Abstract
Background
The Motoric Cognitive Risk Syndrome (MCR) is characterized by slow gait speed and cognitive complaints.
Objectives
The objective of this study was to determine if the presence of MCR increases the risk of falls in older people.
Methods
Individual participant data (n = 6,204) from five longitudinal studies from three countries were used for this analysis. MCR diagnosis was defined as both the presence of objectively measured slow gait speed and subjective cognitive complaints in those without dementia or mobility disability. Falls were prospectively ascertained using phone calls or questionnaires. Log binomial regression was performed to determine if MCR increased the risk of falls separately in each cohort. Random effects meta-analysis was used to pool results from all cohorts.
Results
The mean age of participants was 74.9 (SD 6.8) years and 44% (n = 2728) were male. Overall 33.9% (n = 2104) reported a fall over follow-up. Pooled relative risk of MCR with any falls was RR 1.44 95% CI 1.16, 1.79. The components of MCR, slow gait (RR 1.30 95% CI 1.14, 1.47) and cognitive complaint (RR 1.25, 95% CI 1.07, 1.46) were also associated with an increased risk of any falls. In sub-analyses MCR was associated with any fall independent of previous falls (RR 1.29 95% CI 1.09, 1.53) and with multiple falls (RR 1.77, 95% CI 1.25, 2.51).
Conclusion
MCR is associated with an increased risk of falls. The increase in risk was higher than for its individual components. The simplicity of the MCR makes it an attractive falls risk screening tool for the clinic.
Keywords: Cognition, dementia, falls, gait
INTRODUCTION
Falls occur in over 30% of older people living in the community [1]. A fall can result in a number of adverse outcomes ranging from minor injury to fracture, hospitalization or even death [2]. The long-term effects of falls include fear of falling [3], subsequent loss of mobility, and increased need for care or nursing home placement [2]. Identifying those at risk of falling and subsequently implementing appropriate interventions is an important goal in geriatric care.
Risk factors for falls are multifactorial, and include physical impairments such as reduced gait and balance [4] as well as cognitive impairment in the domains of executive function, attention, processing speed, and memory [5–7]. Gait speed has emerged as an important marker of people at risk of falls as it likely represents an overall summary measure of a person’s ability to compensate for age and disease related impairments in multiple systems [1, 8]. Importantly, the co-existence of both gait and cognitive impairments appears to be multiplicative in increasing the overall risk of falling [5].
Screening older people for falls risk is recommended at least once per year by a health professional [9]. Assessment of gait and balance is currently recommended in the screening phase. Cognitive testing is assessed in the second stage as part of a multifactorial assessment if screening is positive [9, 10]. This may be due to time commitments or the requirement of a specialized health professional to administer and interpret some cognitive tests. However, a simple measure of cognition in combination with a gait assessment may better assist in identifying those most at risk of falling.
Recently, we reported on a new pre-dementia syndrome—the Motoric Cognitive Risk Syndrome (MCR)—characterized both by slow gait and presence of subjective cognitive complaints [11–13]. MCR was shown to have improved predictive validity for dementia compared to its individual components of subjective cognitive complaints or slow gait [11]. MCR may provide a sensitive screening test for falls as its components are also important falls risk factors.
Therefore, the aim of this study was to determine if the presence of MCR increases the risk of falls using data from five longitudinal cohort studies of community-dwelling older people based in three countries. We hypothesized that a diagnosis of MCR would be a stronger risk factor for falls than its individual cognitive and motor components.
MATERIALS AND METHODS
Participants
The MCR falls study includes individual data for participants from five established longitudinal aging studies in the USA (n = 3), Italy (n = 1), and Australia (n = 1). Individual study details have been published previously [14–18], and are briefly summarized here. LonGenity recruited an Ashkenazi Jewish cohort from New York City (USA) and surrounding counties with the aim of identifying longevity associated genotypes [14]. The Einstein Aging Study (EAS) is a population-based study of cognitive ageing in community-dwelling older people from Bronx county (USA) [12, 15]. The Health and Retirement Study (HRS) is a population-based study in the USA, which aims to examine changes in labor force participation and health in retirement [16, 19]. InCHIANTI is a population-based study of older people recruited from the Chianti region (Tuscany, Italy), with the aim of developing tools and treatments to prevent mobility problems [17]. The Tasmanian Study of Cognition and Gait (TASCOG) is a population-based study that aims to determine the neural correlates of cognition, gait and falls in older people from, Tasmania, Australia [18]. For this analysis, participants were included if they were aged ≥60 years, had an assessment of subjective complaints, objectively measured walking speed and ascertainment of falls after the baseline assessment. Participants were excluded if they resided in a nursing home, or were diagnosed with dementia. For LonGenity and EAS cohorts, dementia was diagnosed after review of all clinical and neuropsychological data at consensus case conferences. Self-report or proxy report of a dementia diagnosis was used to exclude participants in the HRS and InCHIANTI studies. A combination of self-report or proxy and available clinical and neuropsychological data was used in TASCOG. All studies obtained written consent from participants and had local institutional ethics approval.
Motoric cognitive risk syndrome diagnosis
MCR diagnosis was defined as both the presence of slow gait speed and cognitive complaints in those without dementia or immobility (inability to ambulate even with assistance or walking aids) [12]. The operational definition and methods used to diagnose MCR is analogous to mild cognitive impairment (MCI) [20]. Both disorders require subjective cognitive complaints. For MCI, objective cognitive impairment is required while for MCR objective gait impairment is necessary. Gait impairment was defined based on walking speed (cm/s) one standard deviation below age and sex specific means. Cohort specific norms were developed (Table 1) to account for differences in populations and testing procedures (as done in our previous MCR validation study and described further in Table 2) [12]. Subjective cognitive complaints were obtained based on responses to items on standardized questionnaires. Subjective cognitive procedures are further outlined in Table 2.
Table 1.
Slow Gait Cut points for each cohort
| Slow Gait Cuts (cm/s)
|
||||
|---|---|---|---|---|
| M ≥75 | M <75 | F ≥75 | F <75 | |
| Australia (TASCOG) | 86.00 | 102.82 | 71.97 | 93.57 |
| USA (LonGenity) | 85.35 | 101.95 | 76.73 | 97.44 |
| USA (HRS) | 48.36 | 61.36 | 42.42 | 53.61 |
| Italy (InCHIANTI) | 69.70 | 93.80 | 56.79 | 85.73 |
| USA (Einstein Aging Study) | 72.2 | 88.0* | 66.4 | 76.7* |
TASCOG, Tasmanian Study of Cognition and Gait; HRS, Health Retirement Study; InCHIANTI, Invecchiare in Chianti; EAS, Einstein Aging Study;
The EAS cohort did not recruit participants younger than age 70, and the cut scores are for participants aged 70 to 74 years.
Table 2.
Participant characteristics in each cohort
| Study (n) | LonGenity (n = 509) | EAS (n = 817) | HRS (n = 3,640) | InCHIANTI (n = 832) | TASCOG (n = 406) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline study year | 2008* | 2004* | 2006 | 1998 | 2005 | |||||
| Age range | 64–94 | 69–100 | 65–105 | 60–95 | 61–86 | |||||
| Age, mean SD | 75.0 | 6.2 | 79.7 | 5.4 | 74.4 | 6.7 | 73.5 | 6.8 | 72.0 | 7.0 |
| Male n, % | 243 | 47.7 | 314 | 38.4 | 1567 | 43.1 | 373 | 44.8 | 231 | 56.9 |
| Education (y) mean, SD | 17.7 | 2.7 | 14.2 | 3.4 | 12.4 | 3.1 | 5.7 | 3.4 | 10.8 | 3.7 |
| Gait speed, (cm/s), mean SD | 110.8 | 19.0 | 94.6 | 23.3 | 78.0 | 25.6 | 103.3 | 25.5 | 113.1 | 22.6 |
| Medical history, n, % | ||||||||||
| Depression | 92 | 21.0 | 91 | 11.1 | 418 | 11.5 | 247 | 29.8 | 16 | 3.9 |
| Diabetes | 44 | 8.7 | 156 | 19.1 | 761 | 20.9 | 109 | 13.1 | 54 | 13.3 |
| Hypertension | 214 | 44.7 | 522 | 63.9 | 2330 | 64.1 | 530 | 63.7 | 202 | 49.8 |
| Stroke | 11 | 2.2 | 67 | 8.2 | 237 | 6.5 | 36 | 4.3 | 35 | 8.6 |
| Arthritis | 211 | 42.1 | 590 | 72.2 | 2522 | 69.3 | 359 | 43.2 | 223 | 55.5 |
| MCR, n % | 56 | 11.0 | 99 | 12.1 | 244 | 6.7 | 57 | 6.9 | 7 | 1.7 |
| Cognitive Complaint | GDS†/self-report‡ | GDS†/self-report‡ | Self-reportx | Disability scale§ | GDS† | |||||
| Cognitive Complaint | 392 | 77.0 | 681 | 83.4 | 1512 | 41.5 | 143 | 17.2 | 45 | 11.1 |
| Gait | GAITRite | GAITRite | 2.5m walk | 4m walk | GAITRite | |||||
| Slow gait | 71 | 14.0 | 122 | 14.9 | 494 | 13.6 | 111 | 13.3 | 55 | 13.6 |
| Falls question | “Have you had any falls in the last 12 months” | “Have you had any falls in the past 2 months” Have you had any falls in the past year” | “Have you fallen down in the last two years” | “Did you ever the falls down in the past months” | “Have you had any falls in months of --” | |||||
| Falls timeframe | follow-up clinic (1–2 years) | 2 monthly telephone calls and at follow-up clinic (1 year) | follow-up clinic (2 years) | follow-up clinic (3 years) | 2 monthly questionnaire (1 year) | |||||
| Falls, n % | 181 | 35.6 | 308 | 37.7 | 1263 | 34.7 | 191 | 23.0 | 161 | 39.7 |
EAS, Einstein Aging Study; HRS, Health Retirement Study; InCHIANTI, Invecchiare in Chianti; TASCOG, Tasmanian Study of Cognition and Gait;
The wave with the first gait assessment for each participant was used.
GDS = Geriatric Depression Scale item “Do you feel you have more problems with memory than most”;
self-rating of memory in past year or past 10 years;
self-rating of memory at current time and over previous 2 years;
Score >1 Instrumental Activities of Daily Living Scale.
Strengths of the components of MCR are that slow gait is defined objectively, independent of clinical gait evaluations that may be prone to variable sensitivity and specificity as well as being examiner dependent. Subjective cognitive complaints do not require formal cognitive testing, but have been reported to be associated with reduced cognitive function and increased risk of dementia [21].
Falls
Falls were ascertained for 12 months after baseline assessment for EAS and TASCOG. Falls were ascertained in EAS every 2 months by telephone and in-person at the 12-month follow-up visits, and for TASCOG every 2 months by questionnaire. In the other cohorts, a standardized question about falls in the past 12 months (InCHIANTI, LonGenity) or past 24 months (HRS) was used at the follow-up subsequent to the MCR ascertainment. The number of falls that were reported over the time period was available in InCHIANTI, TASCOG, and HRS. In TASCOG, EAS, and LonGenity, a fall was defined as an unexpected event in which the participant comes to rest on the ground, floor, or lower level not due to a major intrinsic or extrinsic event. InCHIANTI used a similar definition of a fall coming to the ground that occurred during the last year. HRS did not further define falls over the question “Have you fallen down in the past 2 years”. Falls prior to MCR assessment were available in all cohorts (all previous 12 months other than HRS which was previous 24 months).
Other measures
Age, sex, education, and self-report of physician diagnosis of hypertension, diabetes, heart condition, arthritis, and stroke were obtained using a standardized questionnaire in each study. The presence of a lung condition was also available in EAS and HRS. Depressive symptoms were assessed with the short form of the Geriatric Depression Scale (GDS) for LonGenity, EAS and TASCOG, and The Center for Epidemiologic Studies Depression Scale (CES-D) for HRS and InCHIANTI.
Statistical analysis
T-tests and Chi-squared analyses were used to compare participants included in the study with those excluded due to missing data. Descriptive statistics were used to summarize the characteristics of participants. Log binomial regression was performed to determine if MCR increased the relative risk of falls separately in each cohort. All models were adjusted for age and sex. Further adjustment was made for education and the above listed medical conditions if the relevant variable changed the coefficient of MCR by more than 10%. Weighted analysis was carried out on the HRS data using the 2008 respondent level population weights, updated in 2011 (http://hrsonline.isr.umich.edu/sitedocs/userg/dr-013.pdf). Random effects meta-analysis was used to pool results from each cohort. Heterogeneity between studies was reported as the I2 statistic, and tested using χ2 test. This analysis procedure was repeated in separate models for the two components of MCR (slow gait and cognitive complaint), including an analysis with MCR cases excluded.
The extent to which study-level variables explained heterogeneity in the relationship between MCR and falls was explored using meta-regression. The following variables were considered: Method of measuring gait speed (GAITRite or stopwatch) and the cognitive complaint measurement question (GDS/self-report or Disability scale). To account for the possibility that the findings may have been biased from missing data, we performed the analysis between MCR and falls using inverse propensity weighting. Complete cases are weighted by the inverse of their probability of being a complete case, with those that have a low probability of being a complete case receiving a larger weight. Regression models controlling for baseline information (age, sex, years of education, and all medical conditions described above) were used to estimate the probability of response in each cohort separately, and the reciprocals of these propensities were used as weights in the analysis of risk.
MCR sub-analyses
We conducted two sub-analyses. As previous falls is an important screening question we adjusted models for this variable to determine the independence of the relationship between MCR and future new falls. We also examined the relationship between MCR and prospective multiple falls (0/1 for ≥s2 falls) where this information was available (InCHIANTI, HRS, and TASCOG). STATA 12 was used for all analyses. All studies obtained written and informed consent from participants and were approved by the local ethics committees (EAS and LonGenity: Einstein Institutional Review Board; HRS: University of Michigan and National Institute on Aging Institutional Review Board; InCHIANTI: Instituto Nazionale Riposo e Cura Anziani institutional review board in Italy; TASCOG: The Southern Tasmanian Health and Medical Human Ethics committee).
RESULTS
There were a total of 8,369 participants in the five studies. Participants were excluded if they were <60 years (n = 250), had dementia (n = 192), or resided in a nursing home (n = 49), leaving 7,878 eligible for the study. Participants with missing gait (n = 792), cognitive complaint (n = 1), or missing follow-up falls data (n = 881) were also excluded leaving a total of 6,204 eligible participants. Compared to those with missing data in each cohort: LonGenity participants were younger (p < 0.001), more educated (p = 0.03), and more likely to report a myocardial infarct (p = 0.01); EAS participants were younger (p < 0.001) and less likely to report a history of arthritis (p = 0.01); HRS participants were younger, better educated, and less likely to have diabetes, a heart condition, stroke, depression, a lung condition (all p < 0.001), or arthritis (p = 0.01); InCHIANTI participants were younger and less likely to report a history of arthritis or high blood pressure (p < 0.001). There were no differences between those included in TASCOG versus those with missing data (p > 0.05).
The characteristics of participants in each cohort are shown in Table 2. The mean age of participants was 74.9 years (SD 6.8; range 60–105) and 44.0% (n = 2,728) were male. Mean years of education were 12.1 (4.4). Forty-five percent (n = 2,773) of participants reported a cognitive complaint, 13.8% (n = 853) had slow gait, and 7.5% (n = 463) had a diagnosis of MCR.
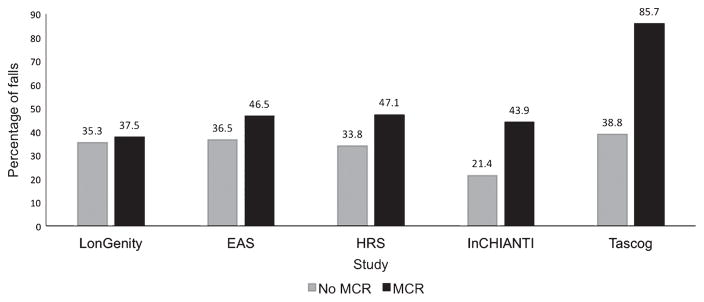
Overall 33.9% (n = 2,104) of participants reported any fall over study follow-up. Figure 1 shows the percentage of incident fallers in each cohort by baseline MCR diagnosis. The percentage of falls was higher in those with a diagnosis of MCR in EAS (p = 0.04), TASCOG (p < 0.001), HRS (p < 0.001), and InChi-anti (p < 0.001), but not in LonGenity (p = 0.74). Data on multiple falls were available in HRS (706/3640, 19.4%); TASCOG (68/406, 16.8%); and InCHI-ANTI (76/832, 9.1%). Number of previous falls in each study were: LonGenity (121/500, 24.2%); EAS (207/808, 25.6%); HRS (1098/3632, 30.2% over 24 months), InCHIANTI (172/832, 20.6%); and TASCOG (66/401 16.5%).
Fig. 1.
MCR status plotted against the percentage of people who reported any fall by cohort. MCR, Motoric Cognitive Risk syndrome; EAS, Einstein Aging Study; HRS, Health Retirement Study; InCHIANTI, Invecchiare in Chianti; TASCOG, Tasmanian Study of Cognition and Gait; Falls were ascertained over a 12-month period except for HRS where the question asked about falls in the previous 2 years.
MCR and any fall by cohort
Table 3 provides the relative risk of falls for MCR status, slow gait, and cognitive complaint in separate models by cohort. After adjustment for age and sex, MCR was associated with increased risk of falls in HRS (RR 1.36, 95% CI 1.18, 1.58), InCHI-ANTI (RR 1.78 95% CI 1.23, 2.55), and TASCOG (RR 2.15 95% CI 1.57, 2.94). Although the risk was increased in LonGenity (RR 1.06 95% CI 0.74, 1.52) and EAS (RR 1.18 95% CI 0.93, 1.49), these associations did not reach statistical significance. The addition of other potential confounders (demographic and medical history described above) did not change the co-efficient of MCR by more than 10% in any of the cohorts and were therefore not included in the models. For the individual components of MCR (Table 3), slow gait was associated with increased risk of falls in HRS, InCHIANTI, and TASCOG, but not for LonGenity and EAS. Subjective cognitive complaints were only associated with increased risk of falls in HRS and InCHIANTI.
Table 3.
Adjusted association of MCR, slow gait, cognitive complaint (separate models) with falls
| LonGenity (n = 509) | EAS (n = 817) | HRS (n = 3640) | InCHIANTI (n = 832) | TASCOG (n = 406) | |
|---|---|---|---|---|---|
|
| |||||
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| MCR | 1.06 (0.74, 1.52) | 1.18 (0.93, 1.49) | 1.37 (1.18, 1.58) | 1.78 (1.23, 2.55) | 2.15 (1.57, 2.94) |
| Slow gait | 1.08 (0.78, 1.49) | 1.10 (0.88, 1.38) | 1.30 (1.16,1.47) | 1.58 (1.17,2.11) | 1.51 (1.18, 1.93) |
| Cognitive complaint | 1.10 (0.82, 1.47) | 1.06 (0.83, 1.35) | 1.23 (1.12, 1.36) | 1.84 (1.36, 2.49) | 1.25 (0.92, 1.69) |
| MCR cases excluded | |||||
| Slow gait | 1.12 (0.60,2.10) | 0.73 (0.36,1.45) | 1.22 (1:03, 1.45) | 1.39 (0.89, 2.18) | 1.40 (1.06, 1.87) |
| Cognitive complaint | 1.09 (0.81, 1.47) | 1.03, 0.80, 1.32) | 1.19 (1.08,1.32) | 1.61 (1.12, 2.32) | 1.10 (0.76, 1.59) |
Models adjusted for age and sex; EAS, Einstein Aging Study; HRS, Health Retirement Study; InCHIANTI, Invecchiare in Chianti; TASCOG, Tasmanian Study of Cognition and Gait; RR, relative risk; MCR, Motoric Cognitive Risk syndrome.
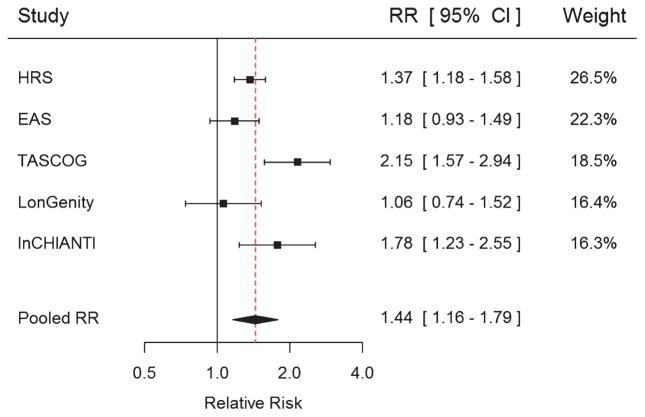
Pooled estimates of MCR and any fall
Figure 2 shows the relative risk of falls for MCR in the individual cohorts as well as the pooled estimates. Pooled relative risk of MCR with any fall was RR 1.44 95% CI 1.16, 1.79 (χ2 13.19 p = 0.01; I2 = 69.7%). In propensity-weighted analysis for missing data this association was largely unchanged for each cohort or for the pooled estimates (RR 1.44 95%CI 1.16, 1.78; χ2 13.22 p = 0.01; I2 = 69.7%). In meta-regression analysis, the method of ascertaining slow gait or cognitive complaint was not associated with the effect of MCR on falls risk (all p > 0.05). For the components of MCR, in separate models slow gait (RR 1.30 95% CI 1.14, 1.47; χ2 6.38 p = 0.17 I2 = 37.3%) and cognitive complaint (RR 1.25 95% CI 1.07, 1.46; χ2 8.94 p = 0.06 I2 = 55.3%) were associated with an increased risk of falls. In analysis with MCR cases excluded, the strength of the association between slow gait (RR 1.25 95% CI 1.09, 1.43; χ2 3.40 p = 0.49 I2 = 0 %), cognitive complaints (RR 1.17 95% CI 1.06, 1.30; χ2 = 4.40 p = 0.35 I2 = 9.1 %), and falls was slightly reduced.
Fig. 2.
Motoric Cognitive Risk syndrome and risk of falls for each cohort and pooled analysis. Models adjusted for age and sex.
Sub-analyses
Adjusting the association between MCR and falls by previous falls in each cohort reduced the strength of associations (LonGenity 1.04 95% CI 0.72, 1.49; EAS RR 1.13 95% CI 0.90, 1.41; HRS RR 1.20, 95% CI 1.05,1.36; InCHIANTI RR 1.68, 95% CI 1.17, 2.40; TASCOG RR 1.73, 95% CI 1.26, 2.37), but MCR remained significantly associated with falls in pooled analysis (RR 1.29 95% CI 1.09, 1.53).
MCR was associated with multiple falls in HRS (RR 1.59, 95% CI 1.30, 1.94) and TASCOG (RR 3.23 95% CI 1.46, 7.16) but not InCHIANTI (RR 1.55 0.76, 3.18). Overall MCR was associated with multiple falls in pooled analysis (RR 1.77, 95% CI 1.25, 2.51). This association reduced in strength but persisted after adjusting for previous falls (RR 1.37 95% CI 1.16, 1.62).
DISCUSSION
By combining five large cohorts of older people across three countries, and with a total of 6,204 participants we show for the first time that the presence of MCR at baseline was associated with a 44% increased risk of future falls in pooled analyses. The increase in risk was higher than for slow gait speed, a recommended falls risk screening test [22]. The simplicity of the MCR makes it an attractive, quick, and inexpensive screening tool for falls in the clinic.
Our study has several strengths. This is the first study to examine the association between MCR and risk of falls. To do this, we combined five longitudinal population based studies of older people from three different countries, increasing power and generalizability of results. Gait speed, cognitive complaints, and falls ascertainment were obtained with standardized protocols and questionnaires in each study. However, there were also limitations to the study. In the pooled analysis, there was a moderate to large amount of heterogeneity, potentially due to the different procedures used to diagnose MCR and ascertain falls, as well as differences in cohorts and their underlying comorbidities. These differences were unavoidable, as each study was designed before the inception of this analysis and the MCR concept. In the included studies, gait speed was either obtained with an electronic mat or derived from time taken to walk over a fixed distance. To account for differences in procedures, we defined slow gait separately in each cohort by age and sex similar to that of MCI [23]. However, this method may limit its applicability to the clinic. The range of methods for diagnosing cognitive complaint highlights the need for a consistent definition of MCR in future studies. Interestingly studies with a question about memory over a longer time frame were less likely to be associated with falls. Although falls were measured prospectively after baseline assessment for MCR, the time from baseline assessment varied between cohorts and questions about falls covered periods ranging from 2–24 months, potentially resulting in recall bias and missing some falls in LonGenity, HRS, and InCHIANTI. However, despite HRS obtaining falls data after 2 years, a recent analysis found an increase in reporting of falls over study waves (1998–2010) that was potentially due to improved reporting, rather than age, disability, or chronic disease factors [24]. Despite limitations, the prevalence of MCR [11] and percentage of falls [25] were consistent with that previously reported in the literature and the relationship between MCR and falls for each cohort was in the expected direction. It is possible that the longer follow-up led to under reporting of falls and therefore our results may provide a conservative estimate of the association between MCR and falls. Additionally, meta-regression analysis did not find measurement differences associated with the effect of MCR on falls, although our power was limited.
MCR was a stronger predictor of falls than its individual components—slow gait speed and subjective cognitive complaints. Findings for the individual components of MCR are consistent with previous studies that have shown slow gait speed [1, 8, 22] and poorer cognitive function, measured using standardized neuropsychological tests [5, 7, 26], increase the risk of falls in older community dwelling people. In concordance, the assessment of gait is a core component of fall risk screenings in older people [9, 10]. In contrast, assessment of cognitive impairment is not generally recommended as part of an initial screening for falls risk in the community setting, but later as part of a multifactorial assessment if screening tests are positive [9, 10]. This may be due to the time required to perform cognitive testing or the need for a qualified health professional. The advantage of a diagnosis of MCR is that it is comprised of a quick and simple question regarding a cognitive complaint that does not require trained staff, combined with a timed gait test that can be performed quickly and inexpensively without any specialized equipment. This simplicity makes it an attractive falls risk screening tool in a wide variety of outpatient settings.
We carried out two sub-analyses. In pooled analysis from three cohorts, MCR increased the risk of multiple falls by 77%. The associations were stronger than for any falls. This is not surprising as we have previously found physical risk factors are more likely to predict multiple, rather than single falls [1]. Secondly, we found that MCR was associated with falls independent of previous falls, indicating the importance of also assessing MCR as part of screening for falls in older people.
The mechanism underlying the relationship between MCR and falls is uncertain, but likely due to factors underlying its individual components. Gait speed has been described as a good summary measure of a person’s overall ability to compensate for decline in multiple body systems [27] including sensorimotor and cognitive function [28, 29], which are also common risk factors for falls. Subjective cognitive complaints may also reflect actual cognitive impairment [30, 31], but in addition are associated with psychological distress [32] and personality traits such as neuroticism, perceived stress, and ineffective coping styles [33]. Interestingly, these psychological factors are associated with increased falls risk, with the underlying mechanisms largely unknown, but potentially related to fear of falling, reductions in physical activity, or centrally acting medications [34–37]. Alternatively it is plausible that the combination of slow gait and subjective cognitive complaints increases an individual’s risk of falling by reducing their overall ability to compensate for physical impairments with adequate cognitive function or vice versa. Finally, the few studies examining correlates of MCR have reported associations with higher levels of chronic disease, dementia, depression, hypertension, diabetes, obesity, and sedentariness as well as stroke [12, 13, 38], suggesting MCR may be a good marker of overall health. A number of these factors provide potential targets for therapeutic interventions to prevent falls. Exercise in particular is recommended to improve gait speed [39], cognition [40], cardiovascular risk [41], and psychological symptoms [42]. However, further studies are required to determine if addressing modifiable risk can reverse MCR and reduce the risk of falls in older people.
In conclusion the results of this and our previous studies [11, 12] suggest MCR is a high-risk clinical syndrome that predicts adverse health outcomes such as falls in older people. Diagnosing MCR provides a quick and inexpensive falls-risk screening test for the clinic, that if present suggests the need for a comprehensive multifactorial falls risk assessment [9].
Acknowledgments
The LonGenity Study was funded by NIH (R00AG037574, 1P01AG034906, R01AG046949, 1R01AG042188, P30AG038072, and NIH R37AG18381), CTSA KL2TR000088, Einstein Glenn, Paul Glenn Foundation, and the American Federation for Aging Research.
The Einstein Aging Study was funded by the National Institute on Aging (1P01AG03949).
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan.
The InCHIANTI Study was funded by the Italian Ministry of Health (grant ICS 110.1/RS97.71) and NIH (grants 263 MD916413, 263 MD 821336, 1ZIAAG001050).
TASCOG was funded by National Health and Medical Research Council (NHMRC) grants 403000 and 491109.
Dr M Callisaya was supported by an NHMRC Early Career Fellowship (APP 1034483).
Prof VK Srikanth is supported by an NHMRC Career Development Fellowship (APP1061457) and a Heart Foundation Future Leader Fellowship (ID 100089).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0230r1).
References
- 1.Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK. Gait, gait variability and the risk of multiple incident falls in older people: A population-based study. Age Ageing. 2011;40:481–487. doi: 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 3.Delbaere K, Crombez G, Vanderstraeten G, Willems T, Cambier D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing. 2004;33:368–373. doi: 10.1093/ageing/afh106. [DOI] [PubMed] [Google Scholar]
- 4.Lord SR, Clark RD, Webster IW. Physiological factors associated with falls in an elderly population. J Am Geriatr Soc. 1991;39:1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin KL, Blizzard L, Srikanth VK, Wood A, Thomson R, Sanders LM, Callisaya ML. Cognitive function modifies the effect of physiological function on the risk of multiple falls–a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:1091–1097. doi: 10.1093/gerona/glt010. [DOI] [PubMed] [Google Scholar]
- 6.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: Results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 7.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: A prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. [Accessed 29 February 2016];Falls in older people: Assessing risk and prevention. 2013 https://www.nice.org.uk/guidance/cg161. [PubMed]
- 11.Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, Bridenbaugh SA, Buchman AS, Callisaya ML, Camicioli R, Capistrant B, Chatterji S, De Cock AM, Ferrucci L, Giladi N, Guralnik JM, Hausdorff JM, Holtzer R, Kim KW, Kowal P, Kressig RW, Lim JY, Lord S, Meguro K, Montero-Odasso M, Muir-Hunter SW, Noone ML, Rochester L, Srikanth V, Wang C. Motoric cognitive risk syndrome: Multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, Katz MJ, Lipton RB, Wang C. Motoric cognitive risk syndrome: Multicenter incidence study. Neurology. 2014;83:2278–2284. doi: 10.1212/WNL.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayers E, Barzilai N, Crandall JP, Milman S, Verghese J. Association of exceptional parental longevity and physical function in aging. Age (Dordr) 2014;36:9677. doi: 10.1007/s11357-014-9677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtzer R, Goldin Y, Zimmerman M, Katz M, Buschke H, Lipton RB. Robust norms for selected neuropsychological tests in older adults. Arch Clin Neuropsychol. 2008;23:531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Produced and distributed by the University of Michigan with funding from the National Institute on Aging. Ann Arbor, MI: 2015. Health, Retirement Study, public use dataset. (grant number NIA U01AG009740) [Google Scholar]
- 17.Zhang F, Ferrucci L, Culham E, Metter EJ, Guralnik J, Deshpande N. Performance on five times sit-to-stand task as a predictor of subsequent falls and disability in older persons. J Aging Health. 2013;25:478–492. doi: 10.1177/0898264313475813. [DOI] [PubMed] [Google Scholar]
- 18.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Sex modifies the relationship between age and gait: A population-based study of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:165–170. doi: 10.1093/gerona/63.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Smith AK, Walter LC, Miao Y, Boscardin WJ, Covinsky KE. Disability during the last two years of life. JAMA Intern Med. 2013;173:1506–1513. doi: 10.1001/jamainternmed.2013.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendonca MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: Who is at risk?: A systematic review. Am J Alzheimers Dis Other Demen. 2016;31:105–114. doi: 10.1177/1533317515592331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, Mayorga LM. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment: Current research and clinical implications. Semin Neurol. 2007;27:22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- 24.Cigolle CT, Ha J, Min LC, Lee PG, Gure TR, Alexander NB, Blaum CS. The epidemiologic data on falls, 1998–2010: More older Americans report falling. JAMA Intern Med. 2015;175:443–445. doi: 10.1001/jamainternmed.2014.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 26.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: A systematic review and meta-analysis. Age Ageing. 2012;41:299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- 27.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, Callisaya ML. Cognitive function, gait, and gait variability in older people: A population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:726–732. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- 29.Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25:292–299. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- 30.Rouch I, Anterion CT, Dauphinot V, Kerleroux J, Roche F, Barthelemy JC, Laurent B. Cognitive complaints, neuropsychological performance and affective disorders in elderly community residents. Disabil Rehabil. 2008;30:1794–1802. doi: 10.1080/09638280701667825. [DOI] [PubMed] [Google Scholar]
- 31.Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: Consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 32.Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: A cross-sectional study. BMC Psychiatry. 2011;11:108. doi: 10.1186/1471-244X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, Livney MG, McCoubrey H, Wolk DA, Kling MA, Arnold SE. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. 2013;28:776–783. doi: 10.1177/1533317513504817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvelde T, McVeigh C, Toson B, Greenaway M, Lord SR, Delbaere K, Close JC. Depressive symptomatology as a risk factor for falls in older people: Systematic review and meta-analysis. J Am Geriatr Soc. 2013;61:694–706. doi: 10.1111/jgs.12209. [DOI] [PubMed] [Google Scholar]
- 35.Peel NM, McClure RJ, Hendrikz JK. Psychosocial factors associated with fall-related hip fractures. Age Ageing. 2007;36:145–151. doi: 10.1093/ageing/afl167. [DOI] [PubMed] [Google Scholar]
- 36.Delbaere K, Close JC, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk of falling among elderly people: Cohort study. BMJ. 2010;341:c4165. doi: 10.1136/bmj.c4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ensrud KE, Blackwell TL, Mangione CM, Bowman PJ, Whooley MA, Bauer DC, Schwartz AV, Hanlon JT, Nevitt MC Study of Osteoporotic Fractures Research G. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–1637. doi: 10.1046/j.1532-5415.2002.50453.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang N, Allali G, Kesavadas C, Noone ML, Pradeep VG, Blumen HM, Verghese J. Cerebral small vessel disease and motoric cognitive risk syndrome: Results from the Kerala-Einstein Study. J Alzheimers Dis. 2016;50:699–707. doi: 10.3233/JAD-150523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 40.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 41.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK American Heart. Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity, Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 42.Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. Effect of exercise on depression severity in older people: Systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201:180–185. doi: 10.1192/bjp.bp.111.095174. [DOI] [PubMed] [Google Scholar]