ABSTRACT
The incidence of melanoma continues to rise with the most rapid increase seen in the elderly population. Historically, elderly patients with advanced melanoma have had dismal clinical outcomes, in part, due to distinct tumor biology, and often ineligibility for effective therapies during their development. In addition, due to relatively few geriatric patients being accrued to clinical trials of novel immunotherapeutics, there is a paucity of data regarding their safety and efficacy. Herein, we present the clinical course of three consecutive nonagenarians (≥90 y old) with metastatic melanoma, who were treated with single-agent or combination immune checkpoint inhibitors. Two patients experienced complete or partial responses with acceptable safety profiles, and one other tolerated therapy well although a significant response was not noted. These cases suggest that with close monitoring, even very elderly patients with advanced cancers and acceptable performance status may tolerate and benefit from immune checkpoint inhibitors.
KEYWORDS: Advanced melanoma, elderly, immunotherapy, ipilimumab, nivolumab, pembrolizumab
Introduction
Over the past 30 y, the incidence of melanoma has risen greater than threefold in the United States. The most rapid increase has occurred in the elderly male population compared to other demographic subsets.1-3 Although melanoma is potentially curable in its early stages following surgical resection, in the metastatic setting, traditional chemotherapy is associated with extremely poor outcomes, particularly for elderly patients and those with co-morbidities.4 The development of immune checkpoint inhibitors has resulted in the expansion of treatment options. Ipilimumab,5-7 a monoclonal antibody to cytotoxic T cell antigen 4 (CTLA4), and nivolumab and pembrolizumab, agents that inhibit the programmed cell death 1 receptor (PD-1),8-14 have all shown superior clinical outcomes over standard chemotherapy with a superior toxicity profile. Moreover, these studies have demonstrated benefits even in patients >75 y of age. Despite these therapeutic advances, the safety and efficacy of immune checkpoint inhibitors in very elderly (>80 y old) patients is not clear. In this report, the clinical course and efficacy of immune therapy in three consecutive nonagenarians (≥90 y old) with metastatic melanoma is presented and discussed. To our knowledge, this is the first report of nonagenarians undergoing successful immunotherapy for metastatic melanoma.
Case presentation
Patient 1
A 90-y-old Caucasian woman was initially seen in the Otolaryngology clinic at the age of 88 in 2013 for unremitting epistaxis. She was found to have a 3 cm fleshy mass in the left nasal cavity and underwent an excisional biopsy, revealing malignant mucosal melanoma with lymphovascular invasion, elevated mitotic rate (13 mitoses per mm2), and lack of infiltrating lymphocytes. Mutational testing revealed no mutations in BRAF, NRAS, and CKIT. A positron emission tomography-computed tomography (PET-CT) scan showed no evidence of distant disease. Following resection, she underwent adjuvant radiation therapy; follow-up PET-CT showed moderately fluorodeoxyglucose (FDG)-avid sinonasal tissue concerning for residual disease. She underwent revision surgery showing residual melanoma in situ. She did well with subsequent surveillance for approximately 1.5 y until PET-CT confirmed stage IV M1c disease with numerous FDG-avid lung, liver, and osseous metastases (at age 90) (Fig. 1A). She was essentially asymptomatic at this time but had an elevated lactate dehydrogenase (LDH) (499 unit/L; normal <226 unit/L) and alkaline phosphatase (237 unit/L; normal range 40–190 unit/L). Magnetic resonance imaging (MRI) of the brain was negative for intracranial metastasis. She lived independently, had concurrent mild hypertension and osteoarthritis, and had an Eastern Cooperative Oncology Group (ECOG) performance status of 2. In view of the poor responses reported for single-agent ipilimumab with mucosal melanoma,15 she was started on combination ipilimumab 3 mg/kg and nivolumab 1 mg/kg every 3 weeks. She received two of the four planned combination doses secondary to grade 2 hepatitis and mucositis that were successfully treated with a 4-week taper of high-dose corticosteroids. An interim PET-CT performed following resolution of her toxicity, demonstrated a complete response (CR; Fig. 1B) as defined by Response Criteria In Solid Tumors (RECIST). She also had normalization of her LDH and alkaline phosphatase. She resumed single-agent nivolumab and has completed 22 doses of therapy as of July 2016, constituting a total of 14 months of anti-PD-1 therapy. A repeat PET-CT done in June 2016 showed ongoing CR. Her performance status improved to 1. She continues to tolerate single-agent nivolumab well with stable anemia, grade 1 rash, and no recurrence of hepatitis or mucositis.
Figure 1.
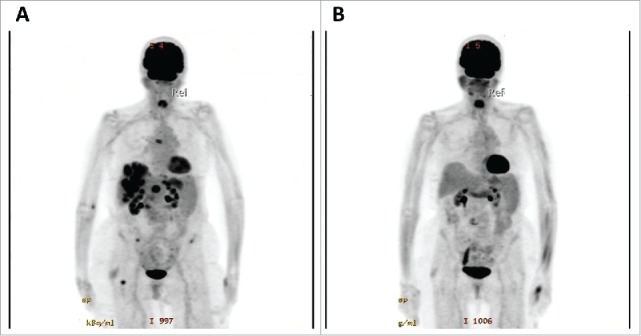
Patient 1 with FDG-avid lung, liver, and osseous metastases prior to treatment (A), and demonstrating complete resolution of metastases following two treatments of combination ipilimumab and nivolumab (B).
Patient 2
An 85-y-old Caucasian female, initially had a history of stage IB (pT2aN0M0) melanoma on her right calf, resected in 2005. Pathology revealed a non-ulcerated superficial spreading malignant melanoma, 1.9 mm in thickness, Clark's level III, no lymphovascular invasion, and 4 mitoses per mm2. Two sentinel lymph nodes were sampled and were negative. She subsequently did well until the spring of 2015, when she developed right inguinal swelling. A PET-CT showed right iliac and inguinal adenopathy as well as nodular lesions in the distal and medial right thigh; biopsy of an inguinal lymph node confirmed melanoma (stage IV M1a). A CT head was negative for metastatic disease. She was evaluated by surgical oncology and was deemed to be unresectable. Her LDH was elevated (299 unit/L; normal <226 unit/L) and her melanoma was BRAF wild type. She had an ECOG performance status of 1 and lived independently. She also had a history of resected early-stage colon cancer many years ago. Given her excellent performance status, she was treated with ipilimumab every 3 weeks, beginning at age 94 y (prior to the approval of anti-PD-1 agents). Although she tolerated therapy well, PET-CT after four doses of ipilimumab showed progressive disease. She was then started on pembrolizumab at 2 mg/kg every 3 weeks. CT imaging after completion of three cycles of therapy revealed a partial response (PR). She developed pneumonia in January 2016 and pembrolizumab was withheld for 2 mo, but she has since resumed therapy and has completed 10 mo of pembrolizumab without progressive disease.
Patient 3
An 88-y-old Caucasian female initially presented to a small community hospital in 2013 with an invasive left lateral anal mucosal melanoma that was 1.7 mm deep with ulceration and had 17 mitoses per mm2. Gene mutation screening revealed no mutations in BRAF or KIT. PET imaging showed no metastases. Prior to referral to our center, a conservative local excision was performed, but further invasive procedures including sentinel lymph node biopsy were omitted given her age. One year later she developed an externally palpable local recurrence along the posterior anal mucosa, which was excised with negative margins. PET imaging was again negative. Two months thereafter, a pelvic MRI revealed a 1.9 cm diameter endocervical mass with a 1 cm diameter extension into the posterior vaginal canal with biopsy demonstrating metastatic melanoma. A brain MRI was normal, and she was asymptomatic aside from occasional vaginal spotting. Neither surgery nor radiotherapy was believed to have curative potential, and each was expected to produce significant morbidity in this elderly patient. She received four standard doses of ipilimumab at 3 mg/kg IV at 3-week intervals and experienced only mild fatigue, but no other immune-related adverse events. One month following the last ipilimumab dose, restaging pelvic MRI and chest CT revealed progression of the endocervical and upper vaginal masses. Two new non-calcified right lung nodules up to 1.1 cm were also noted. Single agent pembrolizumab was initiated and CT scans of the chest, abdomen and pelvis 3 weeks after the fourth dose showed resolution of the lung nodules, but with persistent melanoma involving the cervix and vagina. After six doses of pembrolizumab, a pelvic MRI revealed regional progression of the endocervical and vaginal mass from 5.0 to 7.8 cm and 2.7 to 2.9 cm, respectively. Palliative radiation was delivered to the cervix and vagina with 50 Gy in 20 fractions and pembrolizumab was continued uninterrupted, with no toxicity aside from mild fatigue. After 10 doses of pembrolizumab, pelvic MRI showed partial response of the cervical and vaginal metastases to radiotherapy, but a CT scan of the chest, abdomen and pelvis revealed a new indeterminate 1.4 cm solitary hepatic lesion. The patient's performance status remained excellent so pembrolizumab was continued for a total of 14 doses after which CT imaging demonstrated multiple new subcentimeter hepatic lesions and enlargement of the previously noted hepatic focus from 1.4 to 2.0 cm consistent with worsening hepatic metastases. Thus, after 10 mo on pembrolizumab, and 14 total months on checkpoint inhibitors, immunotherapy was discontinued. She is maintaining an excellent performance status and is currently participating in a clinical trial.
Discussion
The treatment of elderly patients with melanoma is of great importance due to its frequency and rising incidence.16 In particular, older males had a 157% increase in melanoma mortality between 1969 and 1999.2-3 In one retrospective analysis,17 overall mortality and disease-specific mortality at 5 y was statistically inferior in patients ≥70 y of age. Advancing age is associated with more aggressive biologic behavior, as well as a lower percentage of patients with BRAF mutation, especially V600E BRAF mutation, which is the most sensitive to targeted therapies. In several studies, increasing age was significantly associated with poor prognostic markers of Breslow thickness, ulceration, and male gender.17-21 Moreover, with the anticipated growth in the elderly population, the number of older melanoma patients seeking treatment is predicted to lead to increased healthcare costs and utilization of resources,22 underscoring the importance of optimal management of melanoma in the elderly.
The landscape of therapeutic options for the treatment of metastatic melanoma has evolved rapidly since 2011 when the first BRAF inhibitor (vemurafenib) and immune checkpoint inhibitor (ipilimumab) were FDA-approved. The advent of immunotherapies that deliver largely tolerable side effects and durable responses in a sizable fraction of patients has been a major advance. Unfortunately, there is a relative dearth of information available regarding the safety and efficacy of immune checkpoint inhibitors in older patients secondary to fewer geriatric patients participating in clinical trials.23-24 In addition, increasing age is associated with many challenges, including the inferior prognosis associated with aging mentioned above, frequent medical comorbidities, decreased organ reserve, and changes in pharmacokinetics and pharmacodynamics that may limit therapeutic options.25 Historical melanoma therapies have a very poor risk/benefit profile in the elderly. Cytotoxic chemotherapy has minimal clinical activity and an onerous toxicity profile.26 Cytokine therapies such as high-dose interleukin-2 (IL-2) and interferon have severe acute and bothersome chronic side effects, respectively, and are thus very poorly tolerated in the elderly. Moreover, other factors unique to the elderly population that may negatively impact outcome include delayed diagnosis, frailty, diminished functional reserve, decreased participation in preventive screening programs, and loss of spouse.27-28 Therefore, many physicians may be hesitant to recommend aggressive treatment, systemic therapies or clinical trials to elderly patients.
However, despite these many challenges, a subset of older patients with metastatic melanoma have relatively good health and functional status, and may be suitable candidates for immune checkpoint inhibitors. Limited pre-clinical studies have suggested that aging may result in an imbalanced immune system (between native and adaptive arms) that favors antitumor activity.29 Clinical trials have confirmed that elderly patients with advanced melanoma do respond to immunotherapies such as tumor vaccines, and anti-CTLA4 therapy5,30,31 Ipilimumab has shown an improvement in overall survival (OS) in advanced melanoma compared to an experimental peptide vaccine5 and a 5-y OS of ∼20%.32 One subgroup analysis suggested equivalent benefits in patients >70 y.6 However, secondary to its mechanism of action, ipilimumab may induce immune-mediated adverse events such as hepatitis, dermatitis, colitis, pneumonitis, and endocrinopathies in 20–30% of patients.5 This toxicity profile demands careful patient selection especially among elderly patients or those with multiple co-morbidities. Treatment with the newer anti-PD-1 monoclonal antibodies such as nivolumab and pembrolizumab is associated with response rates of 25–45%, many of which are durable, and superior to those observed with chemotherapy13 or ipilimumab. Furthermore, compared to ipilimumab, these anti-PD-1 agents are associated with lower rates of immune-related toxicities, with severe side effects documented in 5–10% of treated patients. Importantly, these studies have demonstrated that elderly patients (even >75 y) benefit at least as much as the younger cohort.12,14,33 More recently, combination therapy with nivolumab and ipilimumab has proven to be superior to monotherapy,34 with an objective response rate of 58% and improved progression-free survival (PFS) (median PFS 11.5 mo). A landmark phase III trial enrolled 118 (12.5%) patients ≥75 y.34 However, a subgroup analysis looking at efficacy in the elderly population has not yet been conducted.
In this case report, we describe the treatment of metastatic melanoma with immune checkpoint inhibitors in the only three nonagenarians treated at two centers. All three patients were Caucasian females, which may reflect the demographics of patients over age 90 (female preponderance) and those at risk for melanoma (predominately Caucasian compared with other racial and ethnic groups).35 Although having a chronological age in the 90s, their functional status was relatively preserved with complete independence in self-care and other activities of daily living. Two patients had classical, RECIST-defined responses to therapy, and the other had prolonged stable disease. This is particularly impressive considering two of the patients had mucosal melanoma which carries a worse prognosis and where response rates to checkpoint inhibitors are less well defined. One patient was treated with combination ipilimumab and nivolumab due to her poor prognostic indicators (elevated LDH, bulky stage IV M1c disease, and mucosal primary). However, we usually prefer single-agent anti-PD-1 for very elderly patients (>80 y) given the high likelihood of toxicity with combination therapy and decreased functional reserve of this subset of patients, although large trials are required to verify efficacy and toxicity of each regimen in the elderly. Interestingly, a CR and PR were seen in two patients, who also had significant immune-mediated adverse events (hepatitis, mucositis, rash, and pneumonia), compared to stable disease in the third patient, who had only mild fatigue. Studies have shown a positive correlation between response to checkpoint inhibitors and the development of immune-related adverse events.36-38 Although the first patient did receive high-dose corticosteroids for immune-related hepatitis, the use of high-dose steroids and other more potent immunosuppression may be a challenge in elderly patients with multiple co-morbidities. At the time of this report, one patient on anti-PD-1 therapy for 14 mo had an ongoing CR. Although the duration of therapy is unknown, a recent study39 suggests that a subset of patients may maintain a durable response after discontinuation of treatment at CR. Ultimately, with close monitoring, and appropriate management of side effects, even very elderly patients with acceptable performance status and advanced melanoma may benefit from immune checkpoint inhibitors.
Disclosure of potential conflicts of interest
Dr. Johnson is on advisory boards for BMS and Genoptix. Dr. Conry is on the Merck Speakers Bureau. Dr. Sosman is on advisory boards for Merck and Array. Drs. Johnpulle and Puzanov report no relevant conflicts of interest.
References
- 1.Tsai S, Balch C, Lange J. Epidemiology and treatment of melanoma in elderly patients. Nat Rev Clin Oncol 2010; 7(3):148-52; PMID:20142815; http://dx.doi.org/ 10.1038/nrclinonc.2010.1 [DOI] [PubMed] [Google Scholar]
- 2.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969-1999. JAMA 2002; 288(14):1719-20; PMID:12365954; http://dx.doi.org/ 10.1001/jama.288.14.1719 [DOI] [PubMed] [Google Scholar]
- 3.Howe HL, Wingo PA, Thun MJ, Ries LA, Rosenberg HM, Feigal EG, Edwards BK. Annual Report to the Nation on the Status of Cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst 2001; 93(11):824-42; PMID:11390532; http://dx.doi.org/ 10.1093/jnci/93.11.824 [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med 2004; 351(10):998-1012; PMID:15342808; http://dx.doi.org/ 10.1056/NEJMra041245 [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrocal A, Arance A, Lopez Martin JA, Soriano V, Muñoz E, Alonso L, Espinosa E, Lopez Criado P, Valdivia J, Martin Algarra S. Ipilimumab for advanced melanoma: experience from the Spanish expanded access program. Melanoma Res 2014; 24(6):577-83; PMID:25046550; http://dx.doi.org/ 10.1097/CMR.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33(17):1889-94; PMID:25667295; http://dx.doi.org/ 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366(26):2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS et al.. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369(2):134-44; PMID:23724846; http://dx.doi.org/ 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC et al.. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomized dose-comparison cohort of a phase 1 trial. Lancet 2014; 384(9948):1109-17; PMID:25034862; http://dx.doi.org/ 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 11.Weber JS, Kudchadkar RR, Gibney GT, De Conti RC, Yu B, Wang W, Sarnaik A, Martinez AJ, Kroeger J, Eysmans C et al.. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. J Clin Oncol 2013; 31(18):2233-365; ASCO Annual Meeting (suppl; abstr 9011) [Google Scholar]
- 12.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372(4):320-30; PMID:25399552; http://dx.doi.org/ 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 13.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD et al.. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16(4):375-84; PMID:25795410; http://dx.doi.org/ 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 14.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD et al.. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16(8):908-18; PMID:26115796; http://dx.doi.org/ 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postow MA, Luke JJ, Bluth MJ, Ramalya N, Panageas KS, Lawrence DP, Ibrahim N, Flaherty KT, Sullivan RJ, Ott PA et al.. Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013; 18(6):762-32; PMID:23716015; http://dx.doi.org/19131946 10.1634/theoncologist.2012-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol 2009; 129(7):1666-74; PMID:19131946; http://dx.doi.org/ 10.1038/jid.2008.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald JB, Dueck AC, Gray RJ, Wasif N, Swanson DL, Sekulic A, Pockaj BA. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer 2011; 2:538-43; PMID:22084644; http://dx.doi.org/ 10.7150/jca.2.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao C, Martin RCG II, Ross MI, Reintgen DS, Edwards MJ, Noyes RD, Hagendoorn LJ, Stromberg AJ, McMasters KM. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol 2004; 11(3):259-64; PMID:14993020; http://dx.doi.org/ 10.1245/ASO.2004.04.015 [DOI] [PubMed] [Google Scholar]
- 19.Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol 1994; 1(6):487-94; PMID:7850555; http://dx.doi.org/ 10.1007/BF02303614 [DOI] [PubMed] [Google Scholar]
- 20.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM et al.. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001; 19(16):3622-34; PMID:11504744 [DOI] [PubMed] [Google Scholar]
- 21.Cohen HJ, Cox E, Manton K, Woodbury M. Malignant melanoma in the elderly. J Clin Oncol 1987; 5(1):100-6; PMID:3806154 [DOI] [PubMed] [Google Scholar]
- 22.Seidler AM, Pennie ML, Veledar E, Culler SD, Chen SC. Economic burden of melanoma in the elderly population: population-based analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare data. Arch Dermatol 2010; 146(3):249-56; PMID:20231494; http://dx.doi.org/ 10.1001/archdermatol.2009.389 [DOI] [PubMed] [Google Scholar]
- 23.Denson AC, Mahipal A. Participation of the elderly population in clinical trials. Cancer Control 2014; 21(3):209-14; PMID:24955704 [DOI] [PubMed] [Google Scholar]
- 24.Shenoy P, Harugeri A. Elderly patients' participation in clinical trials. Perspect Clin Res 2015; 6(4):184-9; PMID:26623388; http://dx.doi.org/ 10.4103/2229-3485.167099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57(1):6-14; PMID:14678335; http://dx.doi.org/ 10.1046/j.1365-2125.2003.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A et al.. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000; 18(1):158-66; PMID:10623706 [DOI] [PubMed] [Google Scholar]
- 27.Ortiz CA, Goodwin JS, Freeman JL. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med Sci Monit 2005; 11(5):163-72; PMID:1587490717702882 [PubMed] [Google Scholar]
- 28.Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin JS. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol A Biol Sci Med Sci 2007; 62(8):892-8; PMID:17702882; http://dx.doi.org/ 10.1093/gerona/62.8.892 [DOI] [PubMed] [Google Scholar]
- 29.Hegde UP, Chakraborty N, Mukherji B, Grant Kels JM. Metastatic melanoma in the older patient: immunologic insights and treatment outcomes. Expert Rev Pharmacoeconomics Outcomes Res 2011; 11(2):185-193; http://dx.doi.org/ 10.1586/erp.11.14 [DOI] [PubMed] [Google Scholar]
- 30.Tagawa ST, Cheung E, Banta W, Gee C, Weber JS. Survival analysis after resection of metastatic disease followed by peptide vaccines in patients with stage IV melanoma. Cancer 2006; 106(6):1353-7; PMID:16475151; http://dx.doi.org/ 10.1002/cncr.21748 [DOI] [PubMed] [Google Scholar]
- 31.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA et al.. Anti-tumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 2005; 23(35):8968-77; PMID:16204013; http://dx.doi.org/ 10.1200/JCO.2005.01.109 [DOI] [PubMed] [Google Scholar]
- 32.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33(17):1889-94; PMID:25667295; http://dx.doi.org/ 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M et al.. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 2015; 372(26):2521-32; PMID:25891173; http://dx.doi.org/ 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schaderdorf D, Dummer R, Smylie M, Rutkowski P et al.. Combined nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1):23-34; PMID:26027431; http://dx.doi.org/ 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanoma in the US. Cancer 2005; 103(5):1000-7; PMID:15651058; http://dx.doi.org/ 10.1002/cncr.20866 [DOI] [PubMed] [Google Scholar]
- 36.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014; 99(11):4078-85; PMID:25078147; http://dx.doi.org/ 10.1210/jc.2014-2306 [DOI] [PubMed] [Google Scholar]
- 37.Lutzky J, Wolchok J, Hamid O, Lebbe C, Pehamberger H, Linette G, de Pril V, Ibrahim R, Hoos A, O'Day S. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials. J Clin Oncol 2009; 27(15):2417-574(15s); ASCO Annual Meeting (suppl; abstr 9034); PMID:1907525419075254 [Google Scholar]
- 38.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE et al.. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treatment with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005; 23(25):6043-6053; PMID:16087944; http://dx.doi.org/ 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua M, Hwu W-J, Weber JS, Gangadhar TC, Joseph RW et al.. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 2016; 34(18):533-2198; ASCO Annual Meeting (suppl; abstr 9503); PMID:2695131026951310 [Google Scholar]


