Abstract
Introduction
Changes in mitochondrial DNA (mtDNA) can serve as a marker of cumulative oxidative stress (OS) due to the mitochondria’s unique genome and relative lack of repair systems. In utero particulate matter ≤2.5µm (PM2.5) exposure can enhance oxidative stress. Our objective was to identify sensitive windows to predict mtDNA damage experienced in the prenatal period due to PM2.5 exposure using mtDNA content measured in cord blood.
Material and Methods
Women affiliated with the Mexican social security system were recruited during pregnancy in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study. Mothers with cord blood collected at delivery and complete covariate data were included (n=456). Mothers’ prenatal daily exposure to PM2.5 was estimated using a satellite-based spatio-temporally resolved prediction model and place of residence during pregnancy. DNA was extracted from umbilical cord leukocytes. Quantitative real-time polymerase chain reaction (qPCR) was used to determine mtDNA content. A distributive lag regression model (DLM) incorporating weekly averages of daily PM2.5 predictions was constructed to plot the association between exposure and OS over the length of pregnancy.
Results
In models that included child’s sex, mother’s age at delivery, prenatal environmental tobacco smoke exposure, birth year, maternal education, and assay batch, we found significant associations between higher PM2.5 exposure during late pregnancy (35–40 weeks) and lower mtDNA content in cord blood.
Conclusions
Increased PM2.5 during a specific prenatal window in the third trimester was associated with decreased mtDNA content suggesting heightened sensitivity to PM-induced OS during this life stage.
Keywords: particulate matter, mitochondrial DNA, distributive lag models, prenatal exposure
1. Introduction
Prenatal exposure to particulate matter less than 2.5 microns in diameter (PM2.5) has been associated with a number of adverse fetal outcomes including reductions in birth weight and preterm birth (Fleisch et al. 2015; Hyder et al. 2014; Lakshmanan et al. 2015; Lamichhane et al. 2015; Morello-Frosch et al. 2010). The underlying mechanism through which exposure to ambient air pollution leads to adverse fetal outcomes has not been completely elucidated, although oxidative stress (OS) is thought to play a central role (Slama et al. 2008). Because mitochondrial DNA (mtDNA) lacks protective histones and has diminished DNA repair capacity compared to nuclear DNA, it is particularly prone to oxidative damage (Shaughnessy et al. 2014). In humans, mitochondria contain multiple copies of maternally-inherited double stranded, circular mitochondrial DNA (mtDNA). To maintain optimal physiological functions, mtDNA content (also referred to as mtDNA copy number) is kept within a relatively stable range. Mitochondria are both major intracellular sources of and primary targets of reactive oxygen species (ROS), and are especially susceptible to even small increases in systemic OS (Lee et al. 2000; Lee et al. 2005; Sinha et al. 2013). Mitochondria may respond to increased energy demands by increasing copy number (Lee et al. 2000; Lee et al. 2005). However, when compensatory mechanisms are overwhelmed, mtDNA content may decrease (Lee et al. 2000; Lee et al. 2005). Also, mtDNA mutations and their resulting biochemical defects accumulate over time. As such, altered mtDNA content may provide a record of past environmental exposures to pro-oxidant chemicals. Therefore, measurement of mtDNA content in cord blood may also serve to assess a particular vulnerable period in which the fetus is susceptible because of rapid development but also due to immature detoxifying enzyme systems (Wells et al. 2009).
Exposure to particulate matter has been associated with changes in mitochondrial DNA content in non-pregnant adults (Hou et al. 2010; Hou et al. 2013) as well as in cord blood and placenta reflecting in utero exposures (Clemente et al. 2015; Janssen et al. 2012). Measurement of this biomarker in cord blood may provide a source of integrated molecular information of fetal exposures over pregnancy. Studies are starting to emerge that link prenatal pro-oxidant environmental exposures to mtDNA content at birth. a study on prenatal smoking reported decreased placental mtDNA content related to increasing number of cigarettes smoked per day (Bouhours-Nouet et al. 2005). Other pro-oxidant exposures such as outdoor air pollutants (e.g., particulate matter,) have also been associated with changes in mtDNA content in the placenta (Clemente et al. 2015; Janssen et al. 2012). Moreover, there may be periods of time in which individuals are more sensitive to pro-oxidant exposure, such as periods of rapid growth when mitosis is highly active. Exposure to pro-oxidant chemicals during these life stages might induce a cellular response, such as reduced mtDNA copy numbers, that is more prominent than when exposure occurs at other times. For example, development occurs as a cascade of gene expression changes that vary at different life stages. Because the developmental processes and sets of genes expressed differ by developmental stage, potential clues to the underlying biological processes can be inferred simply by understanding what life stages are more sensitive to exposure.
We leveraged daily prenatal PM2.5 measures available over pregnancy to more precisely identify sensitive windows in relation to mtDNA content measured in cord blood. We combined these approaches with distributive lag models (DLM) that allow us to statistically model and visualize the exposure timing-dependent pattern of associations. Because there is evidence to suggest sex-specific effects of air pollution exposure during pregnancy (Chiu et al. 2016; Hsu et al. 2015; Lakshmanan et al. 2015) we also examined sex stratified associations.
2. Material and Methods
2.1 Study population
Pregnant women who were receiving prenatal care through the Mexican Social Security System (Instituto Mexicano del Seguro Social –IMSS) between July 2007 and February 2011 were recruited in the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study. The IMSS provides healthcare to affiliated private sector employees, the majority low- to middle-income workers and their families. Eligibility criteria were as follows: less than 20 weeks gestation, at least18 years old, planning to stay in Mexico City for the next 3 years, had access to a telephone, had no medical history of heart or kidney disease, did not consume alcohol daily, and did not use any steroid or anti-epilepsy medications. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. Women provided written informed consent.
2.2 Prenatal PM2.5 levels
Our group has developed an improved satellite based method to estimate daily PM2.5 levels across Mexico City for the years 2004–2014 (Just et al. 2015). Ultrasounds were not routinely performed as standard of care; therefore gestational age was based on last menstrual period (LMP) and by a standardized physical examination to determine gestational age at birth (Capurro et al. 1978). If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam was used instead of the gestational age determined by LMP. Daily exposure to PM2.5 were then estimated for each cohort participant during pregnancy (i.e., individual-level exposure estimates) using a novel spatio-temporal model that incorporates Moderate Resolution Imaging Spectroradiometer (MODIS) satellite-derived Aerosol Optical Depth (AOD) measurements at a 1× 1 km spatial resolution (Just et al. 2015). These remote sensing data are calibrated with municipal ground level monitors of PM2.5, land use regression (LUR) variables, and meteorological data to yield estimates of daily residential PM2.5 levels for each participant. The model was run using day-specific calibrations of AOD data calibrated against ground PM2.5 measurements from 12 monitoring stations covering Mexico City and LUR and meteorological variables (roadway density, temperature, relative humidity, planetary boundary layer and daily precipitation). As in previous studies, mixed effect models with spatial and temporal predictors and day-specific random effects were used to account for temporal variations in the PM2.5–AOD relationship. For days without AOD data, the model was fit with a seasonal smooth function of latitude and longitude and time-varying average incorporating local monitoring. Model performance was assessed using monitor-level leave one-out cross-validation; the model performed well with an R2 of 0.724. Due to day to day variation, daily PM2.5 measures were averaged into weekly measurements as in prior work (Chiu et al. 2016; Hsu et al. 2015). To compare the DLM approach to traditional windows, we also calculated the average PM over clinically defined trimesters (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks-delivery).
2.3 Mitochondrial DNA content
Venous umbilical cord blood was obtained at the time of delivery for 531 of the 948 infants born into the study. Most missing samples were due to births occurring late at night or in the very early morning hours or mothers not reporting the start of labor to the study workers. The first 260 whole blood samples were stored in PAXgene™ Blood DNA Tubes (PreAnalytiX GmbH, Hombrechtikon Switzerland) and extracted using a QIAamp DNA Blood Kit (QIAGEN). The DNA was then stored at −80°C prior to analysis. The next 271 samples were extracted by conventional phenol–chloroform method after red cell lysis by a second laboratory. The second laboratory stored the DNA at 4°C. Multiplex quantitative real-time polymerase chain reaction (qPCR) was used to determine mtDNA copy number/content. The copy number was calculated by simultaneously measuring the abundance of two gene targets - one specific to mtDNA 12S ribosomal RNA and one to nuclear DNA (nDNA) - and calculated as the ratio of the abundance of these two genes. The primers for qPCR analysis of mtDNA were: mtF805 5’-CCACGGGAAACAGCAGTGATT-3’and mtR927 5’-CTATTGACTTGGGTTAATCGTGTGA-3’. We used a commercial kit to quantify nDNA (TaqMan RNase P Control Reagents Kit, Applied Biosystems); because this is a commercial kit, the information on primers and probe are protected Quantitative real-time PCR was performed using the Bio-Rad CFX96 Real-time PCR detection system (BioRad, Hercules, CA). The PCR conditions were set up as follows: hot start at 95°C for 15 min, followed by 39 cycles of 95°C for 15 s, 60°C for 1 min, and melting Curve 65°C. A pool of 300 DNA samples was used to construct standard curves for mtDNA/nDNA and data was deemed acceptable if the R2 of the standard curve was > 0.99 (Zhong et al. 2016). To ensure quality control, each plate included 2 negative controls, each sample was run in triplicate and each plate contained five interplate controls. The within-run and between-run coefficients of variation of this assay were 5% and 7%, respectively.
2.4 Covariates
Thirteen variables derived from prenatal questionnaire results were used to classify study participant families into six levels based on the socioeconomic status (SES) index created by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (AMAI)(Carrasco 2002). These levels were then collapsed into low, medium, and high socioeconomic status. Only 7 mothers in the original cohort (n=948) reported smoking during pregnancy. Prenatal exposure to environmental tobacco smoke was defined as report of a smoker in the home during the second or third trimester of pregnancy. Maternal education was defined as less than high school, some high school or high school graduate and more than high school at enrollment. Assay batch refers to the plate on which the sample was run and was included in the analysis in order to account for potential batch effects. Inclusion of SES into the model did not significantly alter the results, therefore it was excluded from the final model. The covariates included in the final model were sex, maternal age at delivery, maternal education, prenatal exposure to environmental tobacco smoke, year of delivery and assay batch.
2.5 Statistical Analyses
For these analyses, only children who were born full-term, defined as gestational age greater than or equal to 37 weeks and had complete covariate data were included (n=456). Because DNA was extracted using two different methods, we conducted the following normalization procedure. We calculated the ratio of the geometric mean of the two batches and the inverse was applied to the batch that used the phenol-chloroform method because the QIAamp DNA Kit was shown to provide the more highly reproducible results (Andreu et al. 2009). These values were then log-transformed. Variance was not significantly different (Levene test p>0.2) between log-transformed values before and after normalization procedure. Supplemental figure S1 shows the distribution of values before and after the normalization procedure. We fitted distributive lag models (DLMs) to estimate the time-varying association between mtDNA content in cord blood and estimated PM2.5 level during a given week in pregnancy as previously described (Chiu et al. 2016; Hsu et al. 2015). In brief, this method incorporates data from all time points simultaneously and assumes that the association between the outcome and exposure at a given time point, controlling for exposure at all other time points, varies smoothly as a function of time. We fitted the linear distributive lag model , where APij is the estimated PM2.5 level in week j of pregnancy and x1i, …, xpi are the additional covariates for subject I and Yi is the natural log of mtDNA. A maximum lag of 40 weeks was included in the DLM cross-basis starting from the date of estimated conception. For participants with gestational age 37–39 weeks, postnatal exposure was used for missing weeks. Covariates included sex, maternal age at delivery, maternal education, prenatal exposure to environmental tobacco smoke (defined as report of any smoker in the home), year of delivery and assay batch. DLMs that modeled a smooth function using b-splines with 3 degrees of freedom were fit (Gasparrini 2011; Gasparrini et al. 2010) chosen due to its parsimony and best AIC value; additional smoothing did not significantly improve the model. A sensitive window was identified when the pointwise 95% confidence bands did not contain zero. DLMs were implemented using the dlnm package version 2.2.6 in R version 3.2.3 (Vienna, Austria) (Gasparrini 2011) and other analyses were performed in SPSS version 23 (Chicago, IL).
3. Results
The majority of participants did not have more than 12 years of schooling (77%) and half were classified as having low socioeconomic status. More than a third of participants reported exposure to a smoker in the home during pregnancy. Other relevant cohort characteristics are shown in Table 1. These baseline characteristics did not differ significantly between those included in these analyses when compared to the remainder of the base cohort, except for maternal age at delivery (see supplemental material, Table S1).
Table 1.
PROGRESS cohort characteristics
| Characteristic | N=456 |
|---|---|
| Child’s sex, n (% male) | 252 (55) |
| Prenatal ETS exposure, n (%) | 160 (35) |
| Maternal education at enrollment | |
| Less than high school, n (%) | 181 (40) |
| Some high school or high school graduate, n (%) | 170 (37) |
| More than high school, n (%) | 105 (23) |
| Socioeconomic status | |
| Low, n (%) | 235 (52) |
| Medium, n (%) | 171 (37) |
| High, n (%) | 50 (11) |
| Maternal age at enrollment years, median (25th–75th) | 28.0 (24.4–35.5) |
| Average prenatal PM2.5 µg/m3, median (25th–75th) | 23.1 (20.8–24.5) |
| Normalized relative cord mtDNA content, geometric mean (25th–75th) | 1.13 (1.00–1.32) |
Differences in categorical variables tested using Pearson Chi-Square, differences in continuous variables tested using Mann Whitney U test
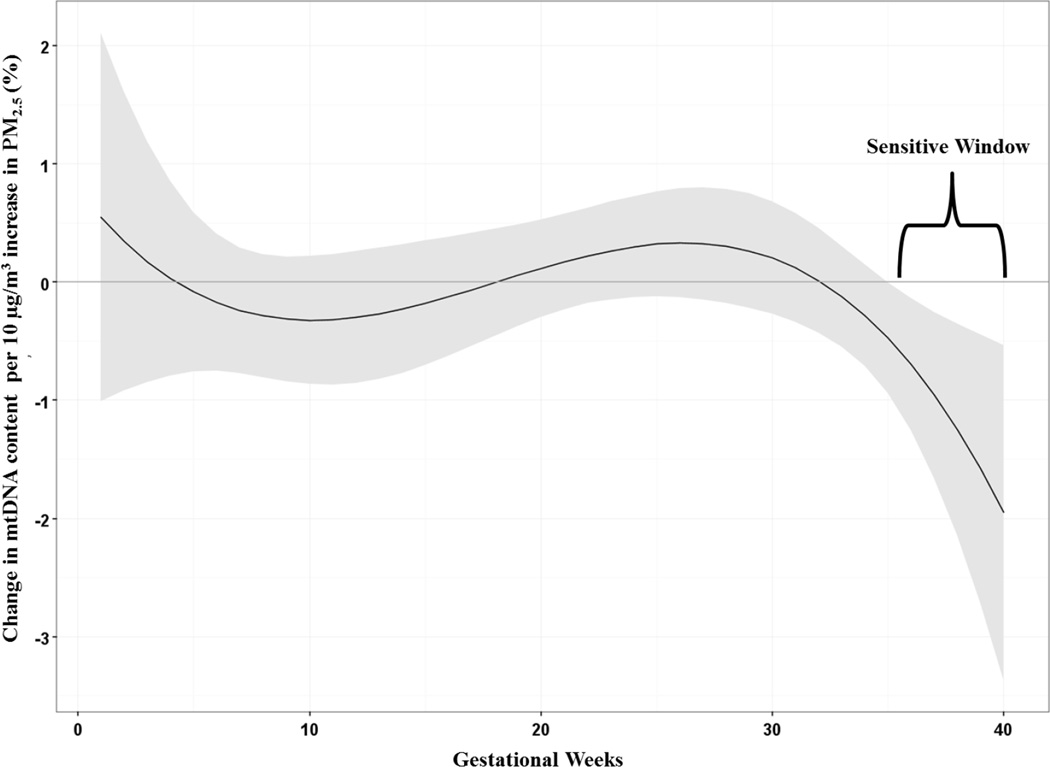
Figure 1 shows the association between a 10 µg/m3 increase in prenatal PM2.5 and cord blood mtDNA content in the sample as a whole, adjusting for maternal age, child’s sex, maternal education, prenatal tobacco smoke exposure, year of birth and assay batch. The DLM identified a significant association between increased PM2.5 exposure in late pregnancy, specifically 35–40 weeks gestation, and reduced mtDNA content in cord blood.
Figure 1.
Associations between weekly prenatal PM2.5 and mtDNA content in cord blood in the entire sample (n=456). Adjusted for sex, maternal age at delivery, year of birth, maternal education, prenatal exposure to environmental tobacco smoke and batch. The y-axis represents the change in mtDNA content associated with a 10 µg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in mtDNA content. Gray areas indicate 95% CIs. A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero.
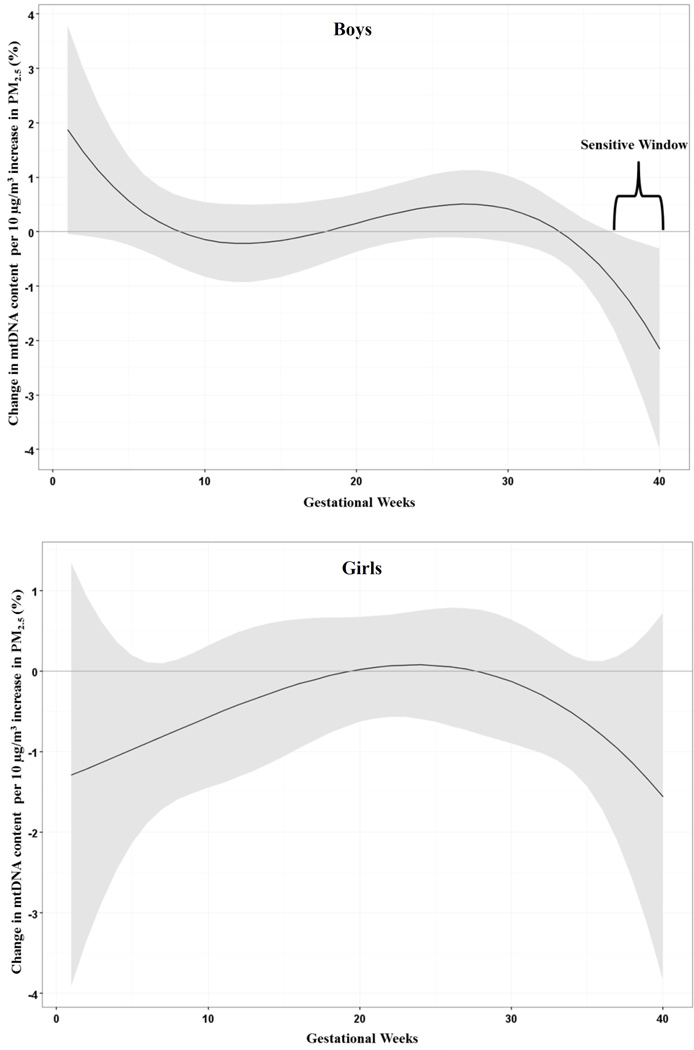
Figure 2 shows sex-specific associations for PM2.5 and mtDNA content in cord blood adjusting for maternal age at delivery, year of birth, maternal education, prenatal exposure to environmental tobacco smoke and assay batch. Distributive lag models run in boys and girls separately, identified a significant association between increased PM2.5 and lower cord blood mtDNA content among boys (37–40 weeks gestation) while there were no significant associations among girls. Post-hoc, we fitted a linear regression model including a PM2.5 *sex interaction term using PM2.5 level averaged over these identified sensitive windows, and we found no significant interaction between PM2.5 and sex.
Figure 2.
Sex-stratified associations between weekly prenatal PM2.5 and mtDNA content in cord blood. Adjusted for maternal age at delivery, year of birth, maternal education, prenatal exposure to environmental tobacco smoke and assay batch. The y-axis represents the change in mtDNA content associated with a 10 µg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in mtDNA content. Gray areas indicate 95% CIs. A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero
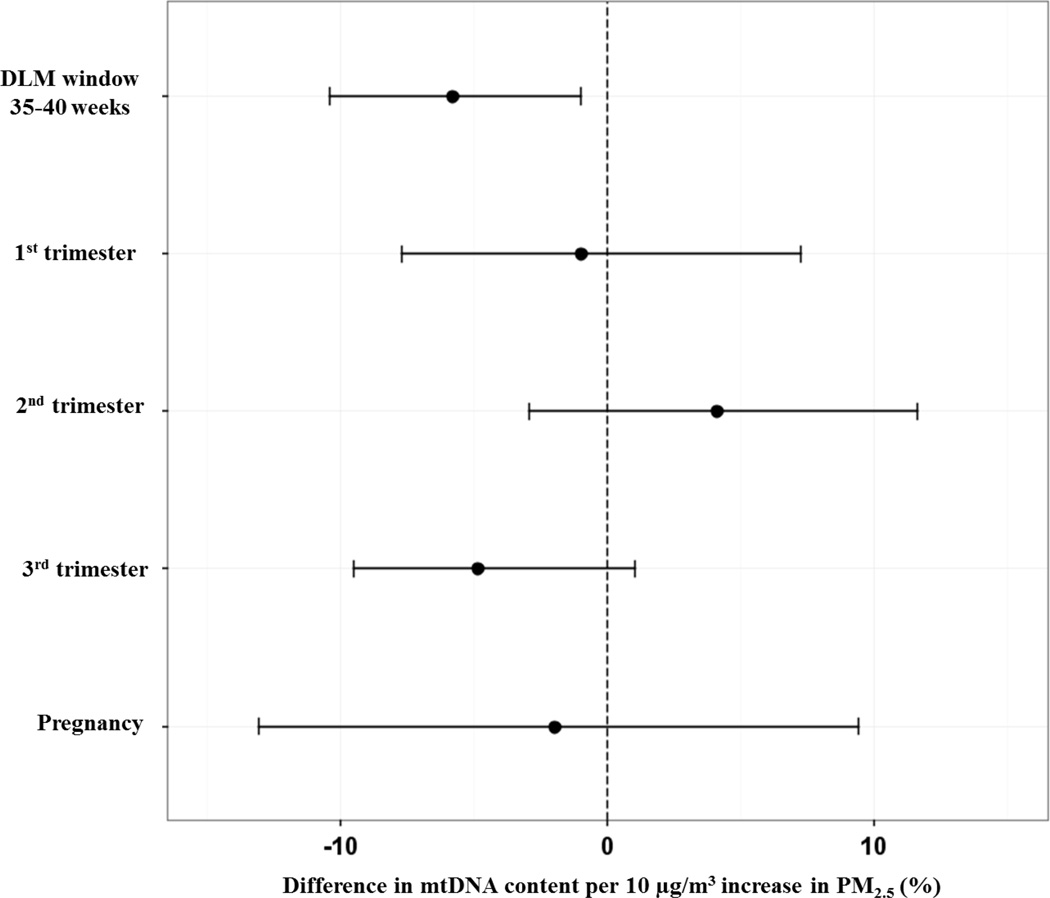
Finally, we assessed the “sensitive window” identified by the DLM by also fitting a linear regression model using PM2.5 levels averaged over the time period when the association was significant based on the pointwise 95% CI bounds. We also estimated the association between PM2.5 averaged over clinically defined trimesters and over all of pregnancy using this same approach. Figure 3 shows a forest plot comparing the difference in mtDNA content for a 10 µg/m3 increment in PM2.5 averaged over the identified window, the 1st, 2nd and 3rd trimester and averaged over the entire pregnancy. Compared to exposure averaged over trimesters, the DLM window average showed more precise estimates.
Figure 3.
Comparison of linear model estimates for percent difference in mtDNA content per 10 µg/m3 higher PM2.5 concentration averaged over the DLM defined window, the clinically defined trimesters and the entire pregnancy. All models adjusted for child’s sex, maternal age at delivery, maternal education, prenatal exposure to environmental tobacco smoke, year of birth and assay batch.
4. Discussion
Our findings suggest that prenatal exposure to PM2.5 during a specific window in late pregnancy is associated with lower mitochondrial DNA content in cord blood, a marker of cumulative oxidative stress. In addition, there was a suggestion that PM2.5 was more strongly associated with differences in mitochondrial DNA content in boys compared to girls. The observed associations remained significant after adjustment for a number of important potential confounders and covariates. These observations may be particularly relevant for susceptibility to health effects that are mediated by white blood cells, such as infections and immune response to allergens among others. If these findings are replicated in independent studies, future research should determine whether air pollution in this window increases the risk of immunological health effects later in life. Because we only measured mtDNA content in WBCs, the effects of oxidative stress from PM on other health endpoints would be speculative. However, if WBC mtDNA content is a biomarker of cumulative oxidative stress, these data may be useful for establishing the baseline dose of OS and relating the impact of subsequent postnatal PM and ROS-inducing environmental agents. OS plays a major role in many different health endpoints and measures of cumulative OS would have value in epidemiological settings as a means to quantify OS regardless of source, and to determine the contribution of environmental exposures such as PM to cumulative OS.
Previous data on the association between ambient air pollution and mtDNA content has been mixed. In a study of foundry workers in Brescia, Italy, occupational exposure to PM10, PM1 and coarse particles were associated with higher mitochondrial DNA content in blood (Hou et al. 2010). In a multicenter cross-sectional study in Italian cities, personal exposure to benzene was also associated with higher mtDNA content (Carugno et al. 2012). More recently, in an occupational study of truck drivers and office workers in Beijing, China, short term (averaged of 2–8 days) personal exposure to elemental carbon (EC) and PM10 was associated with lower mtDNA content in all participants (Hou et al. 2013). In a panel of elderly people living in Belgium, increased annual PM2.5 was associated with decreased mtDNA content in blood (Pieters et al. 2015). Only one other study has reported on the association between prenatal PM exposure and mitochondrial DNA content in cord blood (Janssen et al. 2012). Janssen et al reported that prenatal PM10 during the third trimester of in pregnancy was associated with significantly lower mitochondrial DNA content in placenta but no significant associations were reported in mtDNA content in cord blood (Janssen et al. 2012). Given these results, the advantages of applying a data-driven method rather than pre-assigned time windows are apparent. Our analyses suggest that the direction of the effect estimates using PM2.5 averaged over clinically defined trimesters were generally consistent with the results from DLMs, but the DLMs are more sensitive to the potential significant critical windows. This implies that analyses using exposure metrics arbitrarily defined a priori, such as clinical trimesters, may miss associations if the sensitive window only consists of a portion of a given trimester.
Mitochondrial DNA content can change under different energy demand settings, as mitochondria are the primary source of energy from oxidative phosphorylation. Increases in energy demands can be due in part to high exogenous oxidative stress levels which can overwhelm mitochondrial systems that scavenge endogenous ROS. With prolonged exposure, exogenous ROS can overwhelm the antioxidant capacity of the cell and affect mitochondria quality (Lee et al. 2000). Mild oxidative stress due to exogenous factors, like ambient air pollution, may first lead to increased mtDNA synthesis as a compensatory mechanism in order to ensure cell survival (Lee et al. 2000; Lee et al. 2005). Continued high exposure and oxidative stress induces mtDNA damage and may result in decreased or limited replication of mtDNA due to increased abundance of defective mitochondria (Lee et al. 2005; Meyer et al. 2013). Cellular apoptosis can be initiated by damaged mtDNA and feedback mechanisms are designed to limit the replication of damaged mitochondria in order to protect the cell. Unless these defective mitochondria are repaired or removed, production of excess ROS from damaged mitochondrial respiration may continue, resulting in alterations in the bioenergetic and replicative functions of the cell, eventually leading to cell senescence or apoptosis (Lee et al. 2000; Lee et al. 2005). It is biologically plausible that the results we see are limited to the end of pregnancy due this is the period of fastest growth of the fetus or because there is an accumulation of mtDNA damage over the course of pregnancy via PM exposure. Finally, we cannot rule that the results we see are due to late 3rd trimester exposure being closest in time to the collection of cord blood DNA. It may be that effects are always strongest for most recent exposure. Future work is needed measuring PM and mtDNA content at multiple life stage to determine if the most proximal time of PM exposure is the best predictor of mtDNA content or if earlier life stages predict mtDNA content independent of proximal exposure.
Previous studies have suggested sex-specific differences in response to in utero exposure to particulate matter, including differences in neurodevelopmental outcomes (Chiu et al. 2016), asthma (Clark et al. 2010; Hsu et al. 2015), and autism spectrum disorders (Roberts et al. 2013). In our analyses, we observed that the effect of prenatal particulate matter may differ by sex, with one potential window of susceptibility detected only in males when compared with females. These results are in line with previous evidence suggesting males may be more susceptible to in utero OS than females (Minghetti et al. 2013). However, these results should be interpreted with caution given that in our post-hoc analysis we did not find a significant interaction.
Evidence underscores the central role of oxidative stress (Wells et al. 2009) and the importance of optimal oxidant balance at the maternal-fetal interface in normal development (Herrera et al. 2014). Oxidative stress impacts multiple mitotically stable cellular processes including mtDNA function which have been implicated in programming (Byun et al. 2014; Cameron et al. 2002; Janssen et al. 2014; Shaughnessy et al. 2014). Alterations in mtDNA content/mitochondrial dysfunction are associated with fetal outcomes that are important predictors of health later in life like birth weight (Clemente et al. 2015) and intrauterine growth restriction (Mando et al. 2014). Furthermore, mitochondrial dysfunction is implicated in a variety of diseases and conditions like autism (Chen et al. 2015), diabetes and insulin resistance (Wong et al. 2009; Zheng et al. 2015), glucose metabolism (Weng et al. 2009), cognitive function (Lee et al. 2010; Mengel-From et al. 2014), and chronic kidney disease (Tin et al. 2016).
This study has several strengths. First, we were able to use a novel spatio-temporal satellite method of high spatial and temporal resolution to assign exposure during pregnancy. We leveraged this time-varying exposure data to highlight new data-driven statistical methods that can better delineate the role of exposure timing in air pollution health effects. We were also able to adjust for important confounders. We also acknowledge some limitations. We did not consider air pollution exposures in microenvironments that might lead to personal exposure levels that differed from our ambient exposure estimated at the home. We were not able to adjust for other important traffic-related pollutants like NO2. Another limitation is that we were not able to adjust for blood cell count, particularly platelet count, which is thought to influence variation in mtDNA content by increasing mtDNA without increasing nDNA(Banas et al. 2004). The mtDNA content biomarker would capture effects in both WBCs and platelets and our results are therefore a reflection of the relative contribution of each cell type to the overall mtDNA content. However, Janssen et al (Janssen et al. 2012)reported that none of the blood cell count measures included in their models (platelets, neutrophils, white blood cells or white blood cell ratio were not significant predictors of mtDNA content in cord blood. Also, we cannot rule out potential residual confounding due to unmeasured host and environmental factors that may influence mitochondrial DNA content in cord blood. However, the specificity of our findings to a limited period within pregnancy suggests that such an unmeasured confounder would likely need to vary in time along with PM2.5 and would not be explained by more time-invariant characteristics like socio-demographics. For children born before 40 weeks, the period of exposure is not limited to in utero but includes early postnatal exposure. Finally, the cohort consisted Mexican women belonging mostly to low-income families, in a megacity with substantial PM2.5 exposures that may limit the generalizability of our findings.
In our study, increased PM2.5 at the end of the third trimester was associated with decreased mtDNA content, suggesting heightened sensitivity to PM during this particular time period. Refined determination of time windows in which PM has the greatest magnitude of effect can enhance insight into underlying mechanisms and may better inform future interventions.
Supplementary Material
Highlights.
Examined sensitive windows of prenatal PM2.5 exposure on mtDNA in cord blood.
Sensitive window identified at gestational weeks 35–40
Higher PM2.5 during sensitive window associated with lower mtDNA content.
Findings suggest sex-specific associations.
Acknowledgments
This work was supported by R01ES013744, R01ES014930, R01ES021357, P30 ES023515 and R00 ES023450. MJR was supported by T32 HD049311-09. This study was supported and partially funded by the National Institute of Public Health/Ministry of Health of Mexico and we thank the ABC (American British Cowdray) Hospital in Mexico for providing research facilities. The authors would like to thank the PROGRESS Mexico team and participating parents and children.
Abbreviations
- DLM
Distributive lag models
- mtDNA
Mitochondrial DNA
- PM2.5
Particulate matter <2.5 microns in diameter
- qPCR
Quantitative real time polymerase chain reaction
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maria José Rosa, Email: maria.rosa@mssm.edu.
Allan C. Just, Email: allan.just@mssm.edu.
Marco Sánchez Guerra, Email: m.sanchezguerra@inper.gob.mx.
Itai Kloog, Email: ikloog@bgu.ac.il.
Hsiao-Hsien Leon Hsu, Email: leon.hsu@mssm.edu.
Kasey J. Brennan, Email: kb2891@cumc.columbia.edu.
Adriana Mercado García, Email: adrianam@insp.mx.
Brent Coull, Email: bcoull@hsph.harvard.edu.
Rosalind J. Wright, Email: rosalind.wright@mssm.edu.
Martha María Téllez Rojo, Email: mmtellez@insp.mx.
Andrea A. Baccarelli, Email: ab4303@cumc.columbia.edu.
Robert O. Wright, Email: robert.wright@mssm.edu.
References
- Andreu AL, Martinez R, Marti R, Garcia-Arumi E. Quantification of mitochondrial DNA copy number: Pre-analytical factors. Mitochondrion. 2009;9:242–246. doi: 10.1016/j.mito.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Banas B, Kost BP, Goebel FD. Platelets, a typical source of error in real-time PCR quantification of mitochondrial DNA content in human peripheral blood cells. Eur J Med Res. 2004;9:371–377. [PubMed] [Google Scholar]
- Bouhours-Nouet N, May-Panloup P, Coutant R, Boux de Casson F, Descamps P, Douay O, Reynier P, Ritz P, Malthiery Y, Simard G. Maternal smoking is assocaited with mitochondrial DNA depletion and respiratory chain complex III deficiency in placenta. American Journal Physiology Endocrinology Metabolism. 2005;288:E171–E177. doi: 10.1152/ajpendo.00260.2003. [DOI] [PubMed] [Google Scholar]
- Byun HM, Baccarelli A. Environmental expousre and mitochondrial epigenetics: study design and analytical challenges. Human Genetics. 2014;133:247–257. doi: 10.1007/s00439-013-1417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Demerath EW. Critical periods in human growth and their relationship to diesases of aging. American Journal Physical Anthropology. 2002;35:159–184. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. The Journal of pediatrics. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- Carrasco AV. The AMAI system of classifying households by socio-economic level: ESOMAR. 2002 [Google Scholar]
- Carugno M, Pesatori AC, Dioni L, Hoxha M, Bollati V, Albetti B, Byun HM, Bonzini M, Fustinoni S, Cocco P, Satta G, Zucca M, Merlo DF, Cipolla M, Bertazzi PA, Baccarelli A. Increased Mitochondrial DNA Copy Number in Occupations Associated with Low-Dose Benzene Exposure. Environmental health perspectives. 2012;120:210–215. doi: 10.1289/ehp.1103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li Z, He Y, Zhang F, Li H, Liao Y, Wei Z, Wan G, Xiang X, Hu M, Xia K, Chen X, Tang J. Elevated mitochondrial DNA copy number in peripheral blood cells is associated with childhood autism. BMC psychiatry. 2015;15:50. doi: 10.1186/s12888-015-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, Wright RO, Wright RJ. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int. 2016;87:56–65. doi: 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environmental health perspectives. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente DB, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, Fernandez MF, Fierens F, Gyselaers W, Iniguez C, Janssen BG, Lefebvre W, Llop S, Olea N, Pedersen M, Pieters N, Santa Marina L, Souto A, Tardon A, Vanpoucke C, Vrijheid M, Sunyer J, Nawrot TS. Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRAGE (Belgium) Birth Cohorts. Environmental health perspectives. 2015 doi: 10.1289/ehp.1408981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Gold DR, Oken E. Prenatal Exposure to Traffic Pollution: Associations with Reduced Fetal Growth and Rapid Infant Weight Gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J Stat Softw. 2011;43:1–20. [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Krause B, Ebensperger G, Reyes RV, Casanello P, Parra-Cordero M, Llanos AJ. The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhu ZZ, Zhang X, Nordio F, Bonzini M, Schwartz J, Hoxha M, Dioni L, Marinelli B, Pegoraro V, Apostoli P, Bertazzi PA, Baccarelli A. Airborne particulate matter and mitochondrial damage: a cross-sectional study. Environmental health : a global access science source. 2010;9:48. doi: 10.1186/1476-069X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LF, Zhang X, Dioni L, Barretta F, Dou C, Zheng YN, Hoxha M, Bertazzi PA, Schwartz J, Wu SS, Wang S, Baccarelli AA. Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: a repeated-measure study. Part Fibre Toxicol. 2013;10 doi: 10.1186/1743-8977-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HHL, Chiu YHM, Coull BA, Kloog I, Schwartz J, Lee A, Wright RO, Wright RJ. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children Identifying Sensitive Windows and Sex Differences. Am J Resp Crit Care. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder A, Lee HJ, Ebisu K, Koutrakis P, Belanger K, Bell ML. PM2.5 Exposure and Birth Outcomes Use of Satellite- and Monitor-Based Data. Epidemiology. 2014;25:58–67. doi: 10.1097/EDE.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Byun HM, Cox B, Gyselaers W, Izzi B, Baccarelli A, Nawrot TS. Variation of DNA methylation in candidate age-related targets on the mitochondrial-telomere axis in cord blood and placenta. Placenta. 2014;35:665–672. doi: 10.1016/j.placenta.2014.06.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W, Nawrot TS. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environmental health perspectives. 2012;120:1346–1352. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Tellez-Rojo MM, Moody E, Wang Y, Lyapustin A, Kloog I. Using High-Resolution Satellite Aerosol Optical Depth To Estimate Daily PM2.5 Geographical Distribution in Mexico City. Environmental science & technology. 2015;49:8576–8584. doi: 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Chiu YHM, Coull BA, Just AC, Maxwell SL, Schwartz J, Gryparis A, Kloog I, Wright RJ, Wright RO. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environmental research. 2015;137:268–277. doi: 10.1016/j.envres.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial role in life and death of the cell. Journal of biomedical science. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. The international journal of biochemistry & cell biology. 2005;37:822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park KD, Im JA, Kim MY, Lee DC. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clinica chimica acta; international journal of clinical chemistry. 2010;411:592–596. doi: 10.1016/j.cca.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Mando C, De Palma C, Stampalija T, Anelli GM, Figus M, Novielli C, Parisi F, Clementi E, Ferrazzi E, Cetin I. Placental mitochondrial content and function in intrauterine growth restriction and preeclampsia. American journal of physiology Endocrinology and metabolism. 2014;306:E404–E413. doi: 10.1152/ajpendo.00426.2013. [DOI] [PubMed] [Google Scholar]
- Mengel-From J, Thinggaard M, Dalgard C, Kyvik KO, Christensen K, Christiansen L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Human genetics. 2014;133:1149–1159. doi: 10.1007/s00439-014-1458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JN, Leung MCK, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a Target of Environmental Toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern-Fetal Neo M. 2013;26:259–262. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch R, Jesdale BM, Sadd JL, Pastor M. Ambient air pollution exposure and full-term birth weight in California. Environ Health-Glob. 2010;9 doi: 10.1186/1476-069X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters N, Janssen BG, Dewitte H, Cox B, Cuypers A, Lefebvre W, Smeets K, Vanpoucke C, Plusquin M, Nawrot TS. Biomolecular Markers Within the Core Axis of Aging and Particulate Air Pollution Exposure in the Elderly: A Cross-Sectional Study. Environmental health perspectives. 2015 doi: 10.1289/ehp.1509728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses' Health Study II participants. Environmental health perspectives. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy DT, McAllister K, Worth L, Haugen AC, Meyer JN, Domann FE, Van Houten B, Mostoslavsky R, Bultman SJ, Baccarelli AA, Begley TJ, Sobol RW, Hirschey MD, Ideker T, Santos JH, Copeland WC, Tice RR, Balshaw DM, Tyson FL. Mitochondria, Energetics, Epigenetics, and Cellular Responses to Stress. Environmental health perspectives. 2014;122:1271–1278. doi: 10.1289/ehp.1408418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, Glinianaia S, Hoggatt KJ, Kannan S, Hurley F, Kalinka J, Sram R, Brauer M, Wilhelm M, Heinrich J, Ritz B. Meeting report: atmospheric pollution and human reproduction. Environmental health perspectives. 2008;116:791–798. doi: 10.1289/ehp.11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin A, Grams ME, Ashar FN, Lane JA, Rosenberg AZ, Grove ML, Boerwinkle E, Selvin E, Coresh J, Pankratz N, Arking DE. Association between Mitochondrial DNA Copy Number in Peripheral Blood and Incident CKD in the Atherosclerosis Risk in Communities Study. Journal of the American Society of Nephrology : JASN. 2016 doi: 10.1681/ASN.2015060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJJ, Perstin J, Preston TJ, Wiley MJ, Wong AW. Oxidative Stress in Developmental Origins of Disease: Teratogenesis, Neurodevelopmental Deficits, and Cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- Weng SW, Lin TK, Liou CW, Chen SD, Wei YH, Lee HC, Chen IY, Hsieh CJ, Wang PW. Peripheral blood mitochondrial DNA content and dysregulation of glucose metabolism. Diabetes research and clinical practice. 2009;83:94–99. doi: 10.1016/j.diabres.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Wong J, McLennan SV, Molyneaux L, Min D, Twigg SM, Yue DK. Mitochondrial DNA content in peripheral blood monocytes: relationship with age of diabetes onsetand diabetic complications. Diabetologia. 2009;52:1953–1961. doi: 10.1007/s00125-009-1424-6. [DOI] [PubMed] [Google Scholar]
- Zheng LD, Linarelli LE, Liu L, Wall SS, Greenawald MH, Seidel RW, Estabrooks PA, Almeida FA, Cheng Z. Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clinical epigenetics. 2015;7:60. doi: 10.1186/s13148-015-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Cayir A, Trevisi L, Sanchez-Guerra M, Lin X, Peng C, Bind MA, Prada D, Laue H, Brennan KJ, Dereix A, Sparrow D, Vokonas P, Schwartz J, Baccarelli AA. Traffic-Related Air Pollution, Blood Pressure, and Adaptive Response of Mitochondrial Abundance. Circulation. 2016;133:378–387. doi: 10.1161/CIRCULATIONAHA.115.018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.