ABSTRACT
Therapeutic blockade of PD-1/PD-L1 can have dramatic therapeutic benefit in some patients; however, the prognostic associations of PD-1 and its ligands, in the absence of therapeutic blockade have not been definitively addressed. In particular, associations of PD-L2 with immune infiltrates and with outcome have yet to be explored. We hypothesized that surface expression of both PD-L1 and PD-L2 by melanoma cells would be associated with immune cell infiltration and with overall patient survival, independent of checkpoint blockade therapy. We also characterized the heterogeneity of their distribution within a tumor and within tumors of the same patient. Tissue microarrays of metastatic melanoma samples from 147 patients were quantified for CD8+, CD45, CD4+, CD3, CD163, CD20, CD138, FoxP3, PD-1, PD-L1 and PD-L2 markers by immunohistochemistry. Relationships between the proportions of PD-L1 and PD-L2 expressing tumor cells with the immune cell count, distribution (immunotype) and patient survival were studied. Expressions of both PD-L1 and PD-L2 correlated significantly with increasing densities of immune cells in the tumor specimens and with immunotype. Positive PD-L2 expression was associated with improved overall survival and the simultaneous positive expression of both PD-1 ligands showed a higher association with survival. Significant heterogeneity of PD-L1 and PD-L2 expressions within tumors were observed, however, they were less pronounced with PD-L2. In conclusion, both are markers of immune infiltration and PD-L2, alone or in combination with PD-L1, is a marker for prognosis in metastatic melanoma patients. Larger tumor samples yield more reliable assessments of PD-L1/L2 expression.
KEYWORDS: Immune checkpoint, immune infiltrates, metastatic melanoma, patient outcomes, PD-L1, PD-L2, PD-1, tumor-infiltrating lymphocytes
Abbreviations
- AJCC
American Joint Committee on Cancer
- CD
cluster of differentiation
- CTLA4
cytotoxic T-cell antigen 4
- DAB
3,3′-Diaminobenzidine
- DC
dendritic cells
- DC-LAMP
DC lysosomal-associated membrane protein
- EDTA
ethylenediaminetetraacetic acid
- ETOH
ethanol
- FFPE
formalin-fixed paraffin-embedded
- FoxP3
Forkhead box P3
- LN
lymph node
- mAB
monoclonal antibody
- NED
no evidence of disease, clinically
- NK
natural killer
- PD-1
programmed cell death 1
- PD-L1
PD-1 ligand 1
- PD-L2
PD-1 ligand 2
- TCR
T cell receptor
- TMA
tissue microarray
- TME
tumor microenvironment
Introduction
Melanoma is an immunogenic tumor that is sporadically infiltrated by immune cells. Despite the presence of these immune cells, most melanomas generally continue to grow, indicating that the lymphocytic infiltrates usually fail to control tumor growth. However, the potential for T cells to control tumor outgrowth is evidenced by durable clinical regressions after adoptive transfer of in vitro expanded tumor antigen-specific T cells.1 This has suggested that the metastatic melanoma tumor microenvironment (TME) can suppress the function of the native immune response, resulting in tumor escape from immune-mediated destruction. It is now apparent that T cell responses can be constrained by several mechanisms. Among these, PD-1 is a checkpoint molecule with clinical relevance. Therapeutic blockade of PD-1 can induce dramatic and durable regression of metastatic melanoma and other cancers.2-4
PD-1 is a transmembrane protein that belongs to the CD28 family of the immunoglobulin superfamily and, within hematological populations, is expressed on T and B lymphocytes, NK and myeloid cells.5-8 Expression of PD-1 is induced on T cells shortly after TCR stimulation, and increased numbers of tumor infiltrating PD-1+ lymphocytes have been associated with prolonged survival of patients with metastatic melanoma.9 However, ligation of PD-1 can induce programmed cell death in lymphocytes.7,10,11 or it may induce downregulation of T-cell function.12 PD-1 expression has also been identified on a small fraction of melanoma cells, where its ligation promotes tumor growth.13 The known ligands for PD-1 are the B7 family molecules PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273),14-20 which are cell membrane-bound glycoproteins that share 40% amino acid homology to each other. In normal human tissues, PD-L1 is expressed by myeloid dendritic cells (DC), macrophages, placental trophoblasts, myocardial endothelium and cortical thymic epithelial cells,21,22 whereas PD-L2 is expressed by DC, macrophages, placental endothelium and medullary thymic epithelial cells.23,24 Binding of PD-1 to either ligand can inhibit T-cell proliferation and cytokine secretion.20 PD-L1 can be also be expressed by lymphocytes, and negatively regulates local immunity by inhibiting their activation through binding of CD8025,26 and inducing IL-10 production.16 In contrast to their inhibitory roles, binding of both ligands was found to co-stimulate the pro-inflammatory cytokine IFNγ.17,27 This contradiction could be explained by PD-L1 and PD-L2 ligation of additional receptors other than PD-1.28 Thus, expression of PD-L1 and PD-L2 on tumor cells and PD-L1 on immune cells all have the potential to impact tumor immunity.
Considerable evidence supports an inhibitory role of PD-1/PD-L1 on T-cell function in the TME. Increased expression of PD-L1 has been found in many human carcinomas, melanomas and glioblastomas.29-34 Its expression in the TME may be expected to reduce function of PD-1+ T cells and to have a negative impact on prognosis.35 On the other hand, PD-L1 expression can be induced by interferons secreted in the setting of active cellular immunity and therefore may be related to better patient prognosis.36,37 The associations of PD-L1 expression with patient prognosis and clinical characteristics remain controversial.35,37-39 PD-L1 expression by tumor cells is also associated with clinical response to PD-1 blockade38 and its use as a predictive biomarker for response to PD-1 blockade is encumbered by the spatial heterogeneity of the expression of PD-L1 in tumors.38 PD-L2 ligation also is generally thought to be immunosuppressive; 40,41 however, PD-L2+ B cells can protect against cancer through augmentation of Th1 and Th17 responses.42 There are few reports about PD-L2 expression in human malignant tumors32,43 and we are not aware of published data on prognostic implications of PD-L2 expression in melanoma metastases. A better understanding of the prognostic significance of these two ligands and the heterogeneity of their expression is warranted as they may have important implications for disease management.
PD-L1 expression in the TME is associated with a higher chance of response to PD-1 blockade; however, some tumors lacking PD-L1 expression can respond to anti-PD1 treatment.38,44 The extent to which “false negative” classification, due to heterogeneous expression in metastases, accounts for these observed responses is unclear. Further, the baseline levels of expression of PD-L1 by tumor cells or immune cells may have a prognostic value independent of the likelihood of response to therapy, and this needs to be clarified in order to understand how the baseline features may interact with the predictive value of the expression. We hypothesized that PD-L1 and PD-L2 are more highly expressed in tumors with more prominent and diffuse T cell infiltration, and that they are markers of better prognosis for patients with melanoma metastases. We also hypothesized that PD-L1 expression is consistent within a tumor, between simultaneously sampled tumors and temporally distinct metastases of the same patient.
Materials and methods
Patients and database
We reviewed surgical pathology reports of patients with a diagnosis of melanoma operated on from 1982 to 2007. We selected metastatic melanoma samples from 183 resections obtained from 147 patients with ample clinical follow-up and surgical pathology material to obtain core samples from at least 3–4 tumor regions to construct tissue microarrays (TMAs). We have previously reported patient characteristics, the nature of the immune cell infiltrates in these specimens, and their association with patient survival.9 The observation time was the interval from date of surgery to date of last contact (death or last follow-up). For survival analysis, subjects alive at the time of last follow-up were censored observations at that time. Follow-up periods range from 1–358 mo, respectively. Histopathological and clinical findings were scored according to the 6th edition AJCC Melanoma Staging and Classification.45
Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved from the archives of the Department of Pathology, University of Virginia Health System. TMAs were constructed, as previously reported,9 with quadruplicate or triplicate 1.0 mm diameter tissue cores were taken from each specimen.
TMA tissue sections were deparaffinized in xylene and rehydrated by sequential incubation in ETOH/water solutions. Antigen retrieval was done by treating the slides in a pressure cooker in citrate buffer for antibodies CD45 (1/400, Dako, Carpinteria, CA), CD3 (1/400, Dako), CD20 (1/400, Dako), CD138 (1/100, Dako), CD4+ (1/120, Vector Laboratories, Burlingame, CA), CD163 (1/50, Santa Cruz Biotechnology Inc., Santa Cruz, CA), PD-1 (1/100, R&D Systems, Minneapolis, MN) and in EDTA for antibodies PD-L1 (1/500, clone 5H1),36 PD-L2 (1/800, R&D Systems, AF1224), CD8+ (1/200, Dako) and CD56 (1/100, Invitrogen, Grand Island, NY). Sections were incubated for 60 min at room temperature for primary antibodies against CD45, CD3, CD4+, CD8+, PD-1 and overnight at 4°C for PD-L1 and PD-L2. Envision system (enzyme-conjugated polymer backbone coupled to secondary antibodies) and 3,3′-diaminobenzidine (DAB) chromogen (Dako) were applied to develop the staining. For PD-L1 and PD-L2, Immpress and TSA (Perkin Elmer, Waltham, MA) were used. Sections were counterstained with hematoxylin (Vector) and mounted in Vectamount (Vector). Positive controls for the CD45, CD3, CD8+, CD4+ and PD-1 included lymph node tissue. Positive control for the PD-L1 and PD-L2 included placenta. Negative controls included the use of PBS instead of primary antibody, with all other conditions preserved. Pictures are acquired using Nikon Digital Sight DS-Fi1 (Nikon Corporation, Tokyo, Japan) camera, Nikon Eclipse E400 (Nikon) scope and NIS-Elements viewer (Nikon) software.
Staining for BRAF V600E mutation was done at the University of North Carolina using Leica's Bond autostainer (Leica Biosystems, Nussloch Germany). Antigen retrieval was done for 30 min with pH 9 buffer (Leica, Cat.No. AR9640) then 30 min incubation in the primary antibody clone VE1 (1:400, Spring Bioscience, Pleasanton CA). Detection was done with Leica Bond Polymer Refine Red Detection Kit (Leica, Cat.No. DS9390).
Quantification of PD-L1, PD-L2, PD-1 and TILs
The percentages of tumor cells that stained positive for PD-L1 and PD-L2 were quantified by a board certified pathologist (E.G.) at increments of 5–10% for each core, and mean percentage was calculated for each tumor. Expression of PD-L1 and PD-L2 was considered positive when staining was membranous, or both cytoplasmic and membranous, and ≥5% of tumor cells were stained. The percentages of TILs that stained positive for PD-L1 was also quantified at increments of 5–10% in the cores which had immune cell infiltration. We were able to discern and to discriminate hematoxylin counterstained lymphocytes morphologically, and did not require additional immunostains to determine their identity. Counts of CD45+, CD3+, CD20+, CD138+, CD163+, CD56+, FoxP3+, DC-LAMP+, CD4+, CD8+ and PD-1+ cells were accessed from the previous report9 and were reported per mm2. Images were obtained using an Olympus BX51 microscope coupled to an Olympus BP70 digital camera (Olympus America Inc., Center Valley, PA), and software Image ProPlus 4.5 for Windows. BRAF expression of melanoma cells was assessed whether positive or negative in each core, slides were reviewed by two users (CLS and JO) and an agreement was reached concerning tumors with questionable staining 7/183 (4%).
Statistical analysis
The correlation between the percentage of PD-L1 and PD-L2 expression in tumor and PD-L1 expression in lymphocytes with numbers of CD45+, CD3+, CD20+, CD138+, CD163+, CD56+, FoxP3+, DC-LAMP+, CD4+ and CD8+ lymphocytes was analyzed using Spearman's rank correlation, and with immunotype9 using the Wilcoxon rank sum test. The significance of associations among expression of PD-L1, PD-L2 and clinicopathological variables were assessed by Wilcoxon rank sum test for variables that are divided into two categories (stage, gender, no evidence of disease after surgery and prior vaccination) and Kruskal Wallis tests for those divided into more than two (cell type, metastatic site and age). Survival curves were estimated by the Kaplan–Meier method and survival differences were assessed using the log-rank test, with overall survival defined from the date of surgery of the first available tumor sample to date of death or last follow-up.
To study the heterogeneity of PD-L1 expression within a tumor, we considered the expression in the cores of the same tumor and we selected all tumor samples that had 3–4 available cores (n = 162). The mean and the distribution of the PD-L1 expression in the cores of each tumor were plotted. Paired t-tests were used to compare PD-L1 expression in initial tumors to subsequent tumors of the same patient. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary NC.) and MedCalc Statistical Software version 16.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016).
Results
Patient demographics and melanoma samples
Median age at surgery was 58.9 y. One hundred three patients had stage IIIA/IIIB/IIIC melanoma, and 44 had stage IV disease. For some patients, there were two synchronous (co-occurring tumors, n = 6) or 2–5 metachronous metastases (occurring at different times, 17 patients had 2 metastases, 7 had 3 and 1 had 5). Forty-nine of the patients were enrolled in experimental melanoma vaccine clinical trials.46,47 Of these, 25 received vaccines prior to surgery (median time to surgery 11.4 mo, mean = 14.7 mo). Twenty-three of those received peptide vaccines (Table S1).
PD-L1 and PD-L2 Expression
PD-L1 is expressed by both melanoma cells and tumor-infiltrating immune cells
We evaluated expression of PD-L1 by melanoma cells and by infiltrating immune cells (Table S2). Analysis focused on the first metastasis evaluable from 147 patients; however, tissue from one specimen was missing from the PD-L1 stained TMA slides (therefore evaluable n is equal to 146 for PD-L1 and 147 for PD-L2 stained tumors). Among these, PD-L1 was detected in 72 samples (49%). It was expressed on ≥5% of tumor cells in 66 cases (45%) and in ≥20% of tumor cells in 35 cases (24%, Table S2). Representative images of membranous, membranous and cytoplasmic, and negative staining are shown in Figs. 1A–C, respectively. Distribution of PD-L1 expression by melanoma cells was assessed among several clinical and histological subgroups (Table 1): there were no significant differences based on cell type, AJCC stage, gender, metastatic site, age or clinical status of disease (no evidence of disease vs. with clinical evidence of disease). However, PD-L1 expression was more common in tumors of patients who had previously participated in melanoma vaccine trials (p = 0.034, Table 1).
Figure 1.
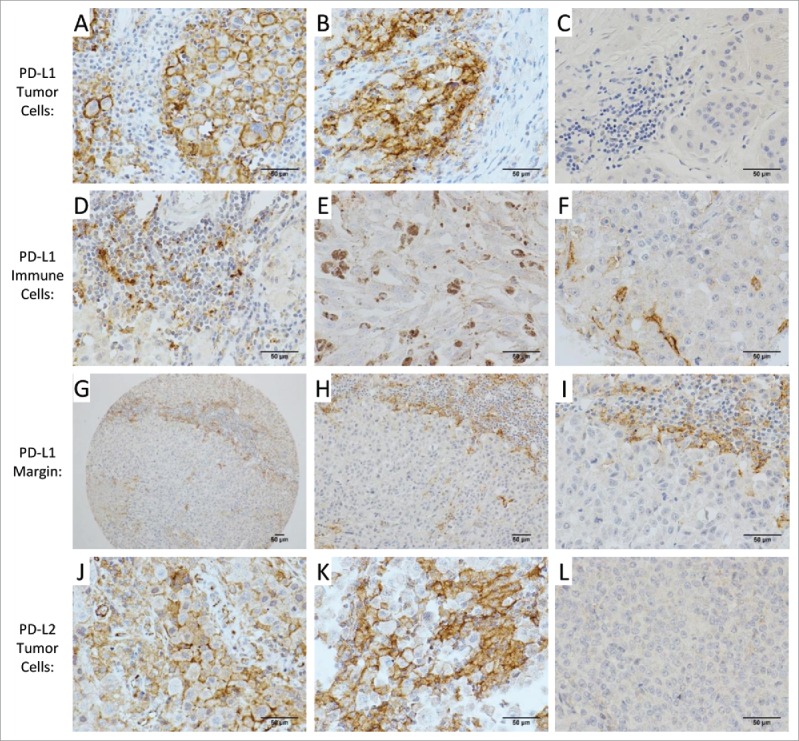
PD-L1 and PD-L2 staining in the melanoma TME. Representative examples of PD-L1 staining of melanoma cells are shown with membranous (A), membranous and cytoplasmic (B), and negative (C) staining patterns. Immune cells that stained for PD-L1 included lymphocytes (D), histiocytes/macrophages (E) and dendritic cells (F). PD-L1 staining of cells at the tumor-immune cell interface is shown at 100× (G), 200× (H) and 400× (I). Representative examples of PD-L2 of melanoma cells are shown with membranous (J), membranous and cytoplasmic (K), and negative (L) staining patterns. For all images in this figure, the chromogen was brown (3,3′-diaminobenzidene; DAB), and images were acquired at 400× except in (G) and (H). Scale bars measure 50 micrometers.
Table 1.
| Distributions of tumors positive for |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD-L1 in ≥5% of tumor cells |
PD-L1 in ≥5% of immune cells |
PD-L2 in ≥5% of tumor cells |
|||||||||
| Clinical features | Total | N positive/Total* | % | p-value | N positive/Total* | % | p-value | N positive/Total | % | p-value | |
| Total | 147 | 66/146 | 45 | — | 66/91 | 73 | — | 35/147 | 24 | — | |
| Cell type | E | 115 | 56/114 | 49 | 0.24 | 57/77 | 74 | 0.018 | 31/115 | 27 | 0.069 |
| M | 21 | 7/21 | 33 | 7/8 | 88 | 1/21 | 5 | ||||
| S | 11 | 3/11 | 27 | 2/6 | 33 | 3/11 | 27 | ||||
| Stage | III | 103 | 42/102 | 41 | 0.29 | 45/63 | 71 | 0.1 | 25/103 | 24 | 0.52 |
| IV | 44 | 24/44 | 55 | 21/28 | 75 | 10/44 | 23 | ||||
| Gender | F | 63 | 27/63 | 43 | 0.87 | 34/44 | 77 | 0.15 | 15/63 | 24 | 0.8 |
| M | 84 | 39/83 | 47 | 32/47 | 68 | 20/84 | 24 | ||||
| Metastatic site | Lymph node | 72 | 34/71 | 48 | 0.47 | 33/51 | 65 | 0.011 | 21/72 | 29 | 0.14 |
| Peritoneum | 1 | 1/1 | 100 | 1/1 | 100 | 0/1 | 0 | ||||
| Small bowel | 6 | 4/6 | 67 | 4/4 | 100 | 1/6 | 17 | ||||
| Skin/SQ | 68 | 27/68 | 40 | 28/35 | 80 | 13/68 | 19 | ||||
| Age | < 50 | 51 | 20/51 | 39 | 0.35 | 22/32 | 69 | 0.57 | 12/51 | 24 | 0.79 |
| ≥ 50, < 70 | 55 | 27/54 | 50 | 28/38 | 74 | 15/55 | 27 | ||||
| ≥ 70 | 41 | 19/41 | 46 | 16/21 | 76 | 8/41 | 20 | ||||
| Prior melanoma vaccines | N | 122 | 51/121 | 42 | 0.034 | 55/75 | 73 | 0.68 | 30/122 | 25 | 0.53 |
| Y | 25 | 15/25 | 60 | 11/16 | 69 | 5/25 | 20 | ||||
| NED after surgery | N | 37 | 18/37 | 49 | 0.83 | 19/23 | 83 | 0.038 | 5/37 | 14 | 0.057 |
| Y | 110 | 48/109 | 44 | 47/68 | 69 | 30/110 | 27 | ||||
Association of clinical features with PD-L1 and PD-L2 expression. Tumors positive (defined as 5% or more of cells staining) for (a) PD-L1 in tumor cells and (b) in immune cells and (c) PD-L2 in tumor cells, their distribution (N) and proportion (%) of positive samples across the different clinical features: (1) cell type (epithelioid/mixed/spindle), (2) clinical stage, (3) gender, (4) metastatic site, (5) age, (6) prior melanoma vaccination and (7) evidence of recurrence of disease post-resection. Associations of expression profiles with the different clinical features are calculated using Kruskal–Wallis and Wilcoxon rank sum tests, and p-values are reported.
Totals for each measure differ from the total of the cohort due to inadequate samples or tumors lacking immune cells on hematoxylin counterstain.
PD-L1 expression was also identified in three types of immune cells: lymphocytes (Fig. 1D), histiocytes (macrophages, Fig. 1E) and dendritic cells (Fig. 1F). PD-L1 positive dendritic cells were found either between tumor cells or concentrated at the intersection of the tumor and immune cell infiltrate. The latter, at low microscope magnification, can sometimes give the false impression of tumor cell membranous staining at the tumor-immune cell infiltrate intersection (Fig. 1G–I). Ninety-one samples contained immune cell infiltrates; of these, PD-L1 was expressed by the immune cells in 70 (77%), in ≥5% of immune cells in 66 (73%), and in ≥20% of immune cells in 54 (59%, Table S2).
Among the various clinical and histologic subsets of melanoma metastases, percent of tumors with ≥5% of immune cells expressing PD-L1 was highest (100%) in small bowel (n = 4) and peritoneal metastases (n = 1), lower in skin and subcutaneous metastases (80%, n = 28) and lowest on lymph node metastases (65%, n = 51, Wilcoxon rank-sum test of expression levels (LN vs. others) p = 0.011, Table 1). PD-L1+ immune cells were also less common if the patient was rendered clinically free of disease at the time of tumor resection (83% vs. 69%, p = 0.038), and were different according to histologic type (33% in spindle cell melanomas, 88% in mixed and 74% in epithelioid, Kruskal–Wallis test p = 0.018) (Table 1).
PD-L2 expression by melanoma cells
PD-L2 was detected in 37 (25%) of the metastatic melanomas: PD-L2 was expressed on ≥5% of tumor cells in 35 (24%) and on ≥20% of tumor cells in 31 (21%) (Table S2). The staining pattern was membranous in most samples (Fig. 1J), and both membranous and cytoplasmic staining in a few samples (Fig. 1K). An example of negative staining is presented in Fig. 1L. PD-L2 staining was also observed in some histiocytes and dendritic cells but was not evident in lymphocytes. Quantification of PD-L2 expression focused on expression in tumor cells, with details shown in Table S2.
BRAF V600E mutation in melanoma cells
BRAF V600E mutation was identified in tumor cells for 47% of the melanoma metastases studied. Its expression did not correlate with either the immune cell subsets or the percent expression of PD-L1 or PD-L2 on tumor cells (Table S3).
PD-L1 and PD-L2 association with distribution (immunotype) and phenotype of immune cells
PD-L1 and PD-L2 association with Immunotype
We recently studied the distribution of intratumoral immune cells within melanoma metastases and categorized them into three immunotypes; no significant immune cell infiltrate (< 50 per mm2, Immunotype A), immune cell infiltrate limited to perivascular cuffing (Immunotype B) and diffuse immune cell infiltrate (Immunotype C): of the metastatic melanomas, 28%, 63% and 9% were Immunotypes A, B and C, respectively.9 Metastatic melanomas with well-defined lymphocytic infiltration (Immunotypes B and C) had significantly higher expression of PD-L1 and PD-L2 on the tumor cells than those devoid of infiltration (Immunotype A) (p < 0.001 and p = 0.020, respectively; Wilcoxon-rank sum test, Fig. 2). Tumors with diffuse immune infiltration (Immunotype C) were also higher expressers of PD-L2 compared to Immunotype B tumors (p = 0.033) and there was a trend to significance with PD-L1 expression (p = 0.058, Fig. 2).
Figure 2.
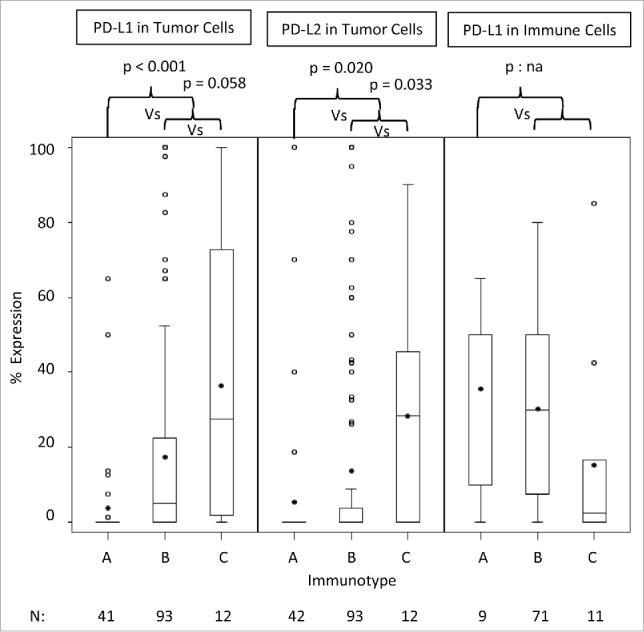
Distribution of PD-L1/PD-L2 expression profiles among Immunotypes A, B and C. The distributions of PD-L1 and PD-L2 expressions in tumor cells and PD-L1 expression in lymphocytes among the 42 Immunotype A tumors, 93 Immunotype B tumors and 12 Immunotype C tumors are represented by box plots (median, quartiles). Means are noted by the dark dots. Comparisons of expression profiles of tumors of Immunotype A versus those with Immunotypes B and C and for those with Immunotype B versus C using the Wilcoxon Test, and p-values are reported. A comparison for PD-L1 expression in TILs was not feasible (p:na) as there was only nine tumors of Immunotype A.
PD-L1 and PD-L2 association with immune cell subsets
In a direct assessment of immune cell subsets in the TME and PD-L1 and PD-L2 expression, there were moderate to strong correlation between PD-L1 expression by tumor cells and the density of CD3+, CD4+, CD8+, PD-1+, FoxP3+ cells and all immune cells CD45+ (r > 0.5, Spearman correlation, p < 0.001, Table 2). In contrast, PD-L1 expression on immune cells was not convincingly associated with the number of infiltrating immune cells, except that there were weak trends to association with numbers of PD-1+ cells and M2 macrophages (CD163+; r = 0.32 r = 0.38, respectively) and possibly an inverse correlation with the number of infiltrating B cells (CD20+, r = −0.34, Table 2). There were significant correlations between PD-L2 expression of the tumor cells and the densities of CD3+, CD8+, CD138+, PD-1+, FoxP3+ cells and all immune cells (CD45+) (p ≤ 0.001), but the Spearman correlation coefficients were lower than for PD-L1 associations ( r < 0.4, Table 2). Tumors with no CD8+ T-cell infiltration were positive for PD-L1 in only 1/9 (11%) of cases, and PD-L2 in 2/9 (22%) of cases. However, tumors with no CD45+ cell infiltrate were negative for PD-L1 and PD-L2 expression (Table S4, Fig. S1). These findings support the hypothesis that PD-L1 and PD-L2 expression on melanoma cells is associated with immune cell infiltration.
Table 2.
| Infiltrating Immune cells | %PD-L1 by Tumor | %PD-L2 by Tumor | %PD-L1 Immune cells |
|---|---|---|---|
| CD45 (immune cells) | 0.57 (<0.001) | 0.26 (0.001) | −0.16 (0.13) |
| CD138 (plasma cells) | 0.44 (<0.001) | 0.28 (<0.001) | −0.04 (0.73) |
| CD20 (B cells) | 0.41 (<0 .001) | 0.18 (0.033) | −0.34 (<0.001) |
| CD3 (T cells) | 0.64 (<0.001) | 0.30 (<0.001) | −0.03 (0.78) |
| CD4+ (CD4+ T cells) | 0.57 (<0.001) | 0.23 (0.005) | 0.08 (0.45) |
| CD8+ (CD8+ T cells) | 0.62 (<0.001) | 0.27 (0.001) | −0.04 (0.72) |
| CD56 (NK cells) | 0.43 (<0.001) | 0.13 (0.11) | 0.19 (0.079) |
| PD-1 | 0.61 (<0.001) | 0.26 (0.001) | 0.38 (<0s.001) |
| Foxp3 (T-regs) | 0.61 (<0.001) | 0.35 (<0.001) | −0.05 (0.67) |
| CD163 (M2 macrophages) | 0.41 (<0.001) | 0.19 (0.019) | 0.32 (0.002) |
| DC-LAMP (mature DC) | 0.27 (0.001) | 0.13 (0.10) | −0.03 (0.76) |
Association of PD-L1 and PD-L2 expression and type of cellular infiltrate. Pearson's correlation coefficients (r) testing associations between the percent of tumor cells expressing PD-L1 and PD-L2 and that of immune cells expressing PD-L1 with the densities of the different immune cell subtypes per mm2 as assessed by IHC. Higher coefficients r > 0.5 are underlined to note higher correlations.
PD-L1 and PD-L2 expression associations with patient survival
To assess the associations of PD-L1 and PD-L2 expressions with patient survival, we performed Kaplan–Meier survival curves and log-rank tests for the 147 patients with metastatic melanoma, based on the protein expression in their first metastases. Survival data was not available for one patient; therefore, n = 145 for PD-L1 and n = 146 for PD-L2. Patients were treated and followed up in an era preceding current effective therapies. Using a commonly applied ≥5% expression cutoff for PD-L1 positivity in melanoma cells, we found no significant difference in survival for PD-L1+ and PD-L1− melanomas (p = 0.35, Fig. 3). In contrast, there was a trend for survival benefit for melanomas with high PD-L1 expression (PD-L12+) based on a criterion that high expression represents at least 20% of melanoma cells expressing PD-L1 (p = 0.053, Fig. 3). PD-L1 expression in ≥5% or ≥20% of tumor-infiltrating immune cells did not correlate with better survival (p = 0.58 and p = 0.46, respectively, Fig. 3).
Figure 3.
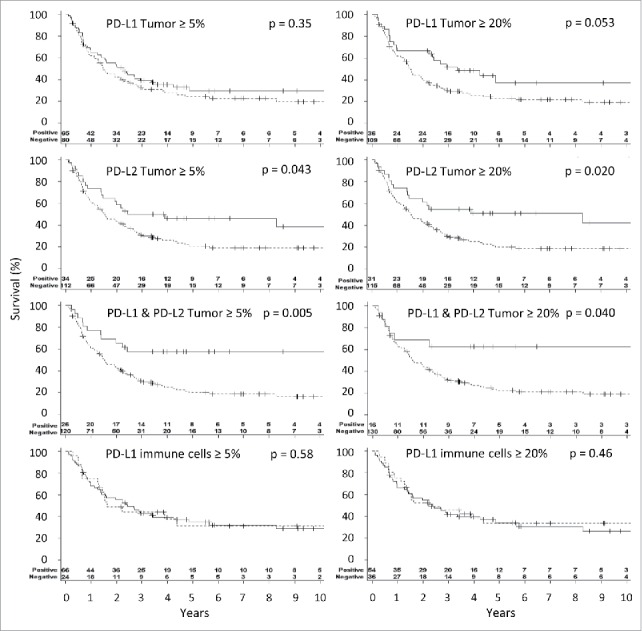
Association of PD-L1 and PD-L2 expression on patient survival. Kaplan–Meier curves illustrating survival for patients whose tumor cells are positive for staining PD-L1, PD-L2 and both and for patients with positive PD-L1 staining in lymphocytes (E). Threshold for positivity was considered at 5% and at 20% of the indicated cells staining positively. Continuous lines represent the population with positive expression and interrupted lines for no expression. Survival was compared using the log-rank test and p-values are reported.
To test our hypothesis that expression of PD-L2 by melanoma cells is associated with survival, we also explored two scenarios, of PD-L2+ melanomas (PD-L2 in ≥5% of tumor cells) and PD-L22+ melanomas (PD-L2 in ≥20% of tumor cells). Survival was significantly prolonged compared to those with PD-L2− tumors with either criterion (p = 0.04 and p = 0.02, Fig. 3). Thus, even relatively infrequent PD-L2 expression on melanoma cells is a significant prognostic indicator.
Interestingly, at the 5% and 20% cutoffs, patients whose tumors are double positive for PD-L1 and PD-L2 have better overall survival than patients with tumors that are not double positive (p = 0.005 and 0.04, respectively, Fig. 3). Altogether, this suggests that assessing PD-L2 expression has an additive prognostic value to the assessment of PD-L1 expression at 5%. This was also the case when studying patients with stage IV disease separately (Fig. S3). Furthermore, in tumors expressing PD-L2 a higher CD8+ T-cell infiltration further identifies tumors of patients with better overall survival (Fig. S4).
Spatial and temporal heterogeneity of tumor PD-L1 and PD-L2 expression
Heterogeneity of PD-L1 and PD-L2 expression in cores of the same tumor: To assess the heterogeneity of expression of PD-1 ligands in each tumor, the different 1 mm-diameter cores of all tumors with 3–4 available cores were assessed for PD-L1 (n = 162) and PD-L2 (n = 167). The mean and range were plotted in Figs. 4A and B. PD-L1 and PD-L2 expressions in at least one core was discordant with that of the mean considering a ≥5% threshold for positivity in 43/162 tumors (27%) and 11/167 (7%), respectively. Considering the ≥20% threshold for positivity, 38/162 tumors (23%) and 17/167 (10%), respectively, at least one core is discordant with the mean. Thus, positive PD-L2 expression is less discordant among different 1 mm2 areas of the same metastasis than that of PD-L1.
Figure 4.
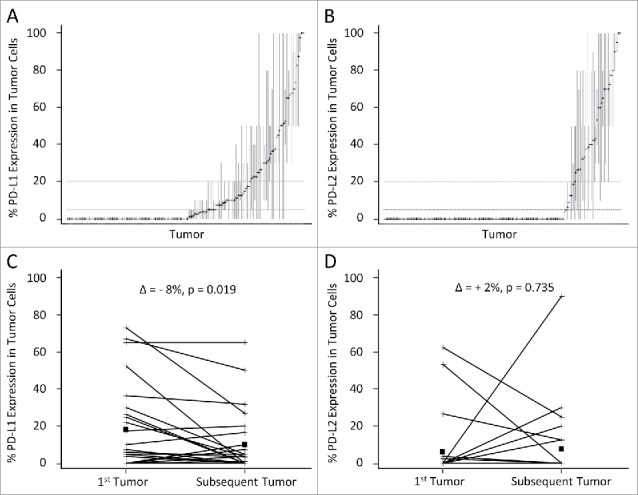
Distribution of PD-L1 and PD-L2 expression in different cores of the same tumor and of metachronous tumors. The percent tumor cells expressing PD-L1 and PD-L2 are represented on the Y-axis. Each tumor with at least three available cores on the TMA is represented on the X-axis expressing PD-L1 (A, n = 162) and PD-L2 (B, n = 167). The mean % expression of the cores from the same tumor is noted by a cross, and the range of the expression on the cores is represented by a vertical line. The horizontal dotted lines express the 5% and 20% thresholds for positivity. Tumors containing a core that is discordant with the mean are those with lines that cross either the 5% or the 20% expression levels. In (C) and (D), the PD-L1 and PD-L2 expression, respectively, in the first resected metastasis and the second resected metastasis of the same patient are connected with a line, means are denoted by squares. The difference of the mean PD-L1 (and PD-L2) expression in the 25 tumors and that of the 25 subsequent tumors is reported (δ), and p-values are obtained from paired T-tests.
Expression in synchronous metastases: Six patients had two tumors excised in the same operation. For five of those patients, both tumors expressed PD-L1 below the 5% cutoff (range 0 to 1.25%), while one had discordant PD-L1 expression in the two synchronous tumors (3.75% vs. 8%). PD-L2 expression differed in two of the six pairs of synchronous tumors: there was no expression in the concordant tumors, while the two discordant tumors had 0 versus 5%, and 0 versus 39% expression. Thus, in this limited dataset, PD-L1 and PD-L2 expression by melanoma cells was usually consistent for two synchronous tumors but was discordant in a potentially significant minority of cases.
Expression in metachronous metastases: Twenty-five patients had metastatic melanomas resected on at least two different occasions. Expression of PD-1 ligands was compared between the first and second metastases. Mean expression of PD-L1 in the first and second metastases was 18% versus 10%, respectively (mean δ = −8%, CI: −15% to −1%, p = 0.019, paired t-test, Fig. 4C). A similar comparison for PD-L2 expression in these metastases showed that PD-L2 was expressed in neither tumor in 16/25 cases, and disparate (either increased or decreased) in 9/25 tumor pairs (Fig. 4D). In contrast to PD-L1 where we seldom saw increased expression with time, in four cases PD-L2 increased substantially. This could reflect different contributions of CD8+ T cell presence on the expression of these two molecules.
Discussion
Advances in immunotherapy for metastatic melanoma rely on an understanding of immune regulation in the TME. In this study, we present further evidence that the PD-L1 and PD-L2 expression in the tumor reflect interactions between the immune response and the tumor cells. This is demonstrated by associations between immune cell infiltrates and tumor-cell expression of PD-L1 and PD-L2, and by higher expression of PD-L1 in tumors from patients who previously received cancer vaccines. The clinical significance of these interactions is supported by associations with patient outcome. We also characterize how the spatial and temporal heterogeneity of ligand expression in the TME influence their usefulness as biomarkers for disease management.
While the relevance of PD-L1/PD-1 as a target is not questioned, more than one factor regulates the expression of PD-L1. Studies have indicated that expression is commonly a consequence of “adaptive resistance:”34 namely, that it is induced as a negative-feedback response to interferon-α and IFNγ produced by activated innate and adaptive immune responses. In addition, others have demonstrated that oncogenic alterations can induce PD-L1 expression.48-50 The correlations we report between lymphocyte infiltration (in particular CD3 and CD8+) and distribution (immunotype) with PD-L1 expression, and the lack of any association with BRAF mutational status, suggest that PD-L1 expression by human melanoma cells can be explained predominantly by adaptive resistance. We only found a single case where PD-L1 expression was observed in greater than 5% of the tumor cells in the absence of any CD8+ T cells, a result consistent to Taube and colleagues' finding.36 The presence of BRAF activating mutation was not significantly associated with the presence of effector, regulatory T cells or myeloid immune cells. These findings stress that the BRAF V600E mutation alone is not sufficient to induce a discernable antitumoral immune response, or to induce PD-L1 expression. Instead, it is intriguing to note that patients that had received a prior melanoma vaccine had significantly greater PD-L1 expression, suggesting that memory T cell populations generated by vaccination may promote immune responses against recurrent tumors, and by extension PD-L1 expression in those tumors. In contrast, the reduced expression of PD-L1 in secondary metachronous tumors may reflect a mechanism of metastatic outgrowth by escaping the immune surveillance. In fact, subsequent melanoma metastases have less prominent CD8+ T cell infiltration than previous metastases (manuscript in preparation). PD-L2 expression corresponded to the patient immunotype, with infrequent expression in tumors without lymphocytic infiltration. It had significant associations with several lymphocyte subsets, but those associations were weaker than for PD-L1 and there was no association with prior cancer vaccination. Thus, our data suggest that expression of PD-L2 may be induced by interactive components of the immune response and/or may be controlled in part by factors independent of those that control PD-L1 expression.
Initial studies in animal tumor models and human materials reported that PD-L1 expression by tumor cells could mediate immune escape by downregulating tumor-reactive T cell responses.11,51 Therapeutic blockade of PD-1 and PD-L1 induces dramatic and durable regression of melanoma and other cancers in patients, a finding that strongly supports this hypothesis.12,52-54 The contribution of PD-L1 expression in tumor progression without PD-1/PD-L1 blockade, however, remains unclear. Some studies reported negative impact of PD-L1 and PD-L2 expression on patient survival in pancreatic cancer31 and renal cell cancer55 in addition to an inverse relationship with tumor infiltrating CD8+ lymphocytes in esophageal cancer.43 However, others did not find a correlation with poor survival32,33 and some showed an association with poor clinicopathological factors and increased immune cell infiltrate.30 Thus, the prognostic relevance of PD-L1 and PD-L2 expression in tumors is still controversial. We find that increased frequency of PD-L1, and in particular PD-L2 expression, by melanoma cells, but not immune cells, correlates with better overall survival.
Our data differ in some details from a report, from a smaller cohort of 56 patients with metastatic melanoma, which found a correlation with survival for patients with PD-L1 expressed on ≥5% of tumor cells.36 It is noted that in the contrasting study some patients received anti-PD-1 mAb therapy after the biopsy which might provide a survival advantage for those patients with ≥5% expression of PD-L1. We found trends for improved survival among patients whose tumor expressed PD-L1, but this more evident with a more stringent cutoff (20% of tumor cells, p = 0.053), whereas the trend was very weak with a cutoff at 5% (p = 0.35, Fig. 3). Discrepancies concerning the percentage of tumor cells expressing PD-L1 and PD-L2 and survival data in different studies may also result from the use of different staining protocols or misinterpretation of the positive staining cells. This issue has been explained in detail by Gadiot et al. in their recent report.39 Positive staining, in our view, should be membranous, which may or may not be accompanied also by cytoplasmic staining.
Our study has revealed a stronger correlation between PD-L2 expression and patient survival than was observed for PD-L1. PD-L2's contribution to T cell immunity is controversial, as both in vitro 17,20 and knockout mouse56,57 studies have demonstrated both positive and negative influences on T cell activation, while two studies with human PBMC have favored the notion that PD-L2 serves as a negative regulator of T cell activation and function.58,59 However, anti-PD-L1 mAbs do not interfere with PD-1/PD-L2 interactions but have had similar impact on clinical outcome in patients with lung cancer and bladder cancer in comparison with anti-PD-1 mAb,4,60-62 suggesting that the PD-1/PD-L2 interaction plays a lesser role in adaptive resistance to tumor immunity than PD-1/PD-L1. As PD-L2 expression also positively correlated with lymphocytic infiltration, we hypothesize that, in vivo, PD-L2-mediated stimulation results in a different outcome than observed with human PBMC studies in vitro. Consistent with this, forced expression of PD-L2 by tumors has been shown to increase tumor control by a PD-1 independent mechanism,63 and fusion proteins targeted to PD-L2 have been shown to confer survival advantages in murine models.64,65 Together these data suggest that the context in which PD-L2 stimulation is delivered and the potential engagement of an alternative receptor could influence whether PD-L2 provides a positive or negative T cell signal.
Expression of PD-L1 by tumor cells predicts a higher chance of therapeutic response to PD-1/PD-L1 blockade with objective response rates of 30–65%; however, even among patients with low or absent expression of PD-L1, there remains about an 11–42% chance of objective tumor regression.66-69 It is conceivable that the positive correlation between higher PD-L1 expression on tumor cells and patient survival may confound findings of longer overall survival and progression-free survival for these patients after PD-1 therapy. However, the expression of PD-L1 by tumor cells appears to have an active role in the effect of PD-1 blockade by indicating higher rates of clinical benefit from therapy, which includes stable disease, partial response and complete response. It will be interesting to study if the combination of PD-L1 and PD-L2 expression on tumor cells predicts objective responses only to PD-1 antibody therapy better than PD-L1 expression alone.
Biomarkers for response to immunotherapies, such as PD-1/PD-L1 checkpoint inhibitors, may enable physicians to avoid exposing patients who are likely not to respond to undesired effects of therapy, with the intent of selecting a more personalized treatment and management strategy for the patient. This selection intent is dependent upon the reliability of the assessment of PD-L expression. A significantly decreased rate of positive PD-L1 expression is observed in small tumor specimens.38 By examining small and larger specimens of the same tumors, we found that, in up to 27% of cases, considering a single 1 mm2 biopsy within a single tumor can yield results different than the assessment of three or four 1 mm2 areas. Therefore, assessing a one millimeter diameter area of the metastatic melanoma for PD-L1 expression can be misleading. If this measure is to affect management decisions, it is preferable to assess the largest tumor area possible. We have found that in most cases, there was significant concordance in expression of PD-L1 or PD-L2 among synchronous or metachronous tumors from the same patient, but in 8 of 25 (32%), there was discordance across a 5% cutoff for PD-L1 expression among metachronous tumors. Others have reported discordance of PD-L1 expression among primary melanomas and their metastases (across a 1% threshold of positivity) in 50% of patients.70 Notably, however, review of the data for that report reveals that 11 of the patients reported to have heterogeneous PD-L1 expression had values confined to the range of 0–5%, leaving 13/46 (28%) with heterogeneity across a 5% cutoff. Thus, heterogeneity rates may differ with different thresholds of positivity. Thus, with respect to patient management, tumors with PD-L1 or PD-L2 expression values near the cutoff for positivity should be dealt with judiciously and may warrant the biopsy of additional metastases. Thus, some of the patients who have been identified as PD-L1 negative may well have some tumor areas with PD-L1 expression. The possibility of sampling error, and the dynamic nature of PD-L1 expression may explain some of the clinical benefit with PD-1 antibody therapy in patients reported to have PD-L1 negative tumors. Regardless of the reason, a growing body of literature demonstrates that patients with PD-L1 negative tumors may respond to PD-1 antibody therapy. Since the toxicity of PD-1 antibody therapy is low, there is little enthusiasm for withholding that therapy from such patients. However, combination checkpoint therapy (antibodies to PD-1 and to CTLA4) offers much greater toxicity than PD-1 therapy alone, and data from a randomized phase III trial has suggested that clinical outcome may be comparable with PD-1 antibody alone in patients whose tumors express PD-L1 above a cutoff, whereas there is clinical benefit of the combination over PD-1 antibody alone in patients with PD-L1-negative tumors.71 Thus, if valid, accurate identification of patients with PD-L1 positive tumors may spare many of them the toxicity of the combination if these data are supported in longer follow-up. It will be valuable also to understand to what extent PD-L2 expression may help to support such treatment decisions.
Conclusion
In summary, while the mechanism by which PD-L2 is regulated in the TME and its contributions to immune cell function await elucidation, our studies reveal the utility of B7-H family members as indicators of tumor immune regulation and prognostic indicators. The increased expression of PD-L1 in tumors of patients who had previously received experimental melanoma vaccines is also provocative and raises the possibility that cancer vaccines may support increased inflammation in the TME, to support subsequent checkpoint blockade therapy. Our data also reveal some heterogeneity of PD-L1 and PD-L2 expression in melanoma metastases, which warrants attention to obtaining adequate tissues for biopsy, possibly from multiple tumors. The prognostic associations of the combination of PD-L1 and PD-L2 support future studies of the predictive value of these ligands in the setting of combination checkpoint blockade therapy.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Yongjuan Xia in the UNC Translational Pathology Laboratory (TPL) for expert technical assistance. The UNC Translational Pathology Laboratory is supported in part, by grants from the National Cancer Institute (3P30CA016086), the UNC University Cancer Research Fund (UCRF).
Funding
This work has been supported in part by T32 CA163177 (JMO), U01 CA178846 (CLS). CA121974 (LC). LC is a consultant/advisory board member/receives consulting fees from MedImmune, NextCure and Pfizer. LC also receives patent/licensing payments from Bristol-Myers Squibb; and currently has sponsored research grants from Boehringer Ingelheim, Pfizer and NextCure. CLS received consulting fees from Castle Biosciences, and receives royalties from the UVA Licensing and Ventures Group for peptide patents. The University of Virginia receives funding for CLS role as scientific advisor for Immatics, Curetech and Polynoma, and the University of Virginia was provided PD-1 antibody from Merck Sharp and Dohme for use in an investigator initiated trial on which CLS is PI.
ORCID
Timothy N. Bullock http://orcid.org/0000-0001-6141-3261
References
- 1.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21:233-40; PMID:19304471; http://dx.doi.org/ 10.1016/j.coi.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD et al.. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/ 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homet Moreno B, Parisi G, Robert L, Ribas A. Anti-PD-1 therapy in melanoma. Seminars Oncol 2015; 42:466-73; PMID:25965365; http://dx.doi.org/ 10.1053/j.seminoncol.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv4; PMID:26936508; http://dx.doi.org/21061197 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Topics Microbiol Immunol 2011; 350:17-37; PMID:21061197; http://dx.doi.org/ 10.1007/82_2010_116 [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Yu Y, Yang S, Zeng B, Zhang Z, Jiao G, Zhang Y, Cai L, Yang R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother 2009; 58:687-97; PMID:18828017; http://dx.doi.org/ 10.1007/s00262-008-0591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11:3887-95; PMID:1396582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8:765-72; PMID:8671665; http://dx.doi.org/ 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- 9.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012; 72:1070-80; PMID:22266112; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007; 19:813-24; PMID:17606980; http://dx.doi.org/ 10.1093/intimm/dxm057 [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793-800; PMID:12091876; http://dx.doi.org/ 10.1038/nm0902-1039c [DOI] [PubMed] [Google Scholar]
- 12.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013; 19:1021-34; PMID:23460533; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q et al.. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 2015; 162:1242-56; PMID:26359984; http://dx.doi.org/ 10.1016/j.cell.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al.. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027-34; PMID:11015443; http://dx.doi.org/ 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunotherapy 2007; 30:251-60; PMID:17414316; http://dx.doi.org/ 10.1097/CJI.0b013e31802e085a [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999; 5:1365-9; PMID:10581077; http://dx.doi.org/ 10.1038/70932 [DOI] [PubMed] [Google Scholar]
- 17.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001; 193:839-46; PMID:11283156; http://dx.doi.org/ 10.1084/jem.193.7.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203:883-95; PMID:16606670; http://dx.doi.org/ 10.1084/jem.20051776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8:239-45; PMID:17304234; http://dx.doi.org/ 10.1038/ni1443 [DOI] [PubMed] [Google Scholar]
- 20.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R et al.. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2:261-8; PMID:11224527; http://dx.doi.org/ 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- 21.Riella LV, Watanabe T, Sage PT, Yang J, Yeung M, Azzi J, Vanguri V, Chandraker A, Sharpe AH, Sayegh MH et al.. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplantation 2011; 11:832-40; PMID:21401869; http://dx.doi.org/ 10.1111/j.1600-6143.2011.03451.x [DOI] [PubMed] [Google Scholar]
- 22.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 2007; 116:2062-71; PMID:17938288; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.709360 [DOI] [PubMed] [Google Scholar]
- 23.Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH et al.. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med 2014; 211:943-59; PMID:24752301; http://dx.doi.org/ 10.1084/jem.20130790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 2003; 170:1257-66; PMID:12538684; http://dx.doi.org/ 10.4049/jimmunol.170.3.1257 [DOI] [PubMed] [Google Scholar]
- 25.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111-22; PMID:17629517; http://dx.doi.org/ 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Wu LJ, Fan HM, Hu FY, Guan YJ, Yang KL. Study on the copy numbers and mRNA expression levels of the programmed death-1 gene in chronic hepatitis B patients. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology 2011; 19:678-82; PMID:22152383; http://dx.doi.org/ 10.3760/cma.j.issn.1007-3418.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 27.Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood 2001; 97:1809-16; PMID:11238124; http://dx.doi.org/ 10.1182/blood.V97.6.1809 [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med 2003; 197:1083-91; PMID:12719480; http://dx.doi.org/ 10.1084/jem.20021752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L et al.. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004; 101:17174-9; PMID:15569934; http://dx.doi.org/ 10.1073/pnas.0406351101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A et al.. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006; 8:190-8; PMID:16611412; http://dx.doi.org/ 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, M WB, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett 2008; 268:98-109; PMID:18486325; http://dx.doi.org/ 10.1016/j.canlet.2008.03.056 [DOI] [PubMed] [Google Scholar]
- 32.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341-7; PMID:19825956; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1652 [DOI] [PubMed] [Google Scholar]
- 33.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004; 10:5094-100; PMID:15297412; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0428 [DOI] [PubMed] [Google Scholar]
- 34.Avril T, Saikali S, Vauleon E, Jary A, Hamlat A, De Tayrac M, Mosser J, Quillien V. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol 2010; 225:22-33; PMID:20493562; http://dx.doi.org/ 10.1016/j.jneuroim.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 35.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/ 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 36.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/21717461 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kronig H, Julia Falchner K, Odendahl M, Brackertz B, Conrad H, Muck D, Hein R, Blank C, Peschel C, Haller B et al.. PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. Int J Cancer 2012; 130:2327-36; PMID:21717461; http://dx.doi.org/ 10.1002/ijc.26272 [DOI] [PubMed] [Google Scholar]
- 38.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; 20:5064-74; PMID:24714771; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011; 117:2192-201; PMID:21523733; http://dx.doi.org/ 10.1002/cncr.25747 [DOI] [PubMed] [Google Scholar]
- 40.Fukaya T, Takagi H, Sato Y, Sato K, Eizumi K, Taya H, Shin T, Chen L, Dong C, Azuma M et al.. Crucial roles of B7-H1 and B7-DC expressed on mesenteric lymph node dendritic cells in the generation of antigen-specific CD4+Foxp3+ regulatory T cells in the establishment of oral tolerance. Blood 2010; 116:2266-76; PMID:20574047; http://dx.doi.org/ 10.1182/blood-2009-10-250472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-29; PMID:20008522; http://dx.doi.org/ 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomihara K, Shin T, Hurez VJ, Yagita H, Pardoll DM, Zhang B, Curiel TJ, Shin T. Aging-associated B7-DC+ B cells enhance anti-tumor immunity via Th1 and Th17 induction. Aging Cell 2012; 11:128-38; PMID:22044484; http://dx.doi.org/ 10.1111/j.1474-9726.2011.00764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K et al.. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 44.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Therapeutics 2015; 37:764-82; PMID:25823918; http://dx.doi.org/ 10.1016/j.clinthera.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S et al.. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:19917835; http://dx.doi.org/ 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillon PM, Olson WC, Czarkowski A, Petroni GR, Smolkin M, Grosh WW, Chianese-Bullock KA, Deacon DH, Slingluff CL Jr. A melanoma helper peptide vaccine increases Th1 cytokine production by leukocytes in peripheral blood and immunized lymph nodes. J Immunother Cancer 2014; 2:23; PMID:25126421; http://dx.doi.org/ 10.1186/2051-1426-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clancy-Thompson E, King LK, Nunnley LD, Mullins IM, Slingluff CL Jr, Mullins DW. Peptide vaccination in Montanide adjuvant induces and GM-CSF increases CXCR3 and cutaneous lymphocyte antigen expression by tumor antigen-specific CD8 T cells. Cancer Immunol Res 2013; 1:332-9; PMID:24377099; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kappaB. Biochem Biophys Res Commun 2015; 463:95-101; PMID:25998384; http://dx.doi.org/ 10.1016/j.bbrc.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 49.Van Allen EM, Golay HG, Liu Y, Koyama S, Wong K, Taylor-Weiner A, Giannakis M, Harden M, Rojas-Rudilla V, Chevalier A et al.. Long-term benefit of PD-L1 blockade in lung cancer associated with JAK3 activation. Cancer Immunol Res 2015; 3:855-63; PMID:26014096; http://dx.doi.org/ 10.1158/2326-6066.CIR-15-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett MT, Anderson KS, Lenkiewicz E, Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE, Reddy SK, Ramanathan RK et al.. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015; 6:26483-93; PMID:26317899; http://dx.doi.org/ 10.18632/oncotarget.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99:12293-7; PMID:12218188; http://dx.doi.org/ 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Seminars in Oncology 2015; 42:587-600; PMID:26320063; http://dx.doi.org/ 10.1053/j.seminoncol.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Exp Opin On Biol Ther 2013; 13:847-61; PMID:23421934; http://dx.doi.org/ 10.1517/14712598.2013.770836 [DOI] [PubMed] [Google Scholar]
- 55.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H et al.. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381-5; PMID:16585157; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 56.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med 2005; 201:1531-41; PMID:15897272; http://dx.doi.org/ 10.1084/jem.20050072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Chen Y, Li J, Zhang R, Wu Y, Zou L, Zhao T, Zhang X, Han J, Chen A et al.. Renal tubular epithelial expression of the coinhibitory molecule B7-DC (programmed death-1 ligand). J Nephrol 2006; 19:429-38; PMID:17048200 [PubMed] [Google Scholar]
- 58.Pfistershammer K, Klauser C, Pickl WF, Stockl J, Leitner J, Zlabinger G, Majdic O, Steinberger P. No evidence for dualism in function and receptors: PD-L2/B7-DC is an inhibitory regulator of human T cell activation. Eur J Immunol 2006; 36:1104-13; PMID:16598819; http://dx.doi.org/ 10.1002/eji.200535344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saunders PA, Hendrycks VR, Lidinsky WA, Woods ML. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur J Immunol 2005; 35:3561-9; PMID:16278812; http://dx.doi.org/ 10.1002/eji.200526347 [DOI] [PubMed] [Google Scholar]
- 60.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE et al.. Safety and efficacy of Durvalumab (MEDI4736), an anti-programmed cell death Ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016; 34:3119-25; PMID:27269937; https://dx.doi.org/25922725 10.1200/JCO.2016.67.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jia M, Feng W, Kang S, Zhang Y, Shen J, He J, Jiang L, Wang W, Guo Z, Peng G et al.. Evaluation of the efficacy and safety of anti-PD-1 and anti-PD-L1 antibody in the treatment of non-small cell lung cancer (NSCLC): a meta-analysis. J Thorac Dis 2015; 7:455-61; PMID:25922725; http://dx.doi.org/ 10.3978/j.issn.2072-1439.2015.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med 2003; 197:1721-30; PMID:12810690; http://dx.doi.org/ 10.1084/jem.20022089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kojima M, Murata S, Mekata E, Takebayashi K, Jaffee EM, Tani T. Fusion protein of mutant B7-DC and Fc enhances the antitumor immune effect of GM-CSF-secreting whole-cell vaccine. J Immunother 2014; 37:147-54; PMID:24598447; http://dx.doi.org/ 10.1097/CJI.0000000000000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mkrtichyan M, Najjar YG, Raulfs EC, Liu L, Langerman S, Guittard G, Ozbun L, Khleif SN. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol 2012; 189:2338-47; PMID:22837483; http://dx.doi.org/ 10.4049/jimmunol.1103085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedman CF, Postow MA. Emerging tissue and blood-based biomarkers that may predict response to immune checkpoint inhibition. Curr Oncol Rep 2016; 18:21; PMID:26922327; http://dx.doi.org/ 10.1007/s11912-016-0509-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD et al.. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16:375-84; PMID:25795410; http://dx.doi.org/ 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 68.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320-30; PMID:25399552; http://dx.doi.org/ 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 69.Asmar R, Yang J, Carvajal RD. Clinical utility of nivolumab in the treatment of advanced melanoma. Ther Clin Risk Manag 2016; 12:313-25; PMID:27013881; http://dx.doi.org/ 10.2147/TCRM.S78039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV et al.. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 2015; 28:245-53; PMID:25477049; http://dx.doi.org/ 10.1111/pcmr.12340 [DOI] [PubMed] [Google Scholar]
- 71.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P et al.. Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/ 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


