Abstract
Background and aims
There is evidence that distinct genetic polymorphisms of LRP5 are associated with low Bone Mineral Density (BMD) and the risk of fracture. However, relationships between LRP5 polymorphisms and micro- and macro-architectural bone characteristics assessed by pQCT have not been studied. The aim of the present study was to investigate the association of Ala1330Val and Val667Met polymorphisms in LRP5 gene with volumetric BMD (vBMD) and macro-architectural bone parameters in a population-based sample of men and women.
Methods
We studied 959 participants of the InCHIANTI study (451 men and 508 women, age range: 21–94 yrs). Trabecular vBMD (vBMDt, mg/cm3), cortical vBMD (vBMDc, mg/cm3), cortical bone area (CBA, mm2) and cortical thickness (Ct.Th, mm) at the level of the tibia were assessed by peripheral quantitative computed tomography (pQCT). Ala1330Val and Val667Met genotypes were determined on genomic DNA by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).
Results
In age-adjusted analyses both LRP 1330-valine and LRP 667-metionin variants were associated with lower vBMDt in men (p<0.05), and lower vBMDt (p<0.05), Ct.Th (p<0.05) and CBA (p<0.05) in women. After adjusting for multiple confounders, only the association of LRP5 1330-valine and 667-metionin with CBA remained statistically significant (p=0.04 and p=0.01, respectively) in women.
Conclusion
These findings suggest that both Ala1330Val and Val667Met LRP5 polymorphisms may affect the determination of geometric bone parameters in women.
Keywords: Cortical bone area, LRP5 gene polymorphism, osteoporosis, peripheral bone quantitative computed tomography (pQCT), volumetric BMD
INTRODUCTION
Osteoporosis is a complex multifactorial disorder characterized by decreased deposition of skeletal calcium, micro- and macro-architectural deterioration of bone tissue which leads to compromised bone strength, and an increased risk of fracture (1). Bone strength is the result of both bone mineral density (BMD) and bone quality, the latter encompassing a number of factors, such as bone turnover, mineralization, microarchitecture, and geometry (2). In combination with several environmental factors, such as diet and lifestyle, genetic factors appear to contribute to bone mass and bone health, thus affecting the risk of fracture in both women and men (3). Studies conducted in twins suggest that up to 80% of the age-specific variance in BMD is genetically determined (4).
Previous studies have suggested that the low-density lipoprotein (LDL) receptor-related protein 5 (LRP5) gene may be implicated in bone mass. Activating mutations of the LRP5 gene characterize an autosomal dominant syndrome characterized by high bone mineral density, wide, deep mandibles, and torus palatinus (5). Conversely, an inherited functional loss of the LRP5 gene causes the osteoporosis-pseudoglioma syndrome, an autosomal recessive disease characterized by low bone mass, with childhood fractures and abnormal eye development (6, 7). Studies in mice and humans have shown that both osteoblast proliferation and function are decreased in the absence of the LRP5 protein (8). LRP5 gene polymorphisms also contribute to age-specific loss in bone mass in the general population (9, 10).
However, all studies on the effect of LRP5 gene variants on bone genotype have been based on DEXA-derived BMD (11–14), in which bone structural variables, which critically affect bone strength, such as trabecular (vBMDt) and cortical (vBMDc) bone volumetric density, cortical thickness (Ct.Th) and bone macro-architecture were not taken into account.
Johnson et al. have recently shown that the LRP5/Wnt signaling plays a major role in bone mechanosensation (15). Accordingly, the tibia from LRP5 G171V transgenic mice responds with robust bone formation in response to loading, with a reduced threshold level of strain required to induce bone formation (16). Interestingly, the bone hallmark of these transgenic mice is diffuse reduction of bone cortical thickness (15).
The aim of our study was to evaluate the influence of Ala1330Val and Val667Met LRP5 gene polymorphisms on volumetric BMD and macro-architectural bone parameters assessed by tibial quantitative computed tomography (QCT) in a large population-based sample of Caucasian men and women.
SUBJECTS AND METHODS
Population sample
InCHIANTI is an epidemiological study performed in two Italian towns located in the Chianti countryside: Greve in Chianti (11,709 inhabitants; rural area) and Bagno a Ripoli (village of Antella, 4704 inhabitants; just outside the urban area of Florence). The study population consisted of a random sample of the population aged 65 years and older living in the two catchment areas, and 30 men and 30 women randomly selected in each decade between 20 and 70 years. A detailed description of the design and data collection methods of InCHIANTI have been previously published (17). Of the 1530 subjects originally sampled, 1305 (83.3%) older than 65 years underwent a pQCT examination (612 men and 693 women). In the present study, we analysed data from 451 older men and 508 older women who consented to provide DNA samples for analysis. Thus, the Participation Rate (calculated as No. participants/No. eligible for the study) were: 73.7% (451/612) for men and 73.3% (508/693) for women. The study protocol was approved by the INRCA Ethical Committee. All subjects received an extensive description of the purposes and known risks of the study procedures, and all gave their informed consent.
Measures
After a home interview, participants underwent a medical examination in a dedicated laboratory. The level of physical activity in the year prior to the interview was classified on an ordinal scale, based on responses to a standard questionnaire, into: 1) hardly any physical activity; 2) mostly sitting (occasionally walks, easy gardening); 3) light exercise (no sweat) 2–4 h/week; 4) moderate exercise (sweat) 1–2 h/week (level 4); 5) moderate exercise >3 h/week; 6) intense exercise (to the limit) >3 times/week. According to this classification, participants were grouped as: 1–3) inactive or undertaking light physical activity; 4–5) having moderate physical activity; 6) having high physical activity.
Data on dietary intake were collected by administering the food frequency questionnaire created for the European Prospective Investigation into Cancer and nutrition (EPIC) study (18). Although this questionnaire was originally developed for and validated in middle-aged persons, a previous study (19) had suggested that this tool provides good estimates of dietary intake also when administered to the older population. Participants were asked to specify how frequently (weekly, monthly, yearly) each specific food and beverage had been consumed in the previous 12 months. Participants were asked to report the quantity of food consumed, using as references colored photographs with different sizes of portions for the main dishes. Specific software created for EPIC transformed data on food consumption into daily intake of energy, macro and micronutrients. Alcohol intake was estimated from the EPIC questionnaire and expressed as g/day. Data on smoking were derived from the interview questionnaire. History of hip fractures in the last year, and (in women) time in years since menopause (YSM) was assessed by self-report.
Standing height and weight were objectively measured in each participant, and a body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters).
Laboratory measures
Blood samples were drawn in the morning after a 12-hour overnight fast and after participants had been sitting for at least 15 minutes, by means of a standardized method that minimizes the risk of erythrocyte hemolysis. Assays of 25(OH)-vitamin D (Vit-D) and parathormone (PTH) were performed on specimens previously stored at −80°C. 25(OH)-vitamin D was measured by RIA (DiaSorin Inc., Still-water, MN, USA), after extraction of samples with acetonitrile. Intra- and inter-assay coefficients of variations (CVs) were 8.1 and 10.2%, respectively. Serum-intact PTH levels were measured with a two-site immunoradiometric assay kit (N-tact PTHSP, DiaSorin). The assay uses two affinity-purified polyclonal antibodies, one specific for the amino-terminal 1–34 portion of the PTH molecule and the other specific for the 39–84 sequence of the hormone. Assay sensitivity was 1.2 ng/L. Intra- and inter-assay CVs were <3.0 and 5.5%, respectively. Total testosterone was assayed with commercial radio-immunologic kits (Diagnostic Systems Laboratories, Webster, TX, USA). For total testosterone, the minimum detection limit was 0.03 nmol/L; intra- and inter-assay coefficients of variation for three different concentrations were 9.6% and 8.1%, 7.8% and 8.6%, and 9.1% and 8.4%. Concentrations of bio-T were calculated with the Vermeulen formula.
Estradiol was measured by ultrasensitive RIA with a minimum detectable concentration (MDC) of 2.2 pg/mL. The intra- and inter-assay coefficients of variation (CVs) were 8% and 10%, respectively.
Lower leg pQCT
Lower leg pQCT was performed in all study participants by means of a recent generation device (XCT 2000, Stratec Medizintechnik, Pforzheim, Germany) (20). A detailed description of the pQCT device and the method used in the InCHIANTI study has already been published. Briefly, we selected transverse scans at 4% of the tibial length from the distal end of the tibia (tibio-talar joint cleft), where trabecular bone is most abundant, and at 38% of the tibial length, where the cortical shell is usually thicker than 2.5 mm, thus allowing accurate detection of bone boundaries. Cross-sectional images from the pQCT were analyses by BonAlyse (BonAlyse Oy, Jyvaskyla, Finland), a software for processing pQCT scans that automatically identifies bone tissue (cortical and trabecular) and assesses its density and geometry. Different tissues in the analysis were separated according to different density thresholds. In particular, areas with density values above 710 mg/cm3 were considered as “cortical bone”, and areas with density values between 180 and 710 mg/cm3 were considered as “trabecular bone”. The following bone parameters were derived from the pQCT images: 1) Trabecular bone density (vBMDt) (mg/cm3) assessed at 4% of the tibia length; 2) Cortical bone density (vBMDc) (mg/cm3) assessed at 38% of the tibia length; 3) Cortical bone area (CBA) (mm2); and 4) Cortical Thickness (Ct.Th) (mm) assessed at the 38% site (21).
The calf muscle cross-sectional area (CSMA) was evaluated from a transverse scan performed at 66% of the tibia length from the distal tip of the tibia, which is the level of largest outer calf diameters, with little variability across individuals.
The precision error of the XCT2000 is below 1% for volumetric trabecular and cortical density and for cortical bone area (22), and between 1 and 3% for composite geometry parameters (22).
Genotyping
Genomic DNA samples were extracted from EDTA peripheral blood by a modified salting-out procedure according to Miller et al. (23).
The investigated single nucleotide polymorphisms (SNPs) were two previously identified LRP5 missense substitutions, exon 9 c.1999 G>A and exon 18 c.3989 C>T (19), causing the amino acid substitutions Valine to Metionine at the residue 667 (V667M) and Alanine to Valine at residue 1330 (A1330V), respectively. These polymorphisms (dbSNP [database of single-nucleotide polymorphisms]: rs2277268 and rs3736228) were defined by polymerase chain reaction-restriction fragments length polymorphism (PCR-RFLP) with two pairs of primers and the endonucleases MaeII and DraIII, respectively.
The accuracy of the genotyping results obtained from RFLP analysis was demonstrated by means of direct DNA sequencing on an ABI Prism 3100 Genetic Analyser (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Genotyping assignments from agarose gel electrophoresis of digested PCR products were successfully performed in a set of 40 DNA samples and were then validated by comparison with results from direct DNA sequencing. In addition, three controls (for each genotype) previously sequenced and identified as heterozygous and homozygous, were included in all cleavage experiments, to verify the efficacy of enzymatic digestion.
Subjects were conventionally classified as mm (genotype G/G) and MM (genotype A/A) homozygotes, mM heterozygotes (genotype G/A) for V667M polymorphism, and dd (genotype T/T) and DD (genotype C/C) homozygotes, dD heterozygotes (genotype T/C) for A1330V polymorphism.
Statistical analysis
Genotype frequencies were tested for Hardy-Weinberg equilibrium by the χ2 test. All analyses were performed separately in men and women. The difference of the means between genotypes was calculated according to gender, by age-adjusted linear regression analysis.
The population was divided into groups based on genotypes TT vs TC vs CC and AA vs AG vs GG of the LRP5 gene polymorphisms. The age-adjusted means values of vBMDt, vBMDc, Ct.Th and CBA were then reported according to genotype in men and women. The difference of the means between the groups was calculated by using an age-adjusted ANOVA.
The relationship of the LRP5 gene polymorphism with the selected bone parameters (vBMDt, vBMDc, Ct.Th, CBA) was estimated by linear regression analysis, after adjustment for multiple potential confounders including age, PTH, Vit-D, bioavailable testosterone, estradiol, calf cross-sectional muscle area, levels of physical activity, height and weight. The interaction term calculated by calf cross-sectional muscle area and LRP5 gene polymorphism was also used in the regression analysis, to test the interaction between these two variables. Covariates were selected with a Pearson Correlation Coefficient of less than 0.05.
All analyses were performed with the SAS statistical package, version 8.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The main characteristics of the study sample are listed in Table 1. Notably, men and women had similar BMI and reported the same average number of previous hip fractures. Among postmenopausal women (87.8%), the average number of years since menopause (YSM) was 25.1 (10.6 SD). HRT had formerly been used only by 35 women and never used by 93.4% of the women. Men were more physically active and more likely to smoke than women. Consistent with previous literature, men had significantly higher values of CBA, Ct.Th, vBMDt and vBMDc than women.
Table 1.
Clinical characteristics and bone parameters of InCHIANTI population.
| Men (n=451) | Women (n=508) | p | |
|---|---|---|---|
| Age (yrs) (mean±SD) | 66.7±15.1 | 68.0±15.2 | ns |
| Body Mass Index (kg/m2) (mean±SD) | 26.9±3.4 | 27.3±4.7 | ns |
| Previous hip fractures (n, %) | 7 (1.6) | 8 (1.6) | ns |
| Physical activity | <0.0001 | ||
| Sedentary (n, %) | 40 (8.9) | 101 (19.9) | |
| Light (n, %) | 351 (77.8) | 384 (75.6) | |
| Moderate/High (n, %) | 60 (13.9) | 23 (4.5) | |
| Calcium intake (g/die) | 893.0±324.7 | 840.1±338.2 | <0.0001 |
| Smokers (n, %) | 99 (22.0) | 58 (11.4) | <0.0001 |
|
| |||
| CBA (mm2) (mean±SD) | 331.2 (48.2) | 273.7 (54.3) | <0.0001 |
| Ct.Th. (mm) (mean±SD) | 5.3 (1.0) | 4.3 (1.1) | <0.0001 |
| BMDt (mg/cm3) (mean±SD) | 225.7 (54.8) | 202.5 (59.7) | <0.0001 |
| BMDc (mg/cm3) (mean±SD) | 1027.0 (58.0) | 998.6 (73.9) | <0.0001 |
|
| |||
| LRP5 genotype (A1330V) | |||
| CC (n, %) | 351 (78%) | 379 (75%) | |
| CT (n, %) | 89 (20%) | 122 (24%) | |
| TT (n, %) | 11 (2%) | 7 (1%) | |
|
| |||
| LRP5 genotype (Val667Met) | |||
| GG (n, %) | 392 (87%) | 432 (85%) | |
| GA (n, %) | 59 (13%) | 74 (14.5%) | |
| AA (n, %) | - | 3 (0.5%) | |
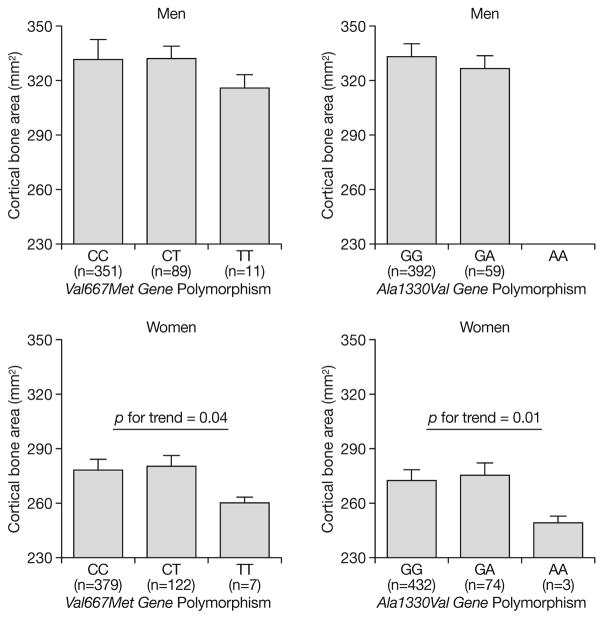
The genotype distribution was in Hardy-Weinberg equilibrium, suggesting that the study population had a homogeneous genetic background. In age-adjusted analysis, LRP5 gene polymorphisms were significantly associated with vBMDt in both men and women, and with Ct.Th and CBA only in women (Tables 2 and 3). Figure 1 shows Ala1330Val and Val667Met LRP5 gene polymorphisms and Cortical Bone Area in both men and women.
Table 2.
General characteristics according to A1330V LRP5 gene polymorphism in men and women.
| Men
|
Women
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | p* | CC | CT | TT | p* | |
| n (%) | 351 (78%) | 89 (20%) | 11 (2%) | 379 (75%) | 122 (24%) | 7 (1%) | ||
| Age (yrs) | 66.0 (15.5) | 68.5 (14.1) | 72.9 (8.5) | 0.18 | 68.2 (15.0) | 66.6 (15.9) | 67.2 (7.9) | 0.16 |
| Body Mass Index (Kg/m2) | 26.6 (3.3) | 27.3 (3.4) | 27.5 (3.8) | 0.61 | 27.3 (4.6) | 27.2 (4.3) | 25.3 (4.0) | 0.51 |
| Calcium intake (g/die) | 889.4 (328.9) | 908.7 (316.7) | 881.9 (268.6) | 0.87 | 872.7 (343.2) | 804.1 (308.7) | 854.5 (366.6) | 0.44 |
| Ct.Th. (mm) (mean±SD) | 5.3 (1.1) | 5.4 (1.1) | 5.1 (0.7) | 0.62 | 4.2 (1.1) | 4.5 (0.9) | 4.0 (1.2) | 0.03 |
| CBA (mm2) (mean±SD) | 280.1 (54.9) | 284.6 (51.0) | 272.8 (59.0) | 0.56 | 272.0 (52.3) | 268.6 (51.0) | 255.8 (48.0) | 0.01 |
| BMDt (mg/cm3) (mean±SD) | 225.8 (56.2) | 229.2 (48.9) | 192.4 (40.6) | 0.03 | 201.9 (60.7) | 205.9 (56.9) | 173.1 (37.0) | 0.03 |
| BMDc (mg/cm3) (mean±SD) | 1026.5 (59.7) | 1031.7 (48.3) | 1030.8 (71.9) | 0.38 | 996.7 (75.1) | 1004.8 (70.7) | 993.2 (50.2) | 0.11 |
| Calf muscle area (mm2) | 7175 (1246) | 7543 (1184) | 7078 (1183) | 0.03 | 5767 (914) | 5822 (945) | 5525 (817) | 0.64 |
p age-adjusted.
Table 3.
General characteristics according to Val667Met LRP5 gene polymorphism in men and women.
| Men
|
Women
|
|||||||
|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | p* | GG | GA | AA | p* | |
| n (%) | 392 (87%) | 59 (13%) | - | 432 (85%) | 74 (14.5%) | 3 (0.5%) | ||
| Age (yrs) | 66.7 (14.6) | 68.9 (14.3) | - | 0.31 | 67.5 (15.8) | 67.3 (16.3) | 70.3 (0.9) | 0.68 |
| Body Mass Index (kg/m2) | 26.9 (3.3) | 27.9 (3.7) | - | 0.86 | 27.2 (4.5) | 26.8 (4.7) | 28.7 (6.0) | 0.71 |
| Calcium intake (g/die) | 889.4 (328.9) | 908.7 (316.7) | - | 0.16 | 889.4 (328.9) | 908.7 (316.7) | 854.5 (366.6) | 0.76 |
| Ct.Th. (mm) (mean±SD) | 5.4 (1.0) | 5.3 (0.7) | - | 0.75 | 4.3 (1.1) | 4.4 (1.0) | 3.5 (0.6) | 0.74 |
| CBA (mm2) (mean±SD) | 333.3 (49.0) | 326.5 (41.4) | - | 0.36 | 272.6 (54.4) | 275.4 (53.3) | 249.4 (65.2) | 0.02 |
| BMDt (mg/cm3) (mean±SD) | 226.4 (55.8) | 219.1 (48.6) | - | 0.04 | 203.4 (58.5) | 200.3 (58.0) | 187.8 (5.1) | 0.02 |
| BMDc (mg/cm3) (mean±SD) | 1028.4 (55.8) | 1020.6 (54.0) | - | 0.46 | 1001.0 (58.5) | 999.0 (73.9) | 989.7 (42.5) | 0.94 |
| Calf muscle area (mm2) | 7251 (1207) | 7365 (1308) | - | 0.24 | 5778 (923) | 5720 (1042) | 5529 (1062) | 0.80 |
p age-adjusted.
Fig. 1.
Ala1330Val and Val667Met LRP5 gene polymorphisms and Cortical Bone Area (CBA) in men and women. In women, for Ala1330Val gene polymorphism, TT vs TC vs CC was associated with lower CBA (B [SE]: −9.21 [4.73]; p for trend=0.04, and for Val667Met gene polymorphism AA vs AG vs GG was associated with lower CBA (B [SE]: −13.8 [5.5]; p for trend=0.01 (age-adjusted ANOVA).
After adjustment for potential confounders (age, height, weight, calcium intake, calf cross-sectional muscle area, levels of physical activity and serum PTH, vit-D, estradiol, bioavailable testosterone), women with TT and AA allele variants showed significantly lower values of other allele variants in CBA, whereas no difference were observed in the male population (Table 4). In a secondary analysis, adjusted for multiple confounders performed in participants >65 years old, we found similar results (for Ala1330Val gene polymorphism, B (SD): 12.4 (4.9); p=0.01 and for Val667Met gene polymorphism, B (SD): 7.1 (2.5); p=0.02). We also tested the interaction between LRP5 gene polymorphisms and calf cross-sectional area (CSAM) in both sexes, and we found no significant association between these polymorphisms and CSAM (Ala1330Val gene polymorphism*CSAM p=0.64 and Val667Met gene polymorphism*CSAM p=0.65 in men, Ala1330Val gene polymorphism*CSAM p=0.48 and Val667Met gene polymorphism*CSAM p=0.76 in women).
Table 4.
Association between LRP5 gene polymorphism and bone parameters in older men and women.
| Dependent Variable vBMDt
|
Dependent Variable vBMDc
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
Men
|
Women
|
|||||
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | |
|
| ||||||||
| LRP5 (TT vs CC) | −7.11 (5.08) | 0.16 | −1.04 (5.58) | 0.85 | 0.57 (5.72) | 0.92 | −3.31 (6.12) | 0.59 |
| LRP5 (AA vs GG) | −15.1 (8.2) | 0.07 | −2.2 (7.8) | 0.78 | −12.1 (9.1) | 0.18 | −1.0 (9.0) | 0.91 |
| Dependent Variable CBA
|
Dependent Variable Ct.Th
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
Men
|
Women
|
|||||
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | |
|
| ||||||||
| LRP5 (TT vs CC) | 5.92 (4.71) | 0.20 | −9.21 (4.73) | 0.04 | 0.08 (0.10) | 0.44 | −6.22 (4.78) | 0.19 |
| LRP5 (AA vs GG) | −10.8 (7.6) | 0.16 | −13.8 (5.5) | 0.01 | −0.15 (0.16) | 0.35 | 0.13 (0.13) | 0.3 |
Parsimonious models obtained by backward selection method from initial models including age, BMI, cross-sectional muscle area, levels of physical activity and serum bioavailable testosterone, estradiol, Vit-D and PTH.
DISCUSSION
Our findings provide novel insights into the possible role of LRP5 polymorphisms in regulating bone structure, indicating that specific LRP5 genotypes affect bone macro-architectural changes in older women, independently of BMD and other potential counfounders. In fact, the LRP5 1330-valine and 667-metionin variants were associated with lower Ct.Th and CBA in women. These results match those of recent studies showing the key role played by LRP5/Wnt signaling in bone mechanosensation (25) and in LRP5 G171V transgenic mice having a diffuse reduction in cortical bone thickness (15).
Recent findings from a genome-wide association study have also shown that the LRP5 gene is associated with decreased bone mineral density, osteoporosis, and an increased risk of osteoporotic fracture, independently of its effect on bone mineral density. These results suggest that the effects of this gene polymorphism on the risk of fracture may be both dependent and independent of bone mineral density (26).
Since the discovery of the LRP5 gene as an important factor in the regulation of bone metabolism, the gene encoding for the receptor has been indicated as a susceptibility gene in regulating BMD and/or fracture risk in the general population (27). Matching our findings, the A1330V polymorphism was significantly linked with DEXA-derived BMD at the Lumbar Spine (LS) and Femoral Neck (FN) in white pre-menopausal women and man with idiopathic osteoporosis, and with LS BMD, LS bone area and FN width in the Rotterdam study (28). Interestingly, the A1330V polymorphism was found to be only marginally associated with BMD at the radial bone in post-menopausal Japanese women (29). Other studies, however, were unable to confirm the association of LRP5 A1330 polymorphism with bone parameters in adult males (30). In a recent review on the genetics of the LRP5 gene, a number of interesting conclusions were proposed (31). First, genetic variations in the LRP5 gene appears to be associated not only with BMD but also with fracture risk in older individuals (31). Second, LRP5 gene variants contribute to BMD variance even in young individuals, suggesting that this gene plays a role in bone development and morphogenesis and therefore has a direct effect on bone mass (31). Thus, in older persons, the rate of variance in BMD may be explained by the combined effects on peak bone mass and differential age-related bone loss. Lastly, the effect of LRP5 gene polymorphisms on bone phenotypes are consistently different in men compared with women (31), suggesting gender-related interactions with this polymorphism. Altogether, these conclusions support our findings.
Geometrical parameters, such as cortical area and cortical thickness, are increasingly recognized as important components of bone strength (32, 33) and there is widespread agreement that differences in bone geometry between the sexes may partially explain the lower rates of fragility fractures in men compared with women. The relevant role of cortical thickness in determining bone strength has recently been confirmed by Mayhew et al., who used computed topography to measure bone distribution in mid-femoral segments post-mortem (34). By establishing a link between genetic variations and bone geometrical parameters, our findings may help to identify bone phenotypes with higher susceptibility to fractures.
To our knowledge, our study is the first that evaluates the influence of LRP5 polymorphisms on the micro- and macro-architectural characteristics of bone assessed by pQCT at the tibia in adult women. However, given the cross-sectional nature of the study, our findings suggest association, but cannot infer causality. Since this is a observational study, we did not perform a sample size calculation a priori but used all available data, on the assumption that finding a significant difference in pQCT parameters associated with LRP5 polymorphisms would improve our understanding in the biological effect although the difference would be small. Additional limitation to this study is that these LRP5 polymorphism variants may be co-inherited markers for a causative polymorphic variant that was not examined in this study. Therefore, this study shows an association of alleles with a bone phenotype, but does not demonstrate a cause/effect relationship. These variants may also have a combined/synergistic effect on CBA, due to a polymorphic variant at another locus within the haplotype block. Lastly, our results should be interpreted with caution, as the number of individuals observed with TT or AA variants is very small. Thus, our findings should be replicated in an independent population.
The existence of a relationship between LRP5 gene polymorphisms and geometric parameters needs to be confirmed in longitudinal studies, by showing that macro-architectural bone changes induced by LRP5 gene polymorphisms at least partially explain higher risk of fracture conferred by LRP5 gene polymorphisms (35). Thus, the potential confounders used here may represent the causal pathway between the influence of LRP5 gene polymorphisms with cortical bone loss.
Limitations notwithstanding, our findings provide novel insights into the possible role of LRP5 gene polymorphisms in regulating bone structure, and suggest that, in women, bone geometry is influenced by this gene, independently of BMD.
Acknowledgments
The InCHIANTI study was supported as a “targeted project” (ICS 110.1\RS97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336), and by U.S. National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). This work was also supported by MIUR 2003 to MLB “Genetic Markers of Osteoporosis in the Italian population”, FIRB PNR 2001–2003 (protocol RB-NE01C5S2) (to MLB) “Identification of Genetic Susceptibility to Multifactorial Diseases in the Italian Population, ISS 2003 SARA project no. 4AF/F10 (to MLB) “Correlation Study between Endocrine Estrogenic Activity and Genetic Polymorphisms”, and the Ente Cassa di Risparmio di Firenze (to MLB). Dr. Chiara Cepollaro received an unrestricted grant from the Agenzia Spaziale Italiana. We thank Dr. Cosimo Roberto Russo for assistance in the methodological aspects of performing pQCT scans. The authors report no conflict of interest.
References
- 1.NIH Consensus Statement Online (2000) Osteoporosis Prevention, Diagnosis, and Therapy. 2000;17:1–36. ( http://consensus.nih.gov/2000/2000Osteoporosis111html.htm) [PubMed] [Google Scholar]
- 2.Woolf AD, Akesson K. Preventing fractures in elderly people. BMJ. 2003;327:89–95. doi: 10.1136/bmj.327.7406.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maggi S, Lauretani F, Ferrucci L, et al. The quality of bone: a “magic natural alloy”. Aging Clin Exp Res. 2004;16(Suppl):3–9. [PubMed] [Google Scholar]
- 4.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–10. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 6.Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002;346:1572–4. doi: 10.1056/NEJM200205163462011. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Slee RB, Fukai N, et al. Osteoporosis-Pseudoglioma Syndrome Collaborative Group. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 8.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Giroux S, Elfassihi L, Cardinal G, Laflamme N, Rousseau F. LRP5 coding polymorphisms influence the variation of peak bone mass in a normal population of French-Canadian women. Bone. 2007;40:1299–307. doi: 10.1016/j.bone.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Grundberg E, Lau EM, Lorentzson M, et al. Large-scale association study between two coding LRP5 gene polymorphisms and bone phenotypes and fractures in men. Osteoporos Int. 2008;19:829–37. doi: 10.1007/s00198-007-0512-z. [DOI] [PubMed] [Google Scholar]
- 11.Sims AM, Shephard N, Carter K, et al. Genetic analyses in a sample of individuals with high or low BMD shows association with multiple Wnt pathway genes. J Bone Miner Res. 2008;23:499–506. doi: 10.1359/jbmr.071113. [DOI] [PubMed] [Google Scholar]
- 12.Hartikka H, Mäkitie O, Männikkö M, et al. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J Bone Miner Res. 2005;20:783–9. doi: 10.1359/JBMR.050101. [DOI] [PubMed] [Google Scholar]
- 13.Koller DL, Ichikawa S, Johnson ML, et al. Contribution of the LRP5 gene to normal variation in peak BMD in women. J Bone Miner Res. 2005;20:75–80. doi: 10.1359/JBMR.041019. [DOI] [PubMed] [Google Scholar]
- 14.Urano T, Shiraki M, Ezura Y, et al. Association of a single-nucleotide polymorphism in low-density lipoprotein receptor-related protein 5 gene with bone mineral density. J Bone Miner Metab. 2004;22:341–5. doi: 10.1007/s00774-003-0492-9. [DOI] [PubMed] [Google Scholar]
- 15.Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: a union made for bone. J Bone Miner Res. 2004;19:1749–57. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- 16.Babij P, Zhao W, Small C, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–74. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 19.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 20.Russo CR, Lauretani F, Bandinelli S, et al. Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–8. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 21.Russo CR, Lauretani F, Seeman E, et al. Structural adaptations to bone loss in aging men and women. Bone. 2006;38:112–8. doi: 10.1016/j.bone.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Sievanen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I. Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J Bone Miner Res. 1998;13:871–82. doi: 10.1359/jbmr.1998.13.5.871. [DOI] [PubMed] [Google Scholar]
- 23.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okubo M, Horinishi A, Kim DH, Yamamoto TT, Murase T. Seven novel sequence variants in the human low density lipoprotein receptor related protein 5 (LRP5) gene. Hum Mutat. 2002;19:186. doi: 10.1002/humu.9012. [DOI] [PubMed] [Google Scholar]
- 25.Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 26.Richards JB, Rivadeneira F, Inouye M, et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet. 2008;371:1505–12. doi: 10.1016/S0140-6736(08)60599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari SL, Deutsch S, Antonarakis SE. Pathogenic mutations and polymorphisms in the lipoprotein receptor-related protein 5 reveal a new biological pathway for the control of bone mass. Curr Opin Lipidol. 2005;16:207–14. doi: 10.1097/01.mol.0000162326.62419.e4. [DOI] [PubMed] [Google Scholar]
- 28.van Meurs JB, Rivadeneira F, Jhamai M, et al. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res. 2006;21:141–50. doi: 10.1359/JBMR.050904. [DOI] [PubMed] [Google Scholar]
- 29.Ezura Y, Nakajima T, Urano T, et al. Association of a single-nucleotide variation (A1330V) in the low-density lipoprotein receptor-related protein 5 gene (LRP5) with bone mineral density in adult Japanese women. Bone. 2007;40:997–1005. doi: 10.1016/j.bone.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Crabbe P, Balemans W, Willaert, et al. Missense mutations in LRP5 are not a common cause of idiopathic osteoporosis in adult men. J Bone Miner Res. 2005;20:1951–9. doi: 10.1359/JBMR.050705. [DOI] [PubMed] [Google Scholar]
- 31.Balemans W, Van Hul W. The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology. 2007;148:2622–9. doi: 10.1210/en.2006-1352. [DOI] [PubMed] [Google Scholar]
- 32.Cepollaro C, Lauretani F, Gozzini A, et al. Relationship of volumetric bone mineral density and structural parameters with ERalpha gene polymorphisms. Calcif Tissue Int. 2007;80:307–15. doi: 10.1007/s00223-007-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauretani F, Bandinelli S, Griswold ME, et al. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–8. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayhew PM, Thomas CD, Clement JG, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 35.van Meurs JB, Trikalinos TA, Ralston SH for the GENOMOS Study. Large-scale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA. 2008;299:127–90. doi: 10.1001/jama.299.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]