1. Introduction
When possible, chronic non cancer pain (CNCP) in older adults should be managed by nonpharmacological modalities in conjunction with non-opioid analgesics. If moderate to severe pain persists despite these approaches, however, non-parenteral opioids (Table 1) may be considered as adjunctive therapy.1,2 This article will discuss the epidemiology of opioid use and their effectiveness for CNCP in older adults, as well as review age-related changes in opioid pharmacokinetics and pharmacodynamics that increase the risks of adverse effects in the elderly. Finally, to assist clinicians with selecting appropriate therapy, the article concludes with an evidence-based approach to optimize opioid prescribing in older adults.
Table 1.
| Full μ agonist opioids | Partial μ agonist opioid | Mixed action opioidsa |
|---|---|---|
|
| ||
| Codeined Fentanylb Hydrocodonec Hydromorphonec Levorphanolb Meperidined Methadoneb Morphinec Oxycodonec Oxymorphonec |
Buprenorphinec | Tapentadold Tramadold |
Mixed action = μ receptor agonist and norepinephrine reuptake inhibitor;
high potency;
moderate potency;
low potency.
2. Epidemiology of Opioid Use and Benefits for CNCP in the Elderly
Approximately 6–9% of community-dwelling older adults use opioids chronically for CNCP.6–8 A recent study using data from the National Ambulatory Medical Care Survey showed that from 1999–2000 to 2009–2010, the percentage of clinic visits for older patients where an opioid was prescribed rose from 4.1% to 9.0%.9 Most commonly, hydrocodone was used in combination with acetaminophen or ibuprofen.9 Additionally, women and individuals diagnosed with arthritis and depression were more likely to use opioids.9 Compared to the community setting, opioid use may be even higher in nursing homes. For example, one study found that 70% of nursing home residents with CNCP received regularly-scheduled opioids.10 Interestingly, there may be a difference between practice settings regarding the potency of opioids most often prescribed. One study found that higher potency opioids (e.g., oxycodone) were more likely to be used among nursing home residents, whereas lower-potency opioids (e.g., tramadol) were more frequently given to community-dwelling older adults.11
Unfortunately, data regarding the efficacy of opioids for CNCP in older adults are limited to short term studies. In a 2010 meta-analysis of 18 randomized, placebo-controlled trials, the majority of studies (78%) focused on the role of opioids for osteoarthritis; the remaining studies evaluated efficacy for neuropathic pain.12 The most commonly studied opioids, in rank order, were: tramadol (n=7), oxycodone (n=5), oxymorphone (n=3), morphine (n=2), codeine (n=1), fentanyl (n=1), and methadone (n=1).12 Overall, pooled data showed a significant small-to-modest improvement in pain intensity and physical function with opioids compared to placebo.12 Despite documented improvements in CNCP from these short term studies, however, a recently published systematic review found no rigorously-conducted long term trials comparing opioids to non-opioid analgesics for CNCP with a duration of greater than one year.13 This represents an important gap in current clinical knowledge, as there is concern that many patients who use opioids chronically may still have high pain intensity and poor function.14
3. Age-related Pharmacokinetic & Pharmacodynamic Changes & Risks of Opioids in the Elderly
To ensure benefits and avoid risks associated with opioid use in older adults, it is critical to understand age-related changes in pharmacokinetics that affect opioid absorption, distribution, metabolism, and elimination. Oral absorption, indicated by bioavailability (i.e., the proportion of drug that reaches systemic circulation), is similar for younger and older adults. For example, the absorption of transdermal buprenorphine and fentanyl does not appear to be altered, despite age-related changes in skin.15,16 An exception is morphine, a drug with high first pass properties, where older subjects have greater oral absorption than their younger counterparts.17 Moreover, opioid distribution is generally not altered in the elderly, despite age-related changes in body composition.18,19
Unlike absorption and distribution, however, age-related changes in metabolism are more apparent. All opioids are metabolized by the liver; Table 2 lists those opioids that undergo phase I and phase II metabolism via respective isoenzymes. In general, drugs metabolized in phase I via the cytochrome P450 enzyme system will undergo oxidation, reduction, or hydrolysis. Age-related reduction in CYP3A4 function may affect opioids, resulting in decreased systemic clearance and subsequent increased elimination half-life. Specifically, the systemic clearance of oxycodone and, possibly, buprenorphine have been shown to decline with age.16,20,21 The effect of age on fentanyl clearance in older adults, however, is unclear.22 Although not explicitly studied in the elderly, both levorphanol and methadone have half-lives exceeding 12 hours; as such, the prescription of these opioids should be restricted to those practitioners with considerable experience.23
Table 2.
| Phase | Isoenzyme | Substrate | Active Metabolite |
|---|---|---|---|
| I | CYP2D6 | Codeinea (prodrug) | Morphine |
| Hydrocodonea (prodrug) | Hydromorphone | ||
| Tramadol (prodrug) | O-desmethyltramadol | ||
|
| |||
| CYP3A4 | Buprenorphine | None | |
| Fentanyl | None | ||
| Meperidine | Normeperidine | ||
| Methadonea | None | ||
| Oxycodone | Oxymorphone and noroxycodone | ||
|
| |||
| II | UGT | Hydromorphone | Hydromorphone-3-glucuronide |
| Levorphanola | None | ||
| Morphine | Morphine-3-and 6 glucuronide | ||
| Oxymorphonea | 6 hydroxy-oxymorphone | ||
| Tapentadol | Tapentadol O-glucuronide | ||
Pharmacokinetics have not been studied in the elderly.
Abbreviation: UGT = uridine diphosphate glucuronosyltransferase.
The effect of age on CYP2D6 metabolism is unclear, although it is well-documented that genetic polymorphisms may result in poor, intermediate, extensive, and ultrarapid metabolism.19,24,25 Theoretically, CYP2D6 poor metabolizers may have reduced efficacy with codeine, hydrocodone, and tramadol, as these medications are prodrugs that require conversion to an active form prior to exerting their pharmacologic effect (Table 2). However, there is no clinical evidence for any opioids that undergo CYP2D6 metabolism to suggest that dose adjustments for opioids used chronically are required.25
During phase II hepatic metabolism, medications have an additional molecule attached to facilitate excretion. For opioids, phase II metabolism by the isoenzyme uridine diphosphate glucuronosyltransferase (UGT) is generally thought to be unaffected by age.26,27 Again, the exception is morphine as age-related reductions in hepatic blood flow lead to decreased clearance and increased half-life of high hepatic extraction drugs.17
The kidneys are involved in the elimination of all opioids. Furthermore, renal function, which can be estimated by glomerular filtration rate (GFR), is reduced with age.18 Codeine, hydromorphone, meperidine, morphine, oxycodone, and tramadol all have renally-cleared, active metabolites (Table 2). Consequently, age-related declines in renal function may lead to opioid toxicity with these opioids due to the accumulation of these active metabolic byproducts. Specifically, meperidine should be avoided in older adults because accumulation of its active metabolite (normeperidine) can cause neurotoxicity. 29 Only tramadol has specific dosing guidelines in those with reduced GFR.29
In addition to the pharmacokinetic changes, it is important to emphasize that among older adults, enhanced pharmacodynamic sensitivity (i.e., more pronounced effects at equivalent doses used in younger adults) is seen with all opioids.19,22 Indeed, pharmacodynamic sensitivity helps explain risks of opioids unique to geriatric patients.6,8,19,29,31 In a meta-analysis of pooled data from 6 observational studies, older adults exposed to opioids had a 38% increased likelihood of fractures (relative risk [RR] 1.38; 95% confidence intervals [CI] 1.15–1.66).32 Moreover, timing of opioid initiation may be an important consideration. Compared with new NSAID users, individuals initiating opioid therapy for arthritis were 4 to 5 times more likely to have a fracture.33–35
Additionally, there is a dose-response relationship between opioid exposure and risk.33,36 One study of 2,341 older adults found that individuals taking ≥ 50 oral morphine equivalents (OME) had a two-fold increased risk of fractures (adjusted hazard ratio [HR] 2.00, 95% CI 1.24–3.24).36 However, this elevated risk is not confined to opioids in isolation. Coadministration of opioids and other agents that affect the central nervous system (benzodiazepine receptor agonists, antipsychotics, tricyclic antidepressants, and selective serotonin reuptake inhibitors [SSRIs]) has also been associated with falls and fractures.37,38 As such, the administration of ≥ 3 of central nervous system agents is now considered a clinically important drug-drug interaction in the 2015 American Geriatrics Society Beers Criteria, an explicit measure of potentially inappropriate prescribing in older adults.29
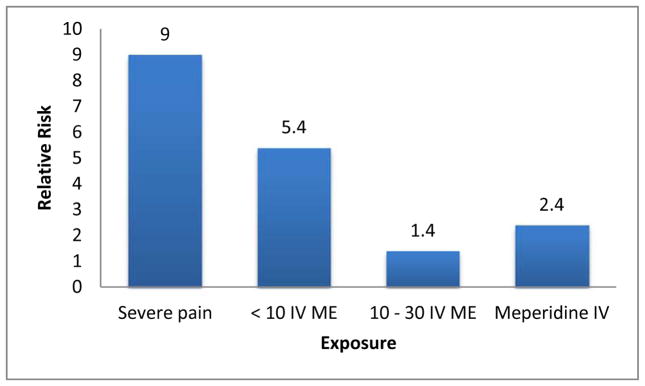
The link between opioids (alone and in combination with other medications affecting the central nervous system) and cognitive decline in older adults is well-established.39 Moreover, a recent meta-analysis showed that opioids also increase the risk of delirium.40 Meperidine was associated with the greatest risk of delirium, which is not surprising given its renally-cleared neurotoxic metabolite. As such, its use in individuals with cognitive impairment or previous delirium is considered a drug-disease interaction per the 2015 American Geriatrics Society Beers Criteria.29 It is important to note, however, that at least in one study, the risk of delirium was inversely associated with opioid dose (Figure 1).41 Lower doses of opioids resulted in greater episodes of delirium, suggesting that undertreating severe pain is a greater risk factor for delirium than the drugs themselves. Again, meperidine was the most problematic, as individuals receiving this medication were more than 2 times as likely to develop delirium as those receiving other opioids. 41
Figure 1.
Adjusted relative risk ratios for incident delirium. 41
Abbreviations: IV = intravenous; ME = morphine equivalents.
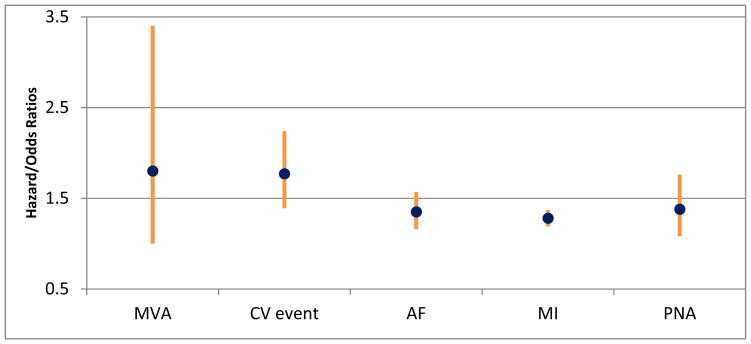
The incidence of many stereotypical adverse drug reactions associated with opioids, such as constipation, do not differ between younger and older adults.6 All patients chronically taking opioid analgesics should receive a daily stimulant laxative (e.g., bisacodyl, senna) to avoid constipation. Additional common symptoms such as nausea and dizziness often subside after a few days of therapy.12 A recent study also found that individuals exposed to tramadol had more than a two-fold increased risk of hospitalization for hypoglycemia (95% CI 1.61–4.23) in the first 30 days after initiation compared with codeine.42 Moreover, tramadol may exacerbate seizures in those with known epilepsy.29 Other adverse drug events identified in studies to be associated with opioid use specific to older adults are highlighted in Figure 2.
Figure 2.
Additional adverse drug events associated with opioid use among older adults.34,43–46
Abbreviations: MVA = motor vehicle accident; CV = cardiovascular event; AF = atrial fibrillation; MI = myocardial infarction; PNA = pneumonia.
Elderly individuals often take multiple medications (i.e., polypharmacy), which increases their risk for adverse events stemming from drug interactions involving opioids.47,48 Because tramadol, meperidine, and fentanyl can increase serotonin levels, case reports suggest they may precipitate serotonin syndrome when given with other medications (e.g., SSRIs) that modulate the serotonin pathway.49 Additionally, methadone has a known risk for torsades de pointes, and is especially problematic when given with other medications that increase the QT interval and/or inhibit CYP3A4 (e.g., certain macrolide antibiotics).50
There is concern that the rapid rise of opioids to treat CNCP in the elderly may lead to abuse and misuse of this analgesic class. However, a recent review assessing the prevalence of opioid misuse in older adults estimated that 1–3% of older adults used opioids inappropriately, which was consistently less than the proportion of their younger counterparts.12 Another study using data from the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) System Poison Center Program evaluated 184,136 calls regarding opioid abuse (intentional incorrect use for the purposes of achieving a psychotropic effect), misuse (intentional incorrect use for reasons other than to achieve a psychotropic effect or suicidal intent), and suicidal intent.51 Compared to controls aged 20–59, adults ≥ 60 years old adults had lower average annual rate of calls and lower proportion of calls for abuse, but a greater proportion of calls as misuse.51 Furthermore, death from opioid overdose is less prevalent in those ≥ 65 year when compared to younger adults. In 2011, for example, 1.3 opioid-related deaths were seen per 100,000 adults ≥ 65 years old, compared with 6.3 to 11.2 deaths per 100,000 adults of younger age groups. 52
4. Conclusion: Optimizing Opioids in the Elderly
When nonpharmacological modalities and non-opioid analgesics do not provide adequate relief for moderate to severe CNCP, opioids should be considered as adjunctive therapy. However, balancing potential risks and benefits associated with opioids in the elderly can be challenging. As such, we recommend the lowest tolerated dose that leads to acceptable relief of pain. Table 3 recommends a clinical algorithm for initiating and maintaining opioid dosage for severe pain. It is important to note that these are suggestions only and that decisions should ultimately depend on severity of pain, functional status, expected length that pain will last, and patient preference. Nonetheless, the guidance outlined in Table 3 regarding appropriate use for severe pain is based on the following principles:
Table 3.
| Dosing Option | Initiation Phase | Maintenance Phase |
|---|---|---|
| 1 |
Tramadol IR 50 mg ½ tab 1–2 times daily AND Tramadol IR 50mg ½ tab every 6 hr as neededb When > 200 mg tramadol is required for analgesia, convert to maintenance phase |
Morphine SRa or oxycodone SR every 12 hr AND Morphine IR or oxycodone IR every 6 hr as needed |
| 2 |
Oxycodone IR 5 mg ½ - 1 tab every 6 hr AND Oxycodone IR 5mg ½ tab every 3 hr as neededb |
Oxycodone SR 1 tab every 12 hr AND Oxycodone IR 1 tab every 6 hr as needed |
| 3 |
Morphine solution 2.5–5 mg every 6 hr AND Morphine solution 2.5 mg every 3 hr as needed |
Morphine SRa 1 tab every 12 hr AND Morphine IR 1 tab every 6 hr as needed |
Special cases: patients with a “true allergy” to codeine/morphine drugs may consider fentanyl patch (12 mcg is approximately equal to 30 mg oral morphine equivalents).
Every 3–7 days, may increase tramadol by 25 mg and oxycodone by 5 mg.
Abbreviations: IR = immediate release; SR = sustained release
Build a personal formulary and become comfortable with a few agents, rather than trying to learn caveats of the entire opioid cadre. We recommend prioritizing those opioids (tramadol, oxycodone, and morphine) in which pharmacokinetic, pharmacodynamic, and efficacy studies have been conducted in the elderly.
Initiation phase doses of opioids should be lower than those employed in a younger population, and subsequent titrations should be made slowly under close supervision.
When initiating therapy in an opioid-naïve patient, avoid using long-acting opioids (methadone, levorphanol, fentanyl patch, or opioids delivered by extended-release dosage forms). In all situations, non-opioid analgesics (i.e., acetaminophen) should be continued to facilitate “opioid-sparing” dosing.
Once a stable daily dose is established, consider changing therapy during the maintenance phase to extended release (ER) opioids on a regularly-scheduled basis in cases of severe pain, with immediate release (IR) opioids prescribed as needed for breakthrough pain. To convert from oxycodone IR to ER, simply add up the total daily dose of IR medication, and then administer ½ of the total amount every 12 hours. When switching from morphine IR to ER, give the full daily dose every 24 hours or ½ the daily dose every 12 hours, depending on the least-expensive formulation available.
Cross-tolerance must be taken into consideration if switching opioids. The conversion factor 50 mg tramadol = 10 mg oxycodone = 10 mg oral morphine equivalents (OME) may be used to convert between opioids. Lower the daily dose of the newly-prescribed opioid by 25–50% OME. For example, when converting from tramadol 200 mg daily to oral morphine, 200 mg = 40 OME. The appropriate dose of morphine is 10 mg every 12 hours, and then 5 mg every 6 hours as needed for breakthrough pain.
Pharmacists and palliative care/pain experts are available to provide consults for challenging cases, including those requiring large doses of opioids (≥ 100–120 OME daily).
Key Points.
Opioids remain a treatment option for moderate to severe chronic non-cancer pain when non-opioid analgesics and nonpharmacological therapies do not provide adequate relief.
Age-related changes in pharmacokinetics (e.g., declines in hepatic and renal function) and pharmacodynamics make older adults more susceptible to adverse consequences associated with opioids, including falls, fractures, and delirium.
To optimize use of opioids, avoid those that have not been studied in older adults, start with the lowest available dose of an immediate-release product, and consult pharmacists or pain experts for challenging cases, including those requiring high doses.
Acknowledgments
Funding: Dr. Naples’s fellowship is supported by a National Institute of Aging grant (T32-AG021885). Dr. Hanlon is supported by National Institute of Aging grants (P30AG024827 and R01AG037451), a grant from the Donoghue Foundation, a grant from the Agency for Healthcare Research and Quality (R18 HS023779), and VA Health Services Research and Development (HSR&D) Service Merit awards (IIR 12-379 and 1 I01 HX001765). Dr. Gellad is supported by VA HSR&D award I01 HX001765.
Contributor Information
Jennifer Greene Naples, Post-doctoral Geriatrics Pharmacotherapy Fellow, University of Pittsburgh School of Medicine, Department of Geriatrics & Gerontology. Mailing address: 3471 Fifth Ave, Kaufmann Medical Building Suite 500, University of Pittsburgh, Pittsburgh, PA 15213.
Walid F. Gellad, Associate Professor of Medicine, University of Pittsburgh School of Medicine, Division of General Medicine, and Center for Health Equity Research and Promotion, VA Pittsburgh Health System. Mailing address: VA Pittsburgh Healthcare System, University Drive (151C), Pittsburgh, PA 15240.
Joseph T. Hanlon, Professor of Geriatric Medicine, Pharmacy, and Epidemiology, and Health Scientist, Center for Health Equity Research and Promotion and Geriatric Research Education and Clinical Center, VA Pittsburgh Health System. Mailing address: 3471 Fifth Ave, Kaufmann Medical Building Suite 500, University of Pittsburgh, Pittsburgh, PA 15213.
References
- 1.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. J Amer Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla A, Adams N, Bone M, et al. British Geriatric Society Guidance on the management of pain in older people. Age Ageing. 2013;42:i1–i57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 3.The Medical Letter: Drugs for Pain. Treat Guidel Med Lett. 2013 Apr;11(128):31–42. [PubMed] [Google Scholar]
- 4.American Society of Health System Pharmacists. [Accessed 11/9/2015];Update to Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. 2011 Available at: http://www.ashp.org/doclibrary/bookstore/p1985/2011-update.aspx.
- 5.Pharmacist’s Letter. [Accessed 11/9/2015];Equianalgesic dosing of opioids for pain management. 2012 Available at: http://prescribersletter.therapeuticresearch.com/pl/ArticlePDF.aspx?cs=&s=PRL&DocumentFileID=0&DetailID=280821&SegmentID=0.
- 6.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–7. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcum ZA, Perera S, Donohue JM, et al. Analgesic use for knee and hip osteoarthritis in community-dwelling elders. Pain Med. 2011;12:1628–1636. doi: 10.1111/j.1526-4637.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karp JF, Lee CW, McGovern J, et al. Clinical and demographic covariates of chronic opioid and non-opioid analgesic use in rural-dwelling older adults: the MoVIES project. Int Psyschogeriatr. 2013;25:1801–10. doi: 10.1017/S104161021300121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman MA, Komaiko KD, Fung KZ, et al. Use of opioids and other analgesics by older adults in the United States, 1999–2010. Pain Med. 2015;16:319–27. doi: 10.1111/pme.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapane KL, Quilliam BJ, Chow W, et al. Pharmacologic management of non-cancer pain among nursing home residents. J Pain Symptom Manage. 2013;45:33–42. doi: 10.1016/j.jpainsymman.2011.12.285. [DOI] [PubMed] [Google Scholar]
- 11.Veal FC, Bereznicki LR, Thmpson AJ, et al. Use of opioid analgesics in older Australian. Pain Med. 2015;16:1519–27. doi: 10.1111/pme.12720. [DOI] [PubMed] [Google Scholar]
- 12.Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1353–69. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen J, Sjøgren P, Bruera E, et al. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125:172–9. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Roy S, Flynn G. Transdermal delivery of narcotic analgesics: pH, anatomical, and subject influences on cutaneous permeability of fentanyl and sufentanil. Pharm Res. 1990;7:842–847. doi: 10.1023/a:1015912932416. [DOI] [PubMed] [Google Scholar]
- 16.Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69:143–149. doi: 10.1007/s00228-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baillie SP, Bateman DN, Coates PE, et al. Age and the pharmacokinetics of morphine. Age Ageing. 1989;18:258–62. doi: 10.1093/ageing/18.4.258. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon JT, Guary DR, Ives TJ. Oral analgesics: efficacy, mechanism of action, pharmacokinetics, adverse effects, drug interactions, and practical recommendations for use in older adults. In: Gibson SJ, Weiner DK, editors. Pain in Older Persons. Progress in Pain Research and Management. 2005. [Google Scholar]
- 19.McLachlan AJ, Bath S, Naganathan V, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol. 2011;71:351–64. doi: 10.1111/j.1365-2125.2010.03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liukas A, Kuusniemi K, Aantaa R, et al. Plasma concentrations of oral oxycodone are greatly increased in the elderly. Clin Pharmacol Ther. 2008;84:462–7. doi: 10.1038/clpt.2008.64. [DOI] [PubMed] [Google Scholar]
- 21.Saari TI, Ihmsen H, Neuvonen PJ, et al. Oxycodone clearance is markedly reduced with advancing age: a population pharmacokinetic study. Br J Anaesth. 2012;108:491–8. doi: 10.1093/bja/aer395. [DOI] [PubMed] [Google Scholar]
- 22.Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987;240:159–66. [PubMed] [Google Scholar]
- 23.Hanlon JT, Weiner D. Methadone for chronic pain in older adults: blast from the past but are we ready for it to return to prime time? Pain Med. 2009;10:287–288. doi: 10.1111/j.1526-4637.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 24.Likar R, Wittels M, Molnar M, et al. Pharmacokinetic and pharmacodynamic properties of tramadol IR and SR in elderly patients: a prospective, age-group-controlled study. Clin Ther. 2006;28:2022–2039. doi: 10.1016/j.clinthera.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Somogyi AA, Coller JK, Barratt DT. Pharmacogenetics of opioid response. Clin Pharmacol Ther. 2015;97:125–7. doi: 10.1002/cpt.23. [DOI] [PubMed] [Google Scholar]
- 26.Durnin C, Hind ID, Ghani SP, et al. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in young and elderly subjects. Proc Western Pharmacol Soc. 2001;44:79–80. [PubMed] [Google Scholar]
- 27.Smit JW, Häufel T, Ravenstijn P, et al. Pharmacokinetics of tapentadol in healthy elderly and young subjects. J Clin Pharmacol. 2010;50:1079. [Google Scholar]
- 28.King S, Forbes K, Hanks GW, et al. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Pall Med. 2011;25:525–32. doi: 10.1177/0269216311406313. [DOI] [PubMed] [Google Scholar]
- 29.American Geriatrics Society. 2015 Beers Criteria Update Expert Panel. AGS Updated Beers Criteria for potentially in appropriate medication use in older adults. J Am Geriatr Soc. 2015 doi: 10.1111/jgs.13702. Epub ahead of print Oct 8. [DOI] [PubMed] [Google Scholar]
- 30.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–24. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang AR, Mallet L. Prescribing opioids in older people. Maturitas. 2013;74:123–9. doi: 10.1016/j.maturitas.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30:171–84. doi: 10.2165/00002018-200730020-00006. [DOI] [PubMed] [Google Scholar]
- 33.Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–8. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170:1979–86. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 35.Rolita L, Spegman A, Tang X, Cronstein BN. Greater number of narcotic analgesic prescriptions for osteoarthritis is associated with falls and fractures in elderly adults. J Am Geriatr Soc. 2013;61:335–40. doi: 10.1111/jgs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310–5. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudreau RM, Hanlon JT, Roumani YF, et al. Central nervous system medication use and incident mobility limitation in community elders: the Health, Aging, and Body Composition study. Pharmacoepidemiol Drug Saf. 2009;18:916–22. doi: 10.1002/pds.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanlon JT, Boudreau RM, Roumani YF, et al. Number and dosage of central nervous system medications on recurrent falls in community elders: the Health, Aging and Body Composition study. J Gerontol Med Sci. 2009;64A:492–8. doi: 10.1093/gerona/gln043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puustinen J, Nurminen J, Löppönen M, et al. Use of CNS medications and cognitive decline in the aged: a longitudinal population-based study. BMC Geriatr. 2011;11:70. doi: 10.1186/1471-2318-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40:23–9. doi: 10.1093/ageing/afq140. [DOI] [PubMed] [Google Scholar]
- 41.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 42.Fournier JP, Azoulay L, Yin H, et al. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med. 2015;175:186–93. doi: 10.1001/jamainternmed.2014.6512. [DOI] [PubMed] [Google Scholar]
- 43.Leveille SG, Buchner DM, Koepsell TD, et al. Psychoactive medications and injurious motor vehicle collisions involving older drivers. Epidemiology. 1994;5:591–8. doi: 10.1097/00001648-199411000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Qureshi WT, O’Neal WT, Khodneva Y, et al. Association between opioid use and atrial fibrillation: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Internal Med. 2015;175:1058–60. doi: 10.1001/jamainternmed.2015.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Setoguchi S, Cabral H, et al. Opioid use for noncancer pain and risk of myocardial infarction amongst adults. J Intern Med. 2013;273:511–26. doi: 10.1111/joim.12035. [DOI] [PubMed] [Google Scholar]
- 46.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59:1899–907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Eng J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 48.Richarz U, Jacobs A, Spina E. How frequently are contraindicated or warned against combinations of drugs prescribed to patients receiving long-term opioid therapy for chronic pain? Pharmacoepidemiol Drug Saf. 2012;21:453–62. doi: 10.1002/pds.2250. [DOI] [PubMed] [Google Scholar]
- 49.Hanlon JT, Wang X, Castle NG, Stone RA, Handler SM, Semla TP, Pugh MJ, Berlowitz DR, Dysken MW. Potential underuse, overuse and inappropriate use of antidepressants in older veteran nursing home patients. J Am Geriatr Soc. 2011;59:1412–20. doi: 10.1111/j.1532-5415.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray WA, Chung CP, Murray KT, et al. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175:420–7. doi: 10.1001/jamainternmed.2014.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug Alcohol Depend. 2015;149:117–21. doi: 10.1016/j.drugalcdep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. [Accessed 11/15/15];NCHS Data Brief. 2014 166:1–8. Available at: http://www.cdc.gov/nchs/data/databriefs/db166.pdf. [PubMed] [Google Scholar]