cunha FQ, Salomao R. 2016. Modulation of monocytes in septic patients: preserved phagocytic activity, increased ROS and NO generation, and decreased production of inflammatory cytokines. Intensive Care Med Exp 4:5. doi: 10.1186/s40635-016-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJW, van der Meer BMJW, Deen PMT, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JWM, Ng A, Joosten LAB, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. 2014. mTOR- and HIF-1-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ifrim DC, Quintin J, Joosten LAB, Jacobs C, Jansen T, Jacobs L, Gow NAR, Williams DL, van der Meer JWM, Netea MG. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21:534–545. doi: 10.1128/CVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arts RJW, Blok BA, Aaby P, Joosten LAB, de Jong D, van der Meer JWM, Benn CS, van Crevel R, Netea MG. 2015. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J Leukoc Biol 98:995–1001. doi: 10.1189/jlb.4MA0215-059R. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida K, Maekawa T, Zhu Y, Renard-Guillet C, Chatton B, Inoue K, Uchiyama T, Ishibashi K, Yamada T, Ohno N, Shirahige K, Okada-Hatakeyama M, Ishii S. 2015. The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory. Nat Immunol 16:1034–1043. doi: 10.1038/ni.3257. [DOI] [PubMed] [Google Scholar]
- 26.Jensen KJ, Larsen N, Sorensen SB, Andersen A, Eriksen HB, Monteiro I, Hougaard D, Aaby P, Netea MG, Flanagan KL, Benn CS. 2015. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 211:956–967. doi: 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.