Abstract
Sloped walking requires altered strategies for maintaining dynamic balance relative to level-ground walking, as evidenced by changes in sagittal-plane whole-body angular momentum (H) in able-bodied individuals. The ankle plantarflexor muscles are critical for regulating H, and functional loss of these muscles from transtibial amputation affects this regulation. However, it is unclear if a powered prosthesis, which more closely emulates intact ankle function than a passive energy-storage-and-return prosthesis, affects H differently during sloped walking. Therefore, our purpose was to investigate H in individuals with unilateral transtibial amputation when using powered and passive prostheses. Overall, the range of H was greater in people with a transtibial amputation relative to able-bodied individuals. On a −10° decline, individuals with amputation did not decrease H as much as able-bodied individuals, and had reduced prosthetic limb braking ground reaction forces and knee power absorption. On a +10° incline, individuals with amputation had a greater relative increase of H than able-bodied individuals, a more anterior placement of the prosthetic foot, and higher peak hip power generation. The powered prosthesis condition resulted in a smaller range of H during prosthetic stance relative to the passive condition, although it was still larger than able-bodied individuals. Our results suggest that prosthetic ankle power generation may help regulate dynamic balance during prosthetic stance, but alone is not sufficient for restoring H to that of able-bodied individuals on slopes. Contributions of knee extensor muscles and the biarticular gastrocnemius in regulating H on slopes should be further investigated.
Keywords: Biomechanics, uphill, downhill, amputee, falls, dynamic balance
I. INTRODUCTION
The biomechanical demands of sloped (uphill/downhill) walking are fundamentally different from level-ground walking. The body center-of-mass (COM) must be raised or lowered during sloped walking, and the risk of slipping on a sloped surface is greater than on level ground due to increased shear ground reaction forces (GRFs) (Redfern et al., 2001). The task demands for sloped walking result in changes in joint kinematics and kinetics (Kuster et al., 1995; Lange et al., 1996; Redfern and Dipasquale, 1997) and electromyography (EMG) (Lange et al., 1996; Lay et al., 2007; Leroux et al., 1999) in comparison to level-ground walking, and may adversely affect dynamic balance in individuals with musculoskeletal impairments.
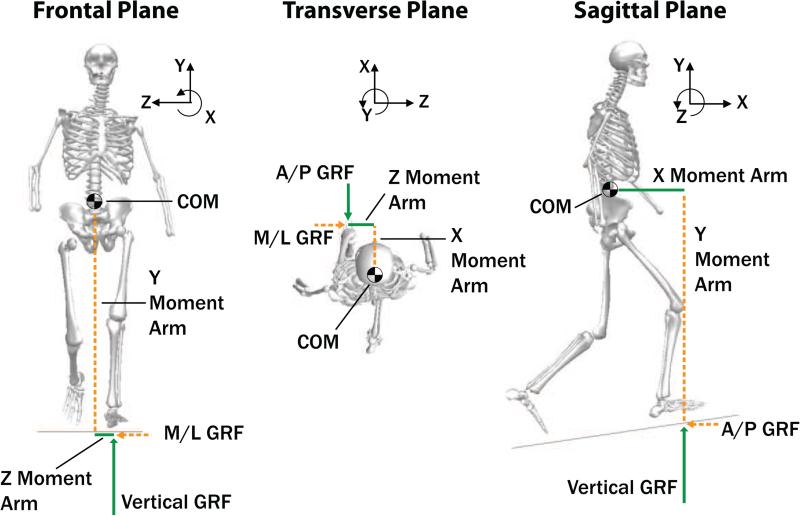
Whole-body angular momentum (H) provides insight into the effects of different walking conditions on dynamic balance, or the ability to maintain equilibrium and avoid falling, during movement. H must be regulated to avoid falling, as observed in level-ground walking (Herr and Popovic, 2008). The time rate of change of H is the sum of the external moments about the body COM,
| (1) |
During walking, the external moment, Mext, is the cross product of the external moment arm (position vector from the body COM to the center-of-pressure) and GRF on each foot (Figure 1), and is controlled through muscle force generation (Neptune and McGowan, 2011). Thus, after a disturbance such as a trip, a rapid response from the muscles is required to generate an external moment to restrain H and avoid falling (Pijnappels et al., 2004). However, populations with balance impairments, such as individuals who have experienced a stroke (Nott et al., 2013) or leg amputation (Silverman and Neptune, 2011), have an increased range of H and compromised muscle function that reduces their ability to regulate dynamic balance. Able-bodied individuals decrease their range of H on declines, potentially as a protective mechanism to counteract the elevated fall risk, but increase their range of H on inclines, where the risk of falling is not as high (Silverman et al., 2012).
Figure 1.
Angular momentum was analyzed in the three anatomical planes. The net external moment about the center-of-mass (COM) results from the ground reaction forces (GRFs) acting between each foot and the ground.
Transtibial amputation (TTA) affects the ability to regulate H (D'Andrea et al., 2014; Pickle et al., 2014; Silverman and Neptune, 2011), likely due to reduced muscle control and proprioception. In particular, the ankle plantarflexors are critical for regulating H (Neptune and McGowan, 2011), but these muscles no longer provide ankle actuation after TTA. Passive energy-storage-and-return prostheses provide reduced body propulsion and leg swing initiation during level-ground walking (Silverman and Neptune, 2012) and result in differences in H (Silverman and Neptune, 2011) in comparison to able-bodied individuals. In contrast, powered prostheses utilize a motorized ankle joint to perform positive net work during stance (Au et al., 2007). Many recent studies have investigated the differences between passive and powered prostheses in a variety of walking conditions (D'Andrea et al., 2014; Esposito et al., 2015; Ferris et al., 2012; Gates et al., 2013; Herr and Grabowski, 2012), but it remains unclear how using a powered prosthesis affects H during sloped walking. Therefore, the purpose of this study was to analyze H in individuals with TTA walking with passive and powered prostheses on a range of slope angles and compared to able-bodied individuals. We hypothesized that individuals with TTA would have a greater range of sagittal-plane H during 0-50% prosthetic limb gait cycle (i.e., during the majority of prosthetic stance) on all slopes when compared to able-bodied individuals. We also hypothesized that using a powered prosthesis would reduce the range of H relative to using a passive prosthesis.
II. METHODS
Ten individuals with TTA (1 female/9 male, 30±5 years, 1.83±0.10 m, 96±7 kg) and ten able-bodied participants (2 female/8 male, 24±5 years, 1.80±0.09 m, 91±10kg) participated in this study. All individuals with TTA were K4-level ambulators capable of walking independently for at least 15 consecutive minutes and were independent walkers for an average of 18.4 (SD=11.1) months prior to the study. Trials were conducted first with the participant's passive energy-storage-and-return prosthesis and then with the BiOM (BiOM, Bedford, MA) powered prosthesis. The BiOM was designed to accommodate a range of activities and surfaces (Au et al., 2007; Ferris et al., 2012; Gates et al., 2013; Herr and Grabowski, 2012; Markowitz et al., 2011; Pickle et al., 2014), and uses a reflexive control scheme whereby the loading of the device determines the motor response and subsequent power production. The passive and powered data collection sessions were separated by an average of 43.4 (SD=18.1) days to allow for acclimation to the BiOM. Participants completed of a range of activities during the initial fitting and training process, including slope ambulation. Device power and timing of power were tuned using average normative biological ankle values. Participants were instructed to practice a variety of activities independently during the acclimation period to ensure comfort and familiarization with device function. All participants provided written informed consent for the protocol approved by the institutional review board at Brooke Army Medical Center.
Whole-body kinematics were captured using a 26-camera motion capture system (Motion Analysis Corp., Santa Rosa, CA) operating at 120 Hz. A set of 57 reflective markers was used to define and track the motion of 13 body segments (Wilken et al., 2012) In addition, virtual markers were created using a digitizing wand (Motion Analysis Corp., Santa Rosa, CA) and were used to identify anatomical landmarks and define local coordinate system orientation. GRF data were captured at 1200 Hz using a 16-ft instrumented walkway inclined at 0°, ±5°, and ±10°. An auditory cue was used to control horizontal (in the laboratory reference frame) walking speed at a Froude number of 0.16 to ensure similar gait across participants (McAndrew et al., 2010). Walking velocity (vw) was based on leg length as , where Fr is the Froude number, g is acceleration due to gravity and l is leg length in meters. The mean (±SD) horizontal velocity was 1.28±0.11 m/s for participants with TTA and 1.21±0.08 m/s for able-bodied participants.
Kinematic marker and force data were filtered using a 4th-order low pass Butterworth filter with cutoff frequencies at 6 Hz and 50 Hz, respectively. Kinematic and GRF data were combined in Visual3D (C-Motion, Inc., Germantown, MD) to compute joint powers using an inverse dynamics approach. Individual segment masses were defined as a percentage of total body mass (Dempster and Aitkens, 1995). In the passive prosthesis trials the prosthetic shank mass was reduced by 30% and that mass was redistributed among the other body segments, the shank COM was shifted 30% more proximal along the shank axis, and the inertial properties adjusted accordingly (Smith et al., 2014). The shank inertial properties were not altered for the powered prosthesis conditions because the mass of BiOM is similar to a biological shank and foot (Eilenberg et al., 2010). H was calculated as
| (2) |
where n is the number of segments, , , and are, respectively, the position, velocity, and angular velocity of the ith segment, and are, respectively, the position and velocity of the whole-body COM, and mi and Ii are the mass and inertia matrix of the ith segment. H was normalized by body height, mass and horizontal walking velocity and expressed as a percentage of the left or prosthetic limb gait cycle for the able-bodied and TTA groups, respectively.
Four gait cycles per participant were analyzed for each group (able-bodied, passive, powered) and slope. The ranges (peak-to-peak values) of H in all three anatomical planes were calculated and compared statistically using R Statistical Computing Software, v. 2.15.1 (R Core Team, 2012). Peak GRFs, external moment arms and joint powers were similarly compared. Values were compared using a linear mixed effects ANOVA (Pinheiro et al., 2015) with slope angle and group (able-bodied, passive, powered) as fixed effects and participant as a random effect. Post-hoc comparisons were performed using least-squares means (Lenth and Hervé, 2015) when significant main or interaction effects were found, and p-values were adjusted using Tukey's method.
III. RESULTS
III.a. Whole-body angular momentum (H)
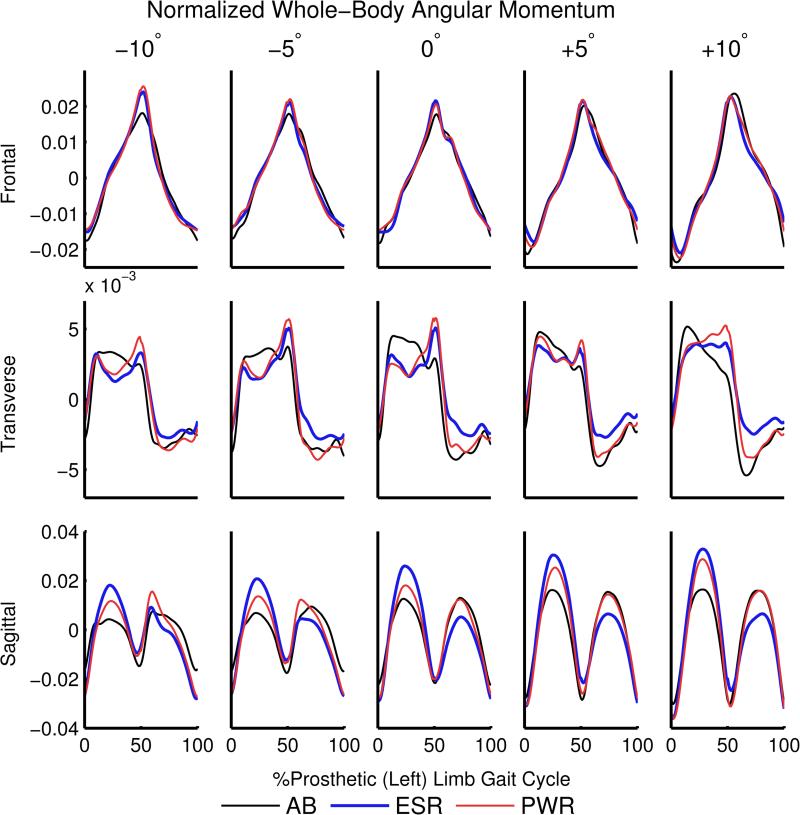
We analyzed the range of H in all three anatomical planes (Figure 2). There were no differences between groups in the range of frontal-plane H over the gait cycle. In the transverse plane, individuals with TTA had a greater range of H during the gait cycle when using a powered prosthesis than when using a passive prosthesis on all slopes. However, the range of H in the transverse plane did not vary substantially with slope, as the only significant difference in comparison to level-ground walking was in individuals with TTA using a powered prosthesis at +10°. The greatest differences between groups occurred in the range of sagittal-plane H, which is also the plane in which slips are most likely to occur during sloped walking. Note that while there were significant differences in peak values of H, particularly in the sagittal plane, we constrained our analysis to range comparisons to describe overall H during gait.
Figure 2.
Whole-body angular momentum (H) over the gait cycle in the three anatomical planes for five sloped walking conditions, normalized by height, mass, and average horizontal walking speed. The range of H was compared across walking condition and participant groups, including able-bodied individuals (AB, black), people using a passive energy-storage-and-return prosthesis (ESR, blue) and people using a powered prosthesis (PWR, red).
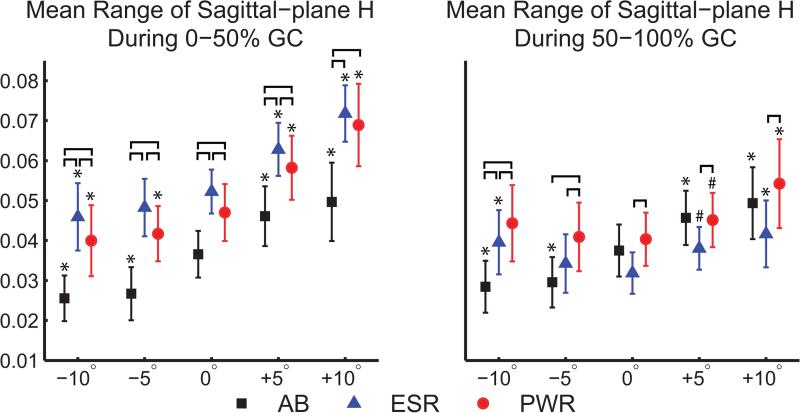
The sagittal-plane range of H during 0-50% gait cycle was significantly greater in the passive and powered prosthesis conditions than in able-bodied people on all slopes (Figure 3). In addition, participants with TTA did not reduce sagittal-plane H as much as able-bodied participants on declines in comparison to level-ground. The range of sagittal-plane H for able-bodied participants during 0-50% gait cycle was 0.037±0.006 at 0° and 0.026±0.006 at −10° (30% decrease). When using a passive prosthesis, the range of sagittal-plane H during 0-50% gait cycle was reduced from 0.052±0.005 at 0° to 0.046±0.008 at −10° (12% decrease) and when using a powered prosthesis the range was reduced from 0.047±0.007 at 0° to 0.040±0.009 at −10° (15% decrease).
Figure 3.
Mean range of sagittal-plane whole-body angular momentum (H) during the 1st and 2nd halves of the prosthetic (left) limb gait cycle (GC), normalized by height, mass, and average horizontal walking speed. Comparisons were performed between slopes and participant groups, including able-bodied individuals (AB, black squares), people using a passive energy-storage-and-return prosthesis (ESR, blue triangles) and people using a powered prosthesis (PWR, red circles). Significant differences between groups are indicated by brackets, and significant differences between each slope and level ground are indicated by ‘#’ (0.001≤p<0.05) and ‘*’ (p<0.001).
The increase in H at +10° relative to 0° was greater in the passive and powered conditions than in able-bodied people. In able-bodied participants, the range of sagittal-plane H during 0-50% gait cycle increased from 0.037±0.006 at 0° to 0.050±0.010 m/s at +10° (35% increase). For people using a passive prosthesis, the range of sagittal-plane H during 0-50% gait cycle increased from 0.052±0.005 at 0° to 0.072±0.007 at +10° (38% increase), and when using a powered prosthesis the range increased from 0.047±0.007 at 0° to 0.069±0.010 at +10° (47% increase).
Although participants with TTA had greater ranges of sagittal-plane H in comparison to able-bodied participants, we also observed lower ranges of sagittal-plane H during 0-50% gait cycle when using a powered compared to passive prosthesis on all slopes except for +10° (Figure 3).
During 50-100% gait cycle on declines, individuals with TTA using both passive and powered prostheses had a greater range of sagittal-plane H relative to able-bodied individuals. In addition, individuals with TTA had a greater range of sagittal-plane H when using the powered compared to the passive prosthesis, which was in contrast to the results from 0-50% gait cycle. On inclines during 50-100% gait cycle there was a significant difference in the range of sagittal-plane H when using a powered compared to passive prosthesis, but there were no significant differences in either the passive or powered prosthesis condition compared to able-bodied participants.
III.b Ground reaction forces and external moment arms
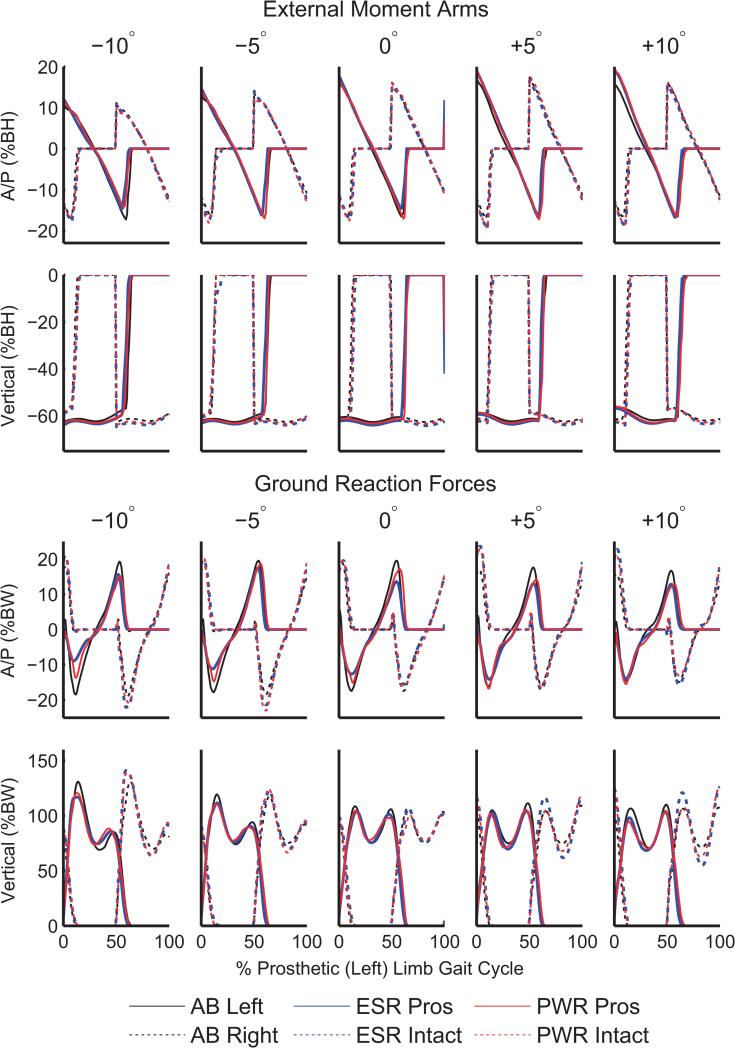
In people with TTA using passive and powered prostheses, the maximum anterior moment arm in the prosthetic limb was greater than the average able-bodied limb at +10° (Figure 4 and Table 1). Few other significant differences between subject groups were observed in external moment arms.
Figure 4.
External moment arms, normalized by body height (BH), and ground reaction forces, normalized by body weight (BW). Results are shown for the left (prosthetic) and right (intact) limb of each participant group: able-bodied (AB), people using a passive energy-storage-and-return prosthesis (ESR), and people using a powered prosthesis (PWR).
Table 1.
Peak external moment arms (percent body height (%BH)), and ground reaction forces (GRFs, percent body weight (%BW)). Able-bodied (AB) values are the average of both limbs, and results for people using an energy-storage-and-return prosthesis (ESR) or a powered prosthesis (PWR) are shown for both the prosthetic and intact limb. The percentage of gait cycle (GC) in this table is for the respective limb (i.e., 0% prosthetic limb GC is 50% intact limb GC).
| −10° | −5° | 0° | +5° | +10° | ||||
|---|---|---|---|---|---|---|---|---|
| A/P Moment Arm (%BH) | Max Anterior, Entire gait cycle | AB | 11.2 (1.7) | 13.8 (1.5) | 16.6 (1.8) | 17.3 (1.8) | 16.2 (2.1) | |
| Prosthetic Limb | ESR | 12.3 (1.0) | 14.9 (0.8) | 18.0 (1.3) | 19.1 (1.4) | 19.6 (2.2) A | ||
| PWR | 12.1 (1.6) | 14.4 (0.8) | 17.0 (1.0) | 19.5 (2.4) A | 19.7 (1.9) A | |||
| Max Posterior, Entire gait | AB | −18.4 (2.9) | −17.5 (1.8) | −17.8 (1.5) | −17.8 (1.4) | −18.1 (2.1) | ||
| Prosthetic Limb | ESR | −16.6 (2.3) | −17.6 (1.6) | −16.1 (2.0) P | −17.8 (2.0) | −18.5 (2.6) | ||
| PWR | −16.2 (1.9) A | −18.4 (2.0) | −18.1 (1.1) P | −18.8 (1.8) | −19.1 (2.6) | |||
| Braking/Propulsion GRF (%BW) | Max Braking, Early stance (0-30% GC) | AB | −19.8 (4.4) | −18.9 (4.4) | −18.0 (4.2) | −16.5 (3.9) | −15.3 (2.9) | |
| Prosthetic Limb | ESR | −10.0 (3.6) A,P | −12.1 (4.2) A,P | −13.1 (2.1) A,P | −14.9 (3.5) P | −14.6 (3.4) | ||
| PWR | −15.4 (6.7) P | −16.0 (5.2) P | −16.0 (2.4) P | −17.8 (3.6) P | −16.1 (3.5) | |||
| Intact Limb | ESR | −26.3 (8.0) A | −23.7 (4.5) | −16.2 (2.7) | −17.6 (3.8) | −16.4 (3.5) | ||
| PWR | −25.2 (7.4) | −24.6 (4.4) A | −17.6 (3.1) | −16.7 (4.7) | −14.5 (4.0) | |||
| Max Propulsion, Late stance (30-60% GC) | AB | 19.4 (3.5) | 19.4 (2.6) | 19.7 (2.1) | 17.6 (2.6) | 16.9 (2.6) | ||
| Prosthetic Limb | ESR | 16.6 (2.9) | 18.2 (2.4) | 13.8 (1.9) A,P | 13.5 (2.8) A,P | 13.7 (5.3) | ||
| PWR | 16.2 (2.7) | 19.6 (2.6) | 18.3 (2.3) P | 15.5 (2.8) P | 14.7 (4.5) | |||
| Intact Limb | ESR | 22.0 (5.2) | 22.0 (4.0) | 20.1 (2.6) | 24.4 (4.2) A | 23.5 (6.3) A | ||
| PWR | 21.9 (4.0) | 22.3 (4.5) | 20.1 (2.9) | 23.7 (5.2) A | 21.8 (5.7) A | |||
| Vertical GRF (%BW) | Max 1st Peak (0-30% GC) | AB | 133.4 (9.8) | 120.6 (7.5) | 109.4 (5.5) | 106.1 (6.7) | 108.1 (5.7) | |
| Prosthetic Limb | ESR | 125.0 (16.6) P | 115.2 (8.1) | 105.7 (3.9) | 103.6 (6.7) | 99.9 (8.9) | ||
| PWR | 132.2 (20.4) P | 117.5 (11.3) | 106.1 (5.5) | 103.4 (7.5) | 97.3 (6.4) A | |||
| Intact Limb | ESR | 150.8 (18.5) A | 127.8 (8.3) | 108.4 (5.9) P | 118.8 (9.9) A,P | 122.7 (9.9) A,P | ||
| PWR | 147.9 (15.4) A | 127.0 (9.7) | 102.7 (5.9) P | 107.1 (10.3) P | 109.9 (8.8) P | |||
| Max 2nd Peak (30-60% GC) | AB | 86.1 (6.6) | 94.0 (5.3) | 105.4 (5.1) | 111.0 (6.3) | 109.9 (7.4) | ||
| Prosthetic Limb | ESR | 89.1 (5.0) | 92.5 (4.9) | 102.4 (3.3) | 105.4 (6.5) | 105.2 (9.2) | ||
| PWR | 91.2 (5.7) | 93.7 (4.7) | 101.8 (2.9) | 107.8 (8.2) | 108.9 (9.1) | |||
| Intact Limb | ESR | 93.1 (7.2) | 96.2 (5.9) | 101.6 (3.8) | 118.9 (9.5) | 127.4 (15.7) A | ||
| PWR | 96.0 (5.8) A | 97.2 (5.8) | 102.9 (4.2) | 120.0 (8.9) A | 128.4 (12.2) A | |||
Bold values indicated significant difference relative to level-ground.
Significant difference (p<0.05) compared to AB.
Significant difference (p<0.05) between ESR and PWR.
On declines, there were several differences between participant groups in the A/P GRF (braking[−]/propulsion[+]). We observed decreased braking in the prosthetic limb on declines when using a passive prosthesis in comparison to both able-bodied individuals and individuals with TTA using a powered prosthesis. At −10°, the maximum braking forces were −10.0±3.6 % body weight (BW) when using a passive prosthesis, which was lower than −15.4±6.7 %BW when using a powered prosthesis and −19.8±4.4 %BW in able-bodied participants. In addition, the first peak of the vertical GRF was increased in the intact limb of people with TTA using both prostheses compared to able-bodied individuals (Table 1) at −10°. However, the first peak of the vertical GRF in the prosthetic limb when using a powered prosthesis was more similar to able-bodied participants than when using a passive prosthesis.
On inclines, the propulsion (positive A/P GRF) provided by the passive prosthesis was lower than the average able-bodied limb, while there was no significant difference between the powered prosthesis and the average able-bodied limb on inclines (Table 1). The first peak vertical GRF in the intact limb was lower when using a powered compared to passive prosthesis. The second peak vertical GRF in the intact limb of people with TTA using both passive and powered prostheses was greater than able-bodied participants at +10°.
III.c. Joint Powers
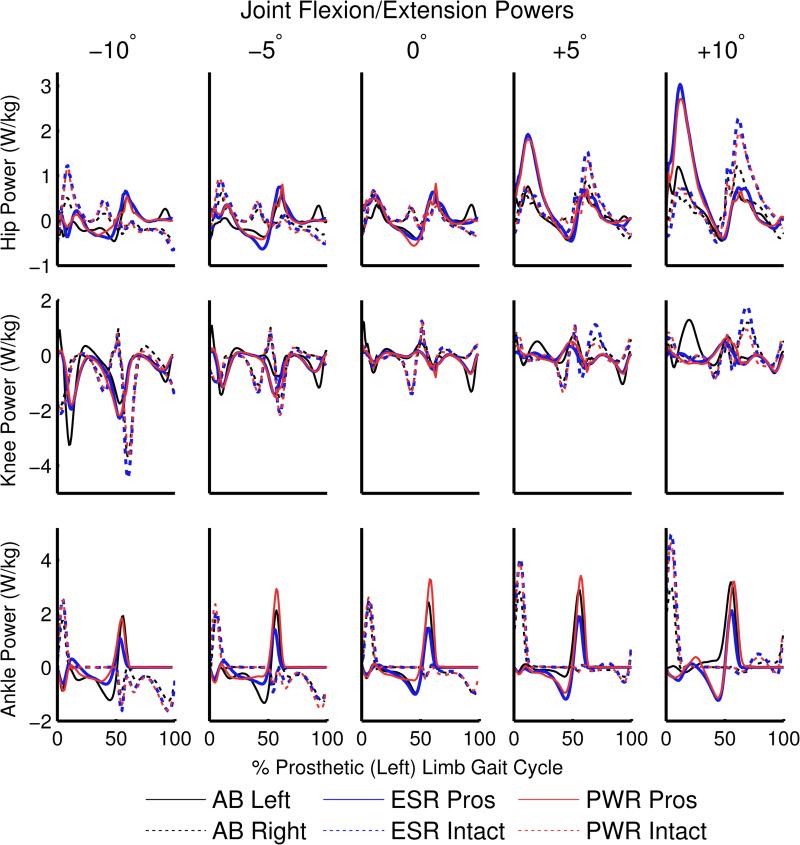
The maximum hip power generated by people with TTA using passive and powered prostheses was greater than able-bodied participants in the intact limb on all inclines and declines except when using the powered prosthesis at +5° (Table 2 and Figure 5). Peak prosthetic limb hip power generation was greater than able-bodied participants at +5° and +10°. At +10° the maximum hip power generated in the intact limb when using a powered prosthesis was significantly less than when using a passive prosthesis (Figure 5).
Table 2.
Mean and peak joint powers. Results for able-bodied (AB) participants are the average of both limbs, and results for people using an energy-storage-and-return prosthesis (ESR) or a powered prosthesis (PWR) are shown for both the prosthetic and intact limb. The percentage of gait cycle (GC) in this table is for the respective limb (i.e., 0% prosthetic limb GC is approximately 50% intact limb GC).
| −10° | −5° | 0° | +5° | +10° | |||
|---|---|---|---|---|---|---|---|
| Max Hip Flex/Ext Power Generated, Entire Gait Cycle | AB | 0.63 (0.13) | 0.68 (0.14) | 0.77 (0.18) | 1.11 (0.32) | 1.57 (0.40) | |
| Prosthetic Limb | ESR | 1.01 (0.45) | 1.08 (0.40) | 0.90 (0.29) P | 2.08 (0.62) A | 3.18 (0.94) A | |
| PWR | 0.94 (0.42) | 1.31 (0.54) A | 1.17 (0.46) P | 2.07 (0.59) A | 2.95 (0.69) A | ||
| Intact Limb | ESR | 1.45 (0.62) A | 1.16 (0.46) A | 0.80 (0.27) | 1.68 (0.55) A | 2.68 (1.00) A,P | |
| PWR | 1.34 (0.52) A | 1.20 (0.48) A | 0.81 (0.27) | 1.49 (0.30) | 2.34 (0.75) A,P | ||
| Mean Knee Flex/Ext Power, 1st Half Stance (0-30% GC) | AB | −0.73 (0.27) | −0.16 (0.13) | 0.10 (0.07) | 0.23 (0.12) | 0.50 (0.26) | |
| Prosthetic Limb | ESR | −0.63 (0.21) | −0.27 (0.10) | 0.02 (0.04) | 0.00 (0.09) A | −0.06 (0.36) A | |
| PWR | −0.55 (0.23) A | −0.23 (0.12) | 0.01 (0.04) | 0.02 (0.06) A | −0.13 (0.24) A | ||
| Intact Limb | ESR | −1.08 (0.49) A,P | −0.32 (0.24) | 0.12 (0.07) | 0.34 (0.15) | 0.62 (0.20) P | |
| PWR | −0.86 (0.30) P | −0.30 (0.15) | 0.09 (0.07) | 0.20 (0.12) | 0.31 (0.17) P | ||
| Max Knee Flex/Ext Power Absorbed, 1st Half Stance (0-30% GC) | AB | −3.84 (1.50) | −1.74 (0.81) | −0.75 (0.45) | −0.52 (0.36) | −0.50 (0.34) | |
| Prosthetic Limb | ESR | −2.12 (0.87) A | −1.22 (0.50) | −0.28 (0.18) | −0.25 (0.12) | −0.56 (0.44) | |
| PWR | −2.02 (0.78) A | −1.24 (0.56) | −0.35 (0.20) | −0.27 (0.15) | −0.71 (0.46) | ||
| Intact Limb | ESR | −4.91 (1.69) A,P | −2.34 (0.83) | −0.63 (0.42) | −0.96 (0.69) | −0.69 (0.45) | |
| PWR | −4.10 (1.20) P | −2.42 (0.73) | −0.54 (0.26) | −0.51 (0.35) | −0.61 (0.42) | ||
| Max Ankle Flex/Ext Power Generated, Entire Gait Cycle | AB | 2.06 (0.50) | 2.18 (0.59) | 2.49 (0.42) | 2.99 (0.54) | 3.30 (0.62) | |
| Prosthetic Limb | ESR | 1.29 (0.37) P | 1.63 (0.43) P | 1.58 (0.30) A,P | 2.02 (0.52) A,P | 2.40 (0.88) A,P | |
| PWR | 2.07 (1.04) P | 3.21 (1.14) A,P | 3.54 (0.90) A,P | 3.68 (1.04) P | 3.99 (1.23) P | ||
| Intact Limb | ESR | 3.06 (1.01) | 2.93 (0.77) | 2.70 (0.54) | 4.32 (1.33) A | 5.47 (2.02) A | |
| PWR | 2.96 (0.80) | 2.81 (0.77) | 2.75 (0.63) | 4.23 (1.29) A | 5.11 (1.22) A | ||
Bold values indicated significant difference relative to level-ground.
Significant difference (p<0.05) compared to AB.
Significant difference (p<0.05) between ESR and PWR.
Figure 5.
Joint flexion and extension powers for the hip, knee, and ankle, normalized by body mass. Positive values indicate power generation, negative values indicate power absorption. Results are shown for the left (prosthetic) and right (intact) limb of each participant group: able-bodied (AB), people using an energy-storage-and-return prosthesis (ESR), and people using a powered prosthesis (PWR).
In the prosthetic limb knee, people using both passive and powered prostheses had lower peak power absorption than able-bodied participants at −10° (Table 2 and Figure 5). People with TTA using a passive prosthesis also had greater power absorption in the intact knee than able-bodied individuals at −10°. At +5° and +10°, people with TTA using both prostheses had lower mean knee power generation in the prosthetic limb during 0-30% gait cycle (1st half of stance) compared to able-bodied individuals.
The peak ankle power generation in the powered prosthesis was higher than or not statistically different from the average able-bodied ankle on all slopes, while the passive prosthesis was lower than the average able-bodied ankle on inclines (Table 2 and Figure 5). Individuals with TTA using both passive and powered prostheses also had greater maximum intact ankle power generation than able-bodied individuals at +5° and +10°.
IV. DISCUSSION
We analyzed the range of H in individuals with and without TTA during sloped walking. We hypothesized that (1) individuals with TTA would have a greater range of sagittal-plane H than able-bodied individuals during 0-50% prosthetic limb gait cycle, and that (2) the powered prosthesis would reduce sagittal-plane H compared to the passive prosthesis.
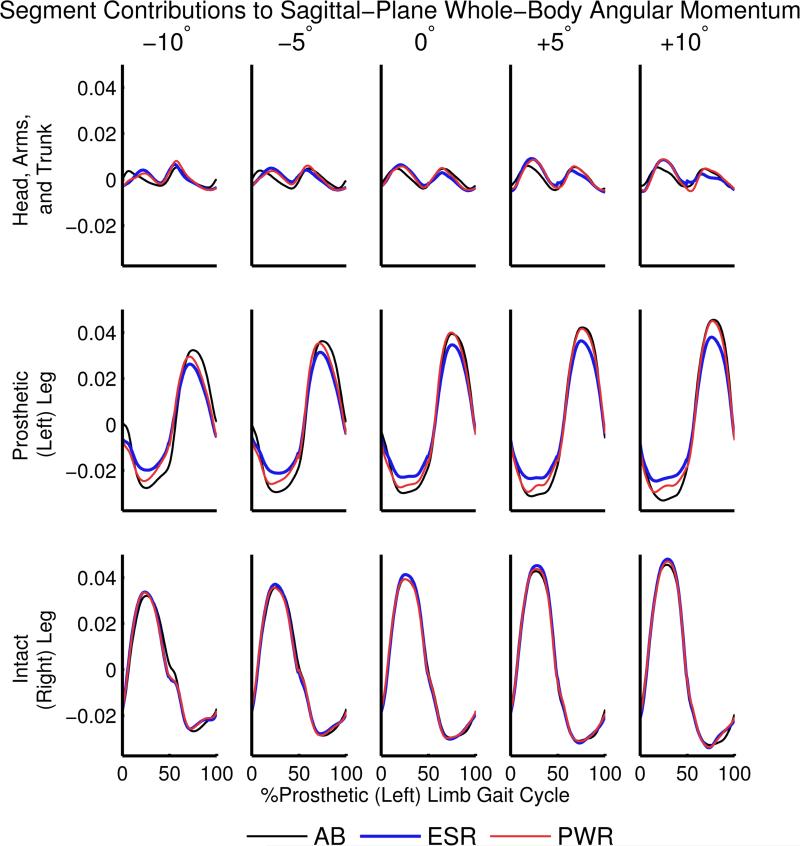
Our first hypothesis was supported. Individuals with TTA had a greater range of sagittal-plane H than able-bodied individuals during 0-50% gait cycle, regardless of prosthesis type. Able-bodied participants had decreased ranges of sagittal-plane H on declines and increased ranges on inclines in comparison to level-ground, as previously reported (Silverman et al., 2012). Individuals with TTA, however, did not reduce H to the same extent as able-bodied people from 0° to −10° and the relative increase in range of sagittal-plane H from 0° to +10° was greater than the relative increase in able-bodied individuals. The increased range of sagittal-plane H in individuals with TTA may suggest increased fall risk during prosthetic stance, which is affected by reduced proprioception and loss of direct muscular control of the ankle. To further investigate this result, we examined the contributions to whole-body H from the head-arms-trunk (HAT) and each limb (thigh, shank, foot) (Figure 6). Greater range of H from participants with TTA during 0-50% gait cycle was related to a more negative prosthetic-limb H just after heel strike and less negative prosthetic-limb H in mid-stance in combination with a later peak of positive H from the HAT relative to able-bodied individuals (Figure 6). The results in individuals with TTA when using a passive prosthesis are partially related to the reduced mass of the passive prosthesis, as the prosthetic limb contributes less negative H during 0-50% gait cycle and less positive H during 50-100% gait cycle on all slopes.
Figure 6.
Segment contributions to sagittal-plane H normalized by height, mass and average horizontal walking speed. Sagittal-plane H is shown for the head-arms-trunk segments and each leg, including the thigh, shank and foot. Results are shown for each participant group, including able-bodied individuals (AB, black), people using a passive energy-storage-and-return prosthesis (ESR, blue) and people using a powered prosthesis (PWR, red).
On the steepest decline (−10°), individuals with TTA had a lower magnitude of peak braking in the prosthetic limb when using a passive prosthesis compared to able-bodied participants, similar to previous studies of level-ground walking (Sanderson and Martin, 1997; Silverman et al., 2008). The braking GRF contributes to a negative external moment (Figure 1), so reduced braking results in a greater positive time rate of change of H, corresponding to backward rotation of the body, during early prosthetic stance. Thus, increased positive H may be a protective mechanism to prevent falling forward on declines, which can lead to serious injury. However, reduced proprioception and volitional control of the prosthetic leg during this period of large H may lead to difficulty maintaining balance in the event of a trip during intact leg swing.
Knee power absorption is important for lowering the body on declines (Kuster et al., 1995), and individuals with TTA had lower peak power absorption in the prosthetic knee relative to able-bodied individuals during 0-30% gait cycle at −10°. Some populations with TTA have knee extensor weakness (Langlois et al., 2014), but the fitness of our participants (young, active military) suggests that socket discomfort may also contribute to knee power reduction. The vasti muscles provide braking during early stance (Neptune et al., 2004) and contribute negatively to the external moment in the sagittal plane (Neptune and McGowan, 2011) during level walking. Thus, the reduction in knee extensor power absorption may contribute to the more positive time rate of change of H in individuals with TTA early in the gait cycle.
On the steepest incline of +10°, the peak anterior moment arm was greater in the prosthetic limb of individuals with TTA than in able-bodied individuals, which suggested spatiotemporal asymmetry and contributed to a more positive external moment during early prosthetic stance. Conversely, the functional loss of the gastrocnemius in individuals with TTA may contribute to the more negative rate of change of H later in prosthetic stance (~30-50% gait cycle) as this muscle contributes to positive H during late stance (Neptune and McGowan, 2011). These gait alterations in individuals with TTA contributed to an overall greater range of sagittal-plane H during 0-50% gait cycle.
Individuals with TTA had greater peak hip power generation than able-bodied people on inclines, regardless of prosthesis type. Hip compensation strategies in individuals with TTA have been observed on level-ground (Silverman et al., 2008; Winter and Sienko, 1988) and in stair ascent (Aldridge et al., 2012; Yack et al., 1999). Our findings suggest that individuals with TTA rely heavily on the hip extensors for walking on inclines. The gluteus maximus contributes to a positive external moment and thus potentially a greater range of H (Neptune and McGowan, 2011) in early stance, suggesting that hip compensations may adversely affect dynamic balance.
Our second hypothesis, that the powered prosthesis would reduce sagittal-plane H compared to a passive prosthesis, was partially supported. Individuals with TTA had lower sagittal-plane range of H during 0-50% gait cycle on all slopes except +10° when using a powered compared to passive prosthesis, suggesting improved ability to regulate H with a powered prosthesis. The powered prosthesis provided significantly more braking than the passive prosthesis, which would contribute to a negative external moment. Increased braking may be at least partially due to the increased ankle power absorption in the powered prosthesis during controlled plantarflexion just after heel-strike (Eilenberg et al., 2010). Passive prostheses lack an ankle joint and cannot perform controlled plantarflexion. Instead, the heel is compressed in early stance, resulting in reduced foot-ground contact area that may adversely affect the ability to generate normative braking force without slipping.
While the range of sagittal-plane H was reduced during 0-50% gait cycle on most slopes when using the powered compared to the passive prosthesis, the range of sagittal-plane H was higher than when using the passive prosthesis during 50-100% gait cycle (Figure 3). The increase in sagittal-plane H is likely due to the higher mass of the powered compared to passive prosthesis, and may suggest increased fall risk. However, H can be sensitive to prosthesis inertial property assumptions during swing when the limb velocity is high. While we accounted for the lower mass of the passive prosthesis based on the literature, this study is limited by assumptions about the inertial properties of the residual limb and both prostheses, which are difficult to measure directly. Greater masses in the prosthetic limb will increase positive H during swing.
Throughout the discussion we have linked a greater range of H to an adverse effect of increased fall risk. While the relationship between H and the required external moment about the body COM is clear, the increases in H in individuals with TTA may be necessary for the movement task. That is, greater ranges of H present a challenge in maintaining dynamic balance, but are not necessarily detrimental given the competing factors that drive movement strategies (e.g., reducing metabolic cost, minimizing fatigue, controlling balance, etc.). Determining the maximum threshold for H that suggests an impending fall is difficult to determine because the available external moment to control H depends on the environment and individual neuromuscular capabilities, and is outside the scope of the current study. However, determining such a threshold is an important area for future work that could help identify safe movement strategies that also meet task requirements. Regardless of these factors, larger ranges of H suggest a greater fall risk if an unexpected perturbation were to occur.
We chose to analyze the range of sagittal-plane H during 0-50% and 50-100% of the prosthetic limb gait cycle rather than comparing prosthetic stance and swing. Note that this division does not imply temporal symmetry in individuals with TTA, but rather describes the sagittal-plane H curve that is characterized by two peaks (Figure 2). Dividing the gait cycle in half is consistent with previous analyses of H in individuals with TTA (Pickle et al., 2014; Silverman and Neptune, 2011) and highlights the contributions of both the prosthetic and intact limbs to controlling H through their interaction with the ground. The results would be largely unchanged when analyzing the entire stance phase for each leg. In addition, while our analysis of the range of H indicated few significant differences in the frontal and transverse planes, an analysis of signal shape may reveal further differences. For example, the upper body influences transverse H (Herr and Popovic, 2008), and further investigation may identify changes in upper body movement between prosthesis conditions. Furthermore, we controlled walking speed at a Froude number of 0.16, which was near the average self-selected walking speed of the participants. We elected to control walking speed to eliminate it as a confounding factor in the analysis. The magnitude of normalized H is smaller with increasing walking speed (Bennett et al., 2010; Silverman and Neptune, 2011). Because the participants with TTA were taller, their absolute walking speed was larger, thus biasing their normalized H results smaller. The results for a greater range of H during prosthetic limb stance are thus expected to be even stronger if absolute walking speed, rather than Froude speed, was held constant.
V. CONCLUSION
We used H to evaluate dynamic balance during sloped walking in individuals with TTA using powered and passive prostheses. Compared to able-bodied individuals, individuals with TTA did not decrease their range of sagittal-plane H to the same extent on declines and had a larger relative increase in the range of sagittal-plane H from 0° to +10°. These findings suggest that, regardless of prosthesis type, individuals with TTA may have greater difficulty restoring balance after a trip or slip on slopes relative to able-bodied individuals, particularly during prosthetic stance when there is a reduced ability to respond to disturbances. Controlled plantarflexion, ankle power generation, and inertial properties similar to a biological limb in a powered prosthesis may provide improvements in the regulation of H for certain sloped walking conditions. However, despite the greater prosthetic ankle power generation when using a powered prosthesis, differences in joint powers and H persist when compared to able-bodied individuals. Thus, restoring ankle power alone may not be sufficient for restoring the regulation dynamic balance to that of able-bodied individuals on slopes. Future research should investigate whether knee extensor strengthening and socket refinements can help restore normative ranges of H. In addition, further investigation into muscle contributions, such as the biarticular gastrocnemius, to H during sloped walking would help identify functional deficits in individuals with TTA.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R03HD075946. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed herein are those of the authors and do not reflect the official policy or position of San Antonio Military Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense and/or the U.S. Government. Additional support was provided by the Center for Rehabilitation Sciences Research, Department of Physical Medicine and Rehabilitation, Uniformed Services University of Health Sciences, Bethesda, MD. The authors would also like to acknowledge Audrey Westbrook and Kelly Ohm for their extensive contributions in data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
There was no conflict of interest in the preparation or publication of this work.
REFERENCES
- Aldridge JM, Sturdy JT, Wilken JM. Stair ascent kinematics and kinetics with a powered lower leg system following transtibial amputation. Gait Posture. 2012;36:291–295. doi: 10.1016/j.gaitpost.2012.03.013. doi:10.1016/j.gaitpost.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Au SK, Weber J, Herr HM. Biomechanical Design of Powered Ankle-Foot Prosthesis. 2007 IEEE 10th International Conference on Rehabilitation Robotics. 2007:298–303. [Google Scholar]
- Bennett BC, Russell SD, Sheth P, Abel MF. Angular momentum of walking at different speeds. Hum. Mov. Sci. 2010;29:114–24. doi: 10.1016/j.humov.2009.07.011. doi:10.1016/j.humov.2009.07.011. [DOI] [PubMed] [Google Scholar]
- D'Andrea S, Wilhelm N, Silverman AK, Grabowski AM. Does Use of a Powered Ankle-foot Prosthesis Restore Whole-body Angular Momentum During Walking at Different Speeds? Clin. Orthop. Relat. Res. 2014;472:3044–3054. doi: 10.1007/s11999-014-3647-1. doi:10.1007/s11999-014-3647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Medicine & Science in Sports & Exercise. 1995;27(12):1692–1697. [PubMed] [Google Scholar]
- Eilenberg MF, Geyer H, Herr HM. Control of a powered ankle-foot prosthesis based on a neuromuscular model. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18:164–73. doi: 10.1109/TNSRE.2009.2039620. doi:10.1109/TNSRE.2009.2039620. [DOI] [PubMed] [Google Scholar]
- Esposito ER, Whitehead JMA, Wilken JM. Step-to-step transition work during level and inclined walking using passive and powered ankle-foot prostheses. Prosthet. Orthot. Int. 2015 doi: 10.1177/0309364614564021. doi:10.1177/0309364614564021. [DOI] [PubMed] [Google Scholar]
- Ferris AE, Aldridge JM, Rábago CA, Wilken JM. Evaluation of a powered ankle-foot prosthetic system during walking. Arch. Phys. Med. Rehabil. 2012;93:1911–8. doi: 10.1016/j.apmr.2012.06.009. doi:10.1016/j.apmr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Gates DH, Aldridge JM, Wilken JM. Kinematic comparison of walking on uneven ground using powered and unpowered prostheses. Clin. Biomech. (Bristol, Avon) 2013;28:467–72. doi: 10.1016/j.clinbiomech.2013.03.005. doi:10.1016/j.clinbiomech.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Herr HM, Grabowski AM. Bionic ankle-foot prosthesis normalizes walking gait for persons with leg amputation. Proc. Biol. Sci. 2012;279:457–64. doi: 10.1098/rspb.2011.1194. doi:10.1098/rspb.2011.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr HM, Popovic M. Angular momentum in human walking. J. Exp. Biol. 2008;211:467–81. doi: 10.1242/jeb.008573. doi:10.1242/jeb.008573. [DOI] [PubMed] [Google Scholar]
- Kuster M, Sakurai S, Wood GA. Kinematic and kinetic comparison of downhill and level walking. Clin. Biomech. 1995;10:79–84. doi: 10.1016/0268-0033(95)92043-l. doi:10.1016/0268-0033(95)92043-L. [DOI] [PubMed] [Google Scholar]
- Lange GW, Hintermeister RA, Schlegel T, Dillman CJ, Steadman R. Electromyographic and kinematic analysis of graded treadmill walking and the implications for knee rehabilitation. J. Orthop. Sports Phys. Ther. 1996;23:294–301. doi: 10.2519/jospt.1996.23.5.294. doi:10.2519/jospt.1996.23.5.294. [DOI] [PubMed] [Google Scholar]
- Langlois K, Villa C, Bonnet X, Lavaste F, Fodé P, Martinet N, Pillet H. Influence of physical capacities of males with transtibial amputation on gait adjustments on sloped surfaces. J. Rehabil. Res. Dev. 2014;51:193–200. doi: 10.1682/JRRD.2013.05.0118. doi:10.1682/JRRD.2013.05.0118. [DOI] [PubMed] [Google Scholar]
- Lay AN, Hass CJ, Richard Nichols T, Gregor RJ, Nichols TR. The effects of sloped surfaces on locomotion: an electromyographic analysis. J. Biomech. 2007;40:1276–85. doi: 10.1016/j.jbiomech.2006.05.023. doi:10.1016/j.jbiomech.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Lenth R, Hervé M. lsmeans: Least-Squares Means [WWW Document]. R Packag. version 2.16. 2015 URL http://cran.r-project.org/package=lsmeans.
- Leroux A, Fung J, Barbeau H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp. Brain Res. 1999;126:359–368. doi: 10.1007/s002210050743. doi:10.1007/s002210050743. [DOI] [PubMed] [Google Scholar]
- Markowitz J, Krishnaswamy P, Eilenberg MF, Endo K, Barnhart C, Herr H. Speed adaptation in a powered transtibial prosthesis controlled with a neuromuscular model. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:1621–1631. doi: 10.1098/rstb.2010.0347. doi:10.1098/rstb.2010.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo-random oscillations of the support surface and visual field. J. Biomech. 2010;43:1470–5. doi: 10.1016/j.jbiomech.2010.02.003. doi:10.1016/j.jbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, McGowan CP. Muscle contributions to whole-body sagittal plane angular momentum during walking. J. Biomech. 2011;44:6–12. doi: 10.1016/j.jbiomech.2010.08.015. doi:10.1016/j.jbiomech.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19:194–205. doi: 10.1016/S0966-6362(03)00062-6. doi:10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Nott CR, Neptune RR, Kautz SA. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture. 2013:129–34. doi: 10.1016/j.gaitpost.2013.06.008. in press doi:10.1016/j.gaitpost.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickle NT, Wilken JM, Aldridge JM, Neptune RR, Silverman AK. Whole-body angular momentum during stair walking using passive and powered lower-limb prostheses. J. Biomech. 2014;47:3380–9. doi: 10.1016/j.jbiomech.2014.08.001. doi:10.1016/j.jbiomech.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieën JH. Contribution of the support limb in control of angular momentum after tripping. J. Biomech. 2004;37:1811–8. doi: 10.1016/j.jbiomech.2004.02.038. doi:10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models. 2015 [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: 2012. Retrieved from http://www.r-project.org. [Google Scholar]
- Redfern MS, Cham R, Gielo-Perczak K, Gronqvist R, Hirvonen M, Lanshammar H, Marpet M, Pai CY-C, Powers CM. Biomechanics of slips. Ergonomics. 2001;44:1138–1166. doi: 10.1080/00140130110085547. doi:10.1080/0014013011008554. [DOI] [PubMed] [Google Scholar]
- Redfern MS, Dipasquale J. Biomechanics of descending ramps. Gait Posture. 1997;6:119–125. doi:10.1016/S0966-6362(97)01117-X. [Google Scholar]
- Sanderson D, Martin P. Lower extremity kinematic and kinetic adaptations in unilateral below-knee amputees during walking. Gait Posture. 1997;6:126–136. [Google Scholar]
- Silverman AK, Fey NP, Portillo A, Walden JG, Bosker G, Neptune RR. Compensatory mechanisms in below-knee amputee gait in response to increasing steady-state walking speeds. Gait Posture. 2008;28:602–9. doi: 10.1016/j.gaitpost.2008.04.005. doi:10.1016/j.gaitpost.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Silverman AK, Neptune RR. Muscle and prosthesis contributions to amputee walking mechanics: a modeling study. J. Biomech. 2012;45:2271–8. doi: 10.1016/j.jbiomech.2012.06.008. doi:10.1016/j.jbiomech.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Silverman AK, Neptune RR. Differences in whole-body angular momentum between below-knee amputees and non-amputees across walking speeds. J. Biomech. 2011;44:379–385. doi: 10.1016/j.jbiomech.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Silverman AK, Wilken JM, Sinitski EH, Neptune RR. Whole-body angular momentum in incline and decline walking. J. Biomech. 2012;45:965–71. doi: 10.1016/j.jbiomech.2012.01.012. doi:10.1016/j.jbiomech.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Smith JD, Ferris AE, Heise GD, Hinrichs RN, Martin PE. Oscillation and reaction board techniques for estimating inertial properties of a below-knee prosthesis. J. Vis. Exp. 2014:1–16. doi: 10.3791/50977. doi:10.3791/50977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken JM, Rodriguez KM, Brawner M, Darter BJ. Reliability and Minimal detectible change values for gait kinematics and kinetics in healthy adults. Gait Posture. 2012;35:301–307. doi: 10.1016/j.gaitpost.2011.09.105. doi:10.1016/j.gaitpost.2011.09.105. [DOI] [PubMed] [Google Scholar]
- Winter DA, Sienko SE. Biomechanics of below-knee amputee gait. J. Biomech. 1988;21:361–367. doi: 10.1016/0021-9290(88)90142-x. [DOI] [PubMed] [Google Scholar]
- Yack HJ, Nielsen DH, Shurr DG. Kinetic Patterns During Stair Ascent in Patients with Transtibial Amputations Using Three Different Prostheses. J. Prosthetics Orthot. 1999;11:57–62. [Google Scholar]