Summary
Objective.
To describe the occurrence of CRKP infections in a tertiary care hospital and to analyse the allelic profiles of the clinical strains involved and the most frequent carbapenemases.
Design.
The study analyzed cases of infection due to CRKP in the period 2013-2014; 147 cases were recorded, most of which (82.31%) were in-hospital infections.
Setting.
A hospital in northern Italy.
Methods.
We retrospectively collected: data on patient characteristics and the microbiological characteristics of CRKP. Isolates from 72 of the in-hospital cases underwent molecular typing (MLST); in addition, in each isolate, a procedure for the detection of the blaKPC gene was carried out.
Results.
The in-hospital death rate was 24.0% in 2013 and 37.5% in 2014. However, the difference between these two values did not prove statistically significant (P > .05).
Analysis of mortality revealed that bloodstream infections were more frequently associated with death than other infections (χ2 = 14.57, P < .001). The age-adjusted Cox proportional hazard model revealed that the patients with bacteremia due to CRKP had a 3-fold higher risk of death (HR 3.11; 95% CI 1.66 - 5.84, P< .001) than those with infections of other sites.
MLST revealed that the prevalent allelic profile was ST 512 (79.62%); the most frequent carbapenemase was KPC-3 (83.8%).
Conclusions.
Our results are in line with those of recent studies, which have shown that the spread of CRKP in Italy is a matter of concern and that further efforts have to be made to prevent the potential dissemination of carbapenemase-producing clones of K. pneumoniae, whenever possible.
Key words: Carbapenem-resistant Klebsiella pneumoniae, Bloodstream infection, Mortality
Introduction
Since the 1970s, the selective pressure exerted by antibiotics has given rise to bacterial species that are increasingly resistant, and the last 20 years have seen a dramatic rise in the number of multi-resistant pathogenic strains [1]. Multidrug-resistant Klebsiella pneumoniae is one of the leading causes of nosocomial infection worldwide. It causes urinary tract infections (UTIs), pneumonia and intra- abdominal infections in hospitalized immunocompromised patients with severe underlying diseases [2] and is responsible for roughly 15% of Gram-negative infections in hospital intensive care units (ICUs) [3].
After the spread of strains resistant to beta-lactams at the end of the 20th century, the diffusion of isolates of K. pneumoniae resistant to carbapenems and colistin is now reducing treatment options and the containment of infections [4]. In recent years, carbapenem-resistant Klebsiella pneumoniae (CRKP) has become a widespread concern and carbapenemase production mediated by blaKPC is the most prevalent mechanism conferring resistance to carbapenems [5]. Outbreaks of CRKP have increasingly been reported in various healthcare settings [4, 6, 7], including long-term acute care hospitals [8].
Risk factors for colonization and infection with CRKP are similar to those associated with other multidrug-resistant organisms [9]. Lengthy hospitalization, antibiotic use, invasive procedures and admission to the ICU [8] are associated with an increased risk of acquisition of CRKP.
Mortality rates due to infections caused by CRKP are high, ranging from 26% to 44% and reaching 70% in cases of bacteremia [10-12]. However, deaths reported to be associated with carbapenem-resistant K. pneumoniae have included several cases in which the patient had a severe underlying disease, and it is frequently difficult to determine whether carbapenem-resistant K. pneumoniae infection was the cause of death.
The aim of the present study was to describe the occurrence of CRKP infections in a northern Italian hospital and to analyse the allelic profiles of the clinical strains involved and the most frequent carbapenemases.
Materials and methods
SETTING
The study was conducted in a nationally renowned, highly specialized Northern Italian hospital organized in accordance with treatment intensity. Structured in pavilions, the hospital has 458 beds (mainly located in 3- and 4-bed rooms) and each year carries out over 15,000 ordinary hospitalizations and more than 8600 medical procedures in Day Hospital and Day Surgery settings.
STUDY DESIGN
The study retrospectively analyzed cases of infection due to CRKP in the period 2013-2014.
Patients who were identified as having CRKP infections within the first 72 h of admission were defined as community- associated cases or, if they had been exposed to healthcare settings during the previous three months, imported healthcare-associated cases. Clinical episodes of infection were considered to be hospital-acquired if they were not present at the time of hospital admission and appeared 72 hours after admission.
A record was made of each case patient's age, gender, history of hospitalizations, antibiotic treatments, duration of hospitalization, the date of the first CRKP detection, the site of infection and co-infections, invasive procedures and outcomes.
If a positive patient had been transferred from one ward to another, acquisition of the infection was attributed to the ward in which the diagnosis of infection was made, as the exact site of acquisition could not be determined.
The incidence of infections was calculated per 1000 days of hospitalization.
CLASSIFICATION OF PATIENTS ACCORDING TO INFECTION RISK AND TYPE OF ISOLATION APPLIED
Patients were grouped into two categories of infection risk: high-risk and medium-risk according to the characteristics of the patient and the site of infection. Patients were deemed to be at high risk if they presented one or more of the following characteristics: presence of excretions/ secretions at the infection site, confinement to bed, lack of self-sufficiency, and great need for assistance. Patients in this category underwent structural or cohort isolation; if this was not possible, functional isolation was implemented.
Patients were defined as being at medium risk if they presented one or more of the following characteristics: presence of reduced secretions or excretions at the infection site, capability of temporal and spatial orientation, ability to cooperate, self-sufficiency, and low-medium need for assistance. These patients underwent functional isolation.
MICROBIOLOGIC METHODS
Bacterial identification and antimicrobial susceptibility testing were carried out by means of the Phoenix 100 Automated Microbiology System (Becton Dickinson Diagnostic Systems, USA).
Confirmatory MIC testing for imipenem, meropenem and ertapenem was carried out by means of Etest (bio- Mérieux SA, France) and the Kirby Bauer disk diffusion method [13]. All collected isolates that were confirmed to be non-susceptible to imipenem and/or meropenem and/or ertapenem according to the EUCAST breakpoints [14] were considered to be Carbapenem-resistant K. pneumoniae and underwent a modified Hodge test [15] to confirm carbapenemase production. In addition, in order to identify which carbapemenase was present, PCR for the blaKPC gene was carried out [16].
BIOMOLECULAR ANALYSIS
The isolates from 72 of the in-hospital cases underwent molecular typing by means of the MLST technique (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html). This technique involves amplifying and sequencing seven housekeeping genes (rpoB, gapA, mdh, pgi, phoE, infB, tonB) and, through comparison with the data available in an online databank, enables a specific ST (Sequence Type) to be assigned to each isolate. The amplification protocol prescribes an initial denaturing phase at 94°C for 5 minutes, followed by 35 cycles at 94°C for 30 seconds, 50°C for 30 seconds and 72°C for 30 seconds, with a final extension at 72°C for 5 minutes.
In addition, in each isolate, a procedure for the detection of the blaKPC gene was carried out through the amplification and sequencing of a DNA fragment of about 1000bp by means of the following primer pairs: blaKPC-Forward 5'-TGTCACTGTATCGCCGTC-3' and blaKPC-Reverse 5'-CTCAGTGCTCTACAGAAAACC-3'. The amplification phase consisted of an initial denaturing phase at 95°C for 5 minutes, followed by 35 cycles at 95°C for 60 seconds, 55°C for 40 seconds and 72°C for 90 seconds, with a final extension at 72°C for 10 minutes. The sequences obtained were compared with those available in the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), which enabled the blaKPC gene to be characterized. For both the identification of the Sequence Type and detection of the blaKPC gene, sequencing was carried out by means of an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, USA).
INFECTION CONTROL MEASURES
In all high-risk patients, rectal swabs were taken by the ward nurse on admission to the ward. Cultures were then sent directly to the bacteriology laboratory for prompt CRKP identification.
The diffusion of the microorganism was monitored by means of continuous integrated microbiological surveillance, starting with laboratory data (alert organism surveillance). Following laboratory identification of an epidemiologically important microorganism, the dedicated software of the surveillance system automatically e-mails the data to all the members of the Hospital Infections Committee, who then implement the interventions deemed necessary, with particular regard to the application of isolation measures.
A validated report is simultaneously sent through the laboratory information system to the hospital facility involved.
Antibiotic therapy was instituted after consultation with the infectious-disease specialist on the basis of case history, patients' clinical features, microbiological isolates and antibiotic sensitivities.
Hospital-wide policies to prevent nosocomial carbapenem- resistant Enterobacteriaceae transmissions were introduced on the basis of an institutional protocol that was developed in accordance with the American Centers for Disease Control and Prevention (CDC) indications [17].
STATISTICAL ANALYSIS
Statistical analysis was carried out by means of STATA SE13TM software (Stata Corp LP, USA). The results were analyzed in terms of descriptive statistics, and differences between groups were evaluated by means of non-parametric chi-square test and Fisher's mid-P exact test.
Kaplan-Meier survival curves were assessed by means of a log-rank analysis, to compare overall survival, and a COX proportional hazard model, to assess the role of possible confounders. A p-value < 5% was considered significant.
Results
In the period of observation, 147 cases of CRKP infection were recorded (75 in 2013 and 72 in 2014) most of which (82.31%) were in-hospital infections.
The overall mean age of the patients was 78.95 ± 12.05 years (range 26-97 years). Women accounted for 50.34% of the patients; their mean age was 81.49 ± 10.51 years (range 42-97 years). The mean age of the male patients was 76.37 ± 12.99 years (range 26-96 years). The difference in age between males and females proved to be statistically significant (t = 2.6265, P < .01).
The mean duration of hospital stay was 35.32 ± 25.04 days (range 1-168).
In 46.28% of the cases of in-hospital infection, the infection was related to an invasive procedure (Tab. I).
Tab. I.
Carbapenem-resistant Klebsiella pneumoniae case characteristics.
| Value | (%) | |
|---|---|---|
| Total no. confirmed | 147 | |
| Unit in which the diagnosis of infection was made | ||
| Medical ward | 55 | (37.41) |
| Geriatric unit | 53 | (36.06) |
| Surgical ward | 30 | (20.41) |
| ICU* | 9 | (6.12) |
| Cases from: | ||
| Hospital | 121 | (82.31) |
| Other healthcare facility | 13 | (8.84) |
| Community | 13 | (8.84) |
| Invasive procedures correlated with infection | 56 | (46.28) |
| Bladder catheter | 42 | (75.00) |
| CVC§ | 5 | (8.92) |
| ERCP^ | 3 | (5.36) |
| Tracheal intubation | 2 | (3.57) |
| Percutaneous surgical drainage | 2 | (3.57) |
| Urethral catheter | 1 | (1.79) |
| PIVC° | 1 | (1.79) |
| Sites of CRKP infection | ||
| Urinary tract infection | 96 | (65.31) |
| Bloodstream infection | 23 | (15.64) |
| Surgical site infection | 15 | (10.20) |
| Airways | 7 | (4.76) |
| Other | 6 | (4.09) |
| Specimen type | ||
| Urine from catheter | 65 | (44.22) |
| Urine | 31 | (21.09) |
| Blood | 25 | (17.00) |
| Other | 6 | (4.08) |
| Bronchial aspirate | 4 | (2.72) |
| Surgical fragment | 4 | (2.72) |
| Abdominal fluid | 4 | (2.72) |
| Pus | 4 | (2.72) |
| Skin swab | 1 | (0.68) |
| Wound swab | 1 | (0.68) |
| Bile | 1 | (0.68) |
| Expectorate | 1 | (0.68) |
ICU = intensive Care Unit
CVC = central venous catheter
ERCP = Endoscopic Retrograde Cholangiopancreatography
PIVC = Peripheral Intravenous Catheter
The Age-adjusted Charlson Comorbidity Index revealed comorbidity values in the hospitalized patients of 6.21 and 6.67 in 2013 and 2014, respectively (P >.05).
Over the two-year observation period, 47.62% of the CRKP-infected patients were deemed at high risk. On considering the two years separately, a statistically significant difference emerged (Χ2 = 16.0481, P < .001) between the percentage of patients classified as being at high infective risk in 2013 (32.00%) and in 2014 (63.89%).
A total of 70.77% of patients were hospitalized in 4-bed rooms, 17.69% in 2-bed rooms and 7.69% in single rooms. It was possible to hospitalize only 3.85% of patients in rooms with dedicated bathrooms (1.54% in 4-bed rooms, 1.54% in 2-bed rooms and 0.77% in single rooms).
Table I reports some characteristics of the cases of infection examined: the unit in which the diagnosis of infection was made, the provenance of the cases, the invasive procedures performed, the sites of infection and the specimen type.
The incidence of infections was 0.442 pts/1000 days of hospitalization in 2013, and 0.513 pts/1000 days of hospitalization in 2014 (P > .05).
In the entire period of observation, we recorded 24 cases of coinfection due to: Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter baumannii, Enterococcus faecium, Proteus mirabilis, Corynebacterium striatum, Enterococcus casselliflavus, Moraxella morganii, Serratia marcescens, and Staphylococcus haemolyticus. Among these microorganisms, we identified ESBL-positive strains of E. faecium, E coli, P. mirabilis and P. aeruginosa; VRE strains of E. casseliflavus and E. faecalis, and strains of MRSA.
With regard to patient outcomes over the two years of observation, 30.61% of the patients died in hospital; 12.24% were transferred to other units in the hospital; 0.68% were transferred to other hospitals; 19.73% were transferred to a residential facility; 4.76% were discharged with home assistance, and 31.97% were discharged home without assistance.
The in-hospital death rate was 24.0% in 2013 and 37.5% in 2014. However, the difference between these two values did not prove statistically significant (P > .05).
Analysis of mortality by means of Kaplan-Meier survival curves revealed that bloodstream infections were more frequently associated with death than were urinary infections and other infections (Fig. 1), the difference being statistically significant (Χ2 = 14.57, P < .001).
Fig. 1.
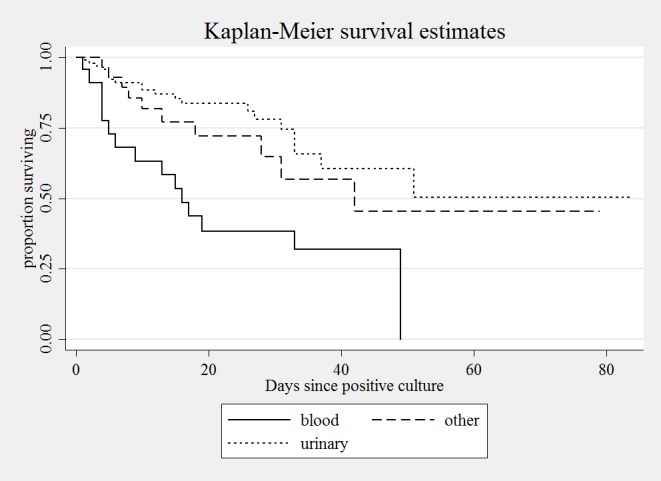
Kaplan-Meier curves of survival probability of patients with carbapenem-resistant Klebsiella pneumoniae infection, by infection site.
Of the 23 cases of bloodsteam infections, 14 (12 hospital and 2 community) were cases of primary bacteremia; 10 of these patients died. The remaining 9 cases were secondary bacteremia, all nosocomial; 5 of these patients died. The mean age of the patients with primary bacteremia was 75.36 ± 10.70 years (range 58-90), while that of the patients with secondary bacteremia was 77.89 ± 9.57 years (range 63-92).
The age-adjusted Cox proportional hazard model revealed that the patients with bacteremia due to CRKP had a 3-fold higher risk of death (Hazard ratio [HR], 3.11; 95% CI 1.66-5.84, P < .001) than those with infections of other sites.
With regard to the results of antimicrobial susceptibility testing, the data on the resistance of the strains are reported in Table II; 26% of strains proved resistant to colistin. The antimicrobial ertapenem was the most frequently reported carbapenem tested for susceptibility (100%), followed by meropenem (98.6%) and imipenem (48.3%).
Tab. II.
Antibiogram for carbapenem-resistant Klebsiella pneumoniae cases reported.
| Antimicrobial | No. resistant/No. tested | (%) |
|---|---|---|
| Aztreonam | 145/147 | (99) |
| Amikacin | 45/55 | (82) |
| Amoxicillinclavulanic acid | 145/147 | (99) |
| Colistin | 38/145 | (26) |
| Cefalexine | 91/92 | (99) |
| Cefepime | 102/147 | (99) |
| Cefixime | 91/92 | (99) |
| Cefotaxime | 145/147 | (99) |
| Ceftazidime | 145/147 | (99) |
| Cefuroxime | 144/145 | (99) |
| Ciprofloxacin | 145/147 | (99) |
| Fosfomycin | 17/130 | (13) |
| Gentamicin | 25/147 | (17) |
| Levofloxacin | 55/55 | (100) |
| Moxifloxacin | 91/92 | (99) |
| Ampicillin | 147/147 | (100) |
| Piperacillin | 55/55 | (100) |
| Piperacillin-tazobactam | 145/147 | (99) |
| Tygecicline | 5/41 | (12) |
| Tobramycin | 135/147 | (92) |
| Thrimetoprim | 76/94 | (81) |
| Thrimetoprim-sulfamethoxazole | 112/147 | (76) |
Genotyping of the strains by means of MLST revealed that the prevalent allelic profile was ST 512 (79.62%), followed by ST 307 (8.97%), ST 101 (3.85%), ST 147 (3.85%), ST 258 (3.85%), ST 15 (1.28%), and ST 45 (1.28%). Detection of the blaKPC gene revealed that the most frequent carbapenemase was KPC-3 (83.8%) and that KPC-2 was less common (16.2%).
With regard to blaKPC gene detection in relation to the allelic profiles, it emerged that STs 258, 101, 147, 15 and 45 displayed only blaKPC-2; that ST 307 were associated only to blaKPC-3; that most ST 512 hosted the blaKPC-3 gene (98%), and that only a small portion hosted blaKPC -2 (2%) (Fig. 2).
Fig. 2.
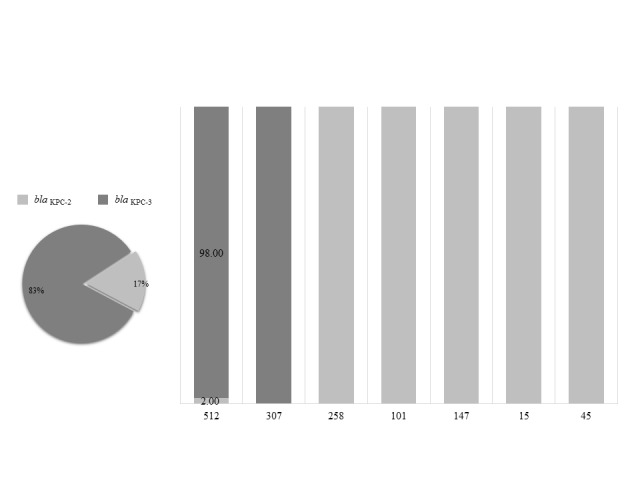
Percentage distribution of carbapenemases in the various allelic profiles isolated
Discussion and conclusions
The emergence and spread of Klebsiella pneumoniae harboring carbapenemases have given rise to several problems regarding infection control and treatment.
Carbapenamase-associated resistance is alarming for a number of reasons. The presence of these enzymes, in addition to signifying resistance to carbapenems, is also associated with additional mechanisms of resistance to other antibiotic classes which, together, result in microbes that are highly multidrug resistant and in some cases panresistant [18-20]. Consequently, they are invariably associated with high treatment failure rates [21].
CRKP has rapidly become a major health concern for hospitalized patients in industrialized countries, and infection rates have been dramatically increasing worldwide over the past 10 years. The first case of carbapenemase-producing Klebsiella pneumoniae in Italy was detected in October 2008. In 2011, the European Antimicrobial Resistance Surveillance Network (EARS-Net) reported that Italy was one of the most seriously affected countries in Europe, with a worrisome increasing trend in CPKP [22-24].
These strains are implicated in nosocomial outbreaks and cause serious infections in ICUs. Moreover, recent national data have shown that CRKP is more frequently isolated from patients outside ICUs, and often from those admitted to geriatric or internal medicine wards [25, 26]. In the present study, we documented the occurrence of 147 cases of CRKP infections in a Northern Italian hospital. These cases were chiefly detected in medical wards (37.41%) and geriatric units (36.06%), followed by surgical wards (20.41%) and ICUs (6.12%). These wards are also frequently involved in CRKP infections in other countries. Indeed, Poulou et al. [27] reported that, of the 73 CRKP infections registered between 2009 and 2011 at a university hospital in Greece, 43.8% were identified in the ICU, 41.1% in medical wards and 15.1% in surgical wards. Moreover, Kanerva described an outbreak of carbapenemase-producing Klebsiella pneumoniae in a primary care hospital in Finland; this was confined to one geriatric ward and involved 142 patients with a mean age of 83 years [28].
Most of the CRKP-positive cases described in the present study involved elderly patients (mean age 78.95 ± 12.05 years). The Age-adjusted Charlson Comorbidity Index revealed values of underlying comorbidity in hospitalized patients of 6.21 in 2013 and 6.27 in 2014 (P > .05), reflecting a high level of complexity of assistance, which remained fairly constant throughout the observation period.
These findings are in line with those of recent studies, which have shown that the spread of CRKP in Italy is becoming a matter of concern in areas of care that were generally considered to be at lower risk. Moreover, one of the principal targets of CRKP is the population of geriatric patients [24, 29, 30], who display a high degree of clinical complexity and a large number of comorbidities and are frequently bedridden or cognitively impaired. Thus, frailty and comorbidity are, in themselves, a major risk factor for CRKP colonization, together with those already described in the literature (length of hospitalization, number of previous hospitalizations and/or previous ICU stays, previous antibiotic use, severity of illness, etc) [21, 30, 31].
In a large, retrospective, matched (1:2) case-control study in five Italian hospitals, Tumbarello et al. identified risk factors for CRKP infections; the strongest predictor of CRKP isolation was a history of ≥ 2 previous acute-care hospitalizations in the year before the index culture. Isolation was also associated with indwelling medical devices, such as urinary catheters, central venous catheters (CVCs) and surgical drains [21]. Invasive procedures are well-known risk factors for infection by CRKP [32]; indeed, the formation of biofilms on these devices is important in the pathogenesis of these bacteria [33, 34]. In 46.28% of the cases of in-hospital infection recorded in the present study, it was possible to correlate the infective event with an invasive procedure. Specifically, 75% of these cases occurred in patients with a urinary catheter; and indeed, the principal specimen type from which CRKP was isolated was catheter urine (44.22%). Lower percentages of infections related to invasive procedures were associated with CVCs (8.92%), endoscopic retrograde cholangiopancreatography (5.36%), and various other indwelling medical devices (overall, 10.72%).
During the two-year observation period, we registered an increase in the incidence of new clinical cases per 1,000 patient days. Fortunately, however, this increase did not reach statistical significance (0.442 cases/1,000 patient days in 2013 versus 0.513 cases/1,000 patient days in 2014; P>.05), despite the fact that a statistically significant difference (Χ2 = 16.0481, P < .001) emerged between the percentages of patients classified as being at high infective risk in 2013 (32.00%) and in 2014 (63.89%).
Following laboratory identification of CRKP, the members of the Hospital Infections Committee implemented the interventions deemed necessary, with particular regard to the application of isolation measures, the disinfection of environmental surfaces and improvement of hand hygiene compliance. Indeed, eliminating surface contamination as a source of patient-to-patient transmission of nosocomial pathogens requires multiple interventions aimed at cleaning/disinfecting the environment and improving adherence to hand hygiene guidelines [35-37].
The mortality rate due to all causes of infection was 24% in 2013 and 37.5% in 2014. This increase, albeit not statistically significant, may have been due to the greater complexity of patients in the second year of observation. In any case, the mortality rates, however evaluated, proved to be in line with the literature data, which report mortality rates between 26% and 44 % [12, 38, 39].
Moreover, it emerged that the infective event most frequently associated with death was bacteremia; this is in agreement with the results of previous studies; in a matched retrospective, historical cohort design study involving 319 patients with infections due to carbapenemresistant K. pneumoniae, Borer et al. [40] found a similar mortality risk ratio: 3.3 (95% CI 2.9-28.5) among case subjects with carbapenem-resistant K. pneumoniae bacteremia.
The results of our study revealed that 26% of strains proved resistant to colistin, a lower value than those reported in similar studies [41, 42].
Regarding the results of genotyping, it emerged that the allelic profile most frequently observed in the hospital was ST 512; this is in line with the results of other studies conducted in Italy [43]. ST 512 (allelic profile: 54- 3-1-1-1-1-79) is a single-locus variant of ST 258 (allelic profile: 3-3-1-1-1-1-79) and is the clone most frequently associated worldwide with the spread of KPCs.
The ST 512 detected in the present study mainly produces KPC-3 carbapenemase; the blaKPC-3-containing strain of K. pneumoniae displays an exceptional combination of multidrug resistance, virulence and ability to spread [44] and the KPC-3-producing K. pneumoniae ST 512 clone has emerged as a successful new lineage, capable of disseminating KPC-3 in Europe [45].
The results of the present study revealed that the characteristics of the predominant strain, together with the high levels of comorbidity of the patients involved and the difficulty of ensuring structural isolation owing to the small number of dedicated rooms, have, at least in part, undermined the success of the measures for prevention and control adopted in the hospital.
ACKNOWLEDGMENTS
The study was supported by a grant from the University of Genoa (University Research Project Grant). The authors declare no conflict of interest.
References
- 1.Perdelli F, Dallera M, Cristina ML, Sartini M, Ottria G, Spagnolo AM, Orlando P. A new microbiological problem in intensive care units: environmental contamination by MRSA with reduced susceptibility to glycopeptides. Int J Hyg Environ Health. 2008;211:213–218. doi: 10.1016/j.ijheh.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, Fadda G, Cauda R. Bloodstream infections caused by extended-spectrum-β-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50:498–504. doi: 10.1128/AAC.50.2.498-504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–3359. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onori R, Gaiarsa S, Comandatore F, Pongolini S, Brisse S, Colombo A, Cassani G, Marone P, Grossi P, Minoja G, et al. Tracking nosocomial Klebsiella pneumoniae infections and outbreaks by whole genome analysis: small-scale Italian scenario within a single hospital. J Clin Microbiol. 2015;53:2861–2868. doi: 10.1128/JCM.00545-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, Endimiani A, Navon-Venezia S, Hothi J, Slim J, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55:593–599. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, Shao C, Li J, Fan H, Bai Y, Wang Y. Outbreak of multidrug resistant NDM-1-producing Klebsiella pneumoniae from a neonatal unit in Shandong Province, China. PLoS One. 2015;10:e0119571–e0119571. doi: 10.1371/journal.pone.0119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kola A, Piening B, Pape UF, Veltzke-Schlieker W, Kaase M, Geffers C, Wiedenmann B, Gastmeier P. An outbreak of carbapenem- resistant OXA-48 - producing Klebsiella pneumoniae associated to duodenoscopy. Antimicrob Resist Infect Control. 2015;4:8–8. doi: 10.1186/s13756-015-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquez P, Terashita D, Dassey D, Mascola L. Populationbased incidence of carbapenem-resistant Klebsiella pneumoniae along the continuum of care, Los Angeles County. Infect Control Hosp Epidemiol. 2013;34:144–150. doi: 10.1086/669087. [DOI] [PubMed] [Google Scholar]
- 9.Hussein K, Sprecher H, Maschiach T, Oren I, Kassis I, Finkelstein R. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol. 2009;30:666–667. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 10.Amit S, Mishali H, Kotlovsky T, Schwaber MJ, Carmeli Y. Bloodstream infections among carriers of carbapenem-resistant Klebsiella pneumoniae: etiology, incidence and predictors. Clin Microbiol Infect. 2015;21:30–34. doi: 10.1016/j.cmi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Cristina ML, Spagnolo AM, Orlando P, Perdelli F. The role of the environment in the spread of emerging pathogens in at-risk hospital wards. Rev Med Microbiol. 2013;24:104–112. [Google Scholar]
- 12.Hoxha A, Kärki T, Giambi C, Montano C, Sisto A, Bella A, D'Ancona F Study Working Group, author. Attributable mortality of carbapenem-resistant Klebsiella pneumoniae infections in a prospective matched cohort study in Italy, 2012-2013. J Hosp Infect. 2016;92:61–66. doi: 10.1016/j.jhin.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 14. The European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2013. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf. Accessed 12.01.16.
- 15. Centers for Disease Control and Prevention (CDC) 2015. Modified hodge test for carbapenemase detection in enterobacteriaceae. http://www.cdc.gov/HAI/pdfs/labSettings/HodgeTest_Carbapenemase_Enterobacteriaceae.pdf. Accessed 12.01.16.
- 16.Anderson KF, Lonsway DR, Rasheed JK, Biddle J, Jensen B, McDougal LK, Carey RB, Thompson A, Stocker S, Limbago B, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007;45:2723–2725. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) , author. Guidance for control of infections with carbapenem-resistant or carbapenemase- producing Enterobacteriaceae in acute care, facilities. Morb Mortal Wkly Rep. 2009;58:256–260. [PubMed] [Google Scholar]
- 18.Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL the European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group, author. Carbapenemase- producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45) doi: 10.2807/1560-7917.ES.2015.20.45.30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062. [DOI] [PubMed] [Google Scholar]
- 19.Chang LW, Buising KL, Jeremiah CJ, Cronin K, Poy Lorenzo YS, Howden BP, Kwong J, Cocks J, Blood A, Greenough J, et al. Managing a nosocomial outbreak of carbapenemresistant Klebsiella pneumoniae: an early Australian hospital experience. Intern Med J. 2015;45:1037–1043. doi: 10.1111/imj.12863. [DOI] [PubMed] [Google Scholar]
- 20.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumbarello M, Trecarichi EM, Tumietto F, Bono V, Rosa FG, Bassetti M, Losito AR, Tedeschi S, Saffioti C, Corcione S, et al. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2014;58:3514–3520. doi: 10.1128/AAC.02373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliotti C, Cappelli V, Carretto E, Marchi M, Pan A, Ragni P, Sarti M, Suzzi R, Tura GA, Moro ML Emilia-Romagna Group for CPE Control, author. Control of carbapenemase-producing Klebsiella pneumoniae: a region-wide intervention. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.43.20943. pii: 20943. [DOI] [PubMed] [Google Scholar]
- 23.Pan YJ, Lin TL, Lin YT, Su PA, Chen CT, Hsieh PF, Hsu CR, Chen CC, Hsieh YC, Wang JT. Identification of capsular types in carbapenem-resistant Klebsiella pneumoniae strains by wzc sequencing and implications for capsule depolymerase treatment. Antimicrob Agents Chemother. 2015;59:1038–1047. doi: 10.1128/AAC.03560-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridolfo AL, Rimoldi SG, Pagani C, Marino AF, Piol A, Rimoldi M, Olivieri P, Galli M, Dolcetti L, Gismondo MR. Diffusion and transmission of carbapenem-resistant Klebsiella pneumoniae in the medical and surgical wards of a university hospital in Milan, Italy. J Infect Public Health. 2016;9:24–33. doi: 10.1016/j.jiph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Nouvenne A, Ticinesi A, Lauretani F, Maggio M, Lippi G, Guida L, Morelli I, Ridolo E, Borghi L, Meschi T. Comorbidities and disease severity as risk factors for carbapenem-resistant Klebsiella pneumoniae colonization: report of an experience in an in an internal medicine unit. PLoS One. 2014;9:e110001–e110001. doi: 10.1371/journal.pone.0110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter SN, Frasson I, Franchin E, Bergo C, Lavezzo E, Barzon L, Cavallaro A, Palù G. KPC-mediated resistance in Klebsiella pneumoniae in two hospitals in Padua, Italy, June 2009-December 2011: massive spreading of a KPC-3-encoding plasmid and involvement of non-intensive care units. Gut Pathog. 2012;4:7–7. doi: 10.1186/1757-4749-4-7. doi: 10.1186/1757-4749-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulou A, Voulgari E, Vrioni G, Xidopoulos G, Pliagkos A, Chatzipantazi V, Markou F, Tsakris A. Imported Klebsiella pneumoniae carbapenemase-producing K. pneumoniae clones in a Greek hospital: impact of infection control measures for restraining their dissemination. J Clin Microbiol. 2012;50:2618–2623. doi: 10.1128/JCM.00459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanerva M, Skogberg K, Ryynänen K, Pahkamäki A, Jalava J, Ollgren J, Tarkka E, Lyytikäinen O. Coincidental detection of the first outbreak of carbapenemase-producing Klebsiella pneumoniae colonisation in a primary care hospital, Finland, 2013. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.26.21172. pii: 21172. [DOI] [PubMed] [Google Scholar]
- 29.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, Pollini S, Network EuSCAPE-Italy, Grundmann H, Pantosti A, Rossolini GM. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.42.20939. pii: 20939. [DOI] [PubMed] [Google Scholar]
- 30.Nouvenne A, Ticinesi A, Meschi T. Carbapenemase-producing Klebsiella pneumoniae in elderly frail patients admitted to medical wards. Ital J Med. 2015;9:116–119. [Google Scholar]
- 31.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saidel-Odes L, Borer A. Limiting and controlling carbapenem- resistant Klebsiella pneumoniae. Infect Drug Resist. 2013;7:9–14. doi: 10.2147/IDR.S44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy CN, Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012;7:991–1002. doi: 10.2217/fmb.12.74. [DOI] [PubMed] [Google Scholar]
- 34.Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun. 2013;81:3009–3017. doi: 10.1128/IAI.00348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlando P, Cristina ML, Dallera M, Ottria G, Vitale A, Badolati G. Surface disinfection: evaluation of the efficacy of a nebulization system spraying hydrogen peroxide. J Prev Med Hyg. 2008;49:116–119. [PubMed] [Google Scholar]
- 36.Ottria G, Dallera M, Aresu O, Manniello MA, Parodi B, Spagnolo AM, Cristina ML. Environmental monitoring programme in the cell therapy facility of a research centre: preliminary investigation. J Prev Med Hyg. 2010;51:133–138. [PubMed] [Google Scholar]
- 37.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 38.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis. 2014;20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spagnolo AM, Orlando P, Panatto D, Perdelli F, Cristina ML. An overview of carbapenem-resistant Klebsiella pneumoniae: epidemiology and control measures. Rev Med Microbiol. 2014;25:7–14. [Google Scholar]
- 40.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. Attributable mortality rate for carbapenem- resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 41.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect. 2016;22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Duin D, Doi Y. Outbreak of Colistin-Resistant, Carbapenemase- producing Klebsiella pneumoniae: are we at the end of the road? J Clin Microbiol. 2015;53:3116–3117. doi: 10.1128/JCM.01399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulcrano G, Iula DV, Luca C, Roscetto E, Vollaro A, Rossano F, Catania MR. Clonal dissemination of Klebsiella pneumoniae ST512 carrying blaKPC-3 in a hospital in southern Italy. APMIS. 2014;122:42–46. doi: 10.1111/apm.12097. [DOI] [PubMed] [Google Scholar]
- 44.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, Shalit I, Carmeli Y Israel Carbapenem-Resistant Enterobacteriaceae Working Group, author; Israel Carbapenem-Resistant Enterobacteriaceae Working Group, author. Containment of a countrywide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52:848–855. doi: 10.1093/cid/cir025. [DOI] [PubMed] [Google Scholar]
- 45.López-Cerero L, Egea P, Gracia-Ahufinger I, González-Padilla M, Rodríguez-López F, Rodríguez-Baño J, Pascual A. Characterisation of the first ongoing outbreak due to KPC-3-producing Klebsiella pneumoniae (ST512) in Spain. Int J Antimicrob Agents. 2014;44:538–540. doi: 10.1016/j.ijantimicag.2014.08.006. [DOI] [PubMed] [Google Scholar]


