Abstract
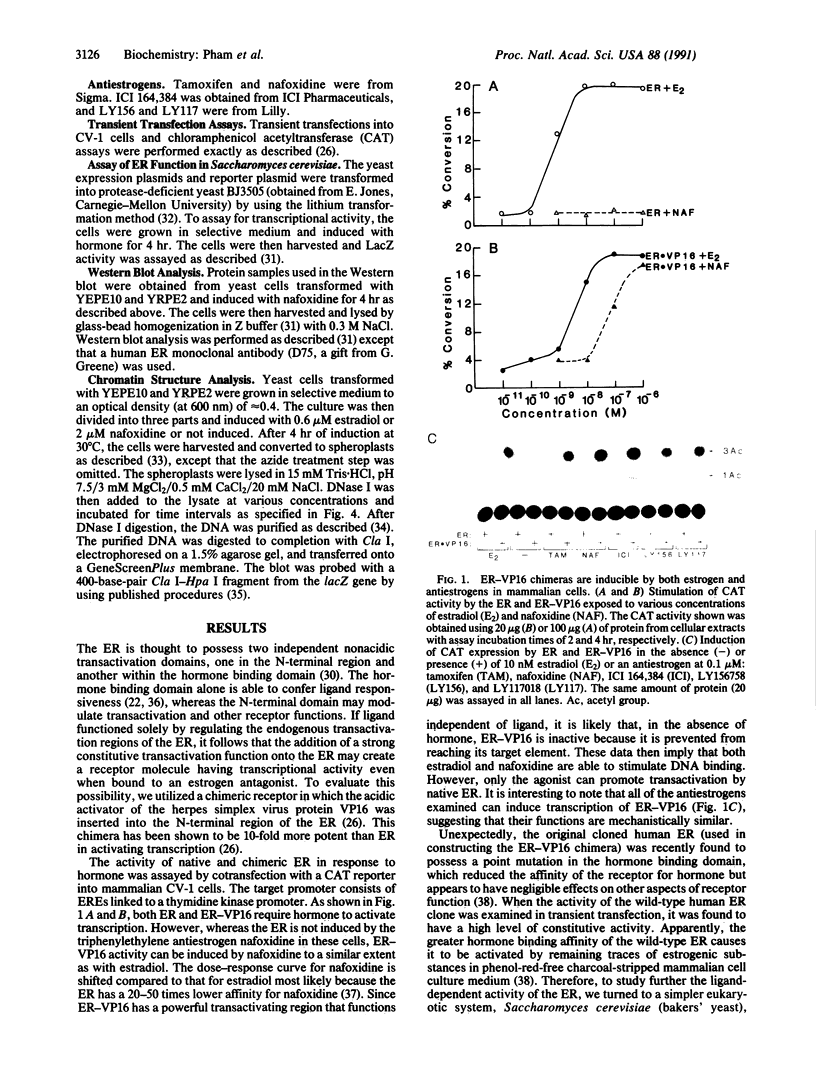
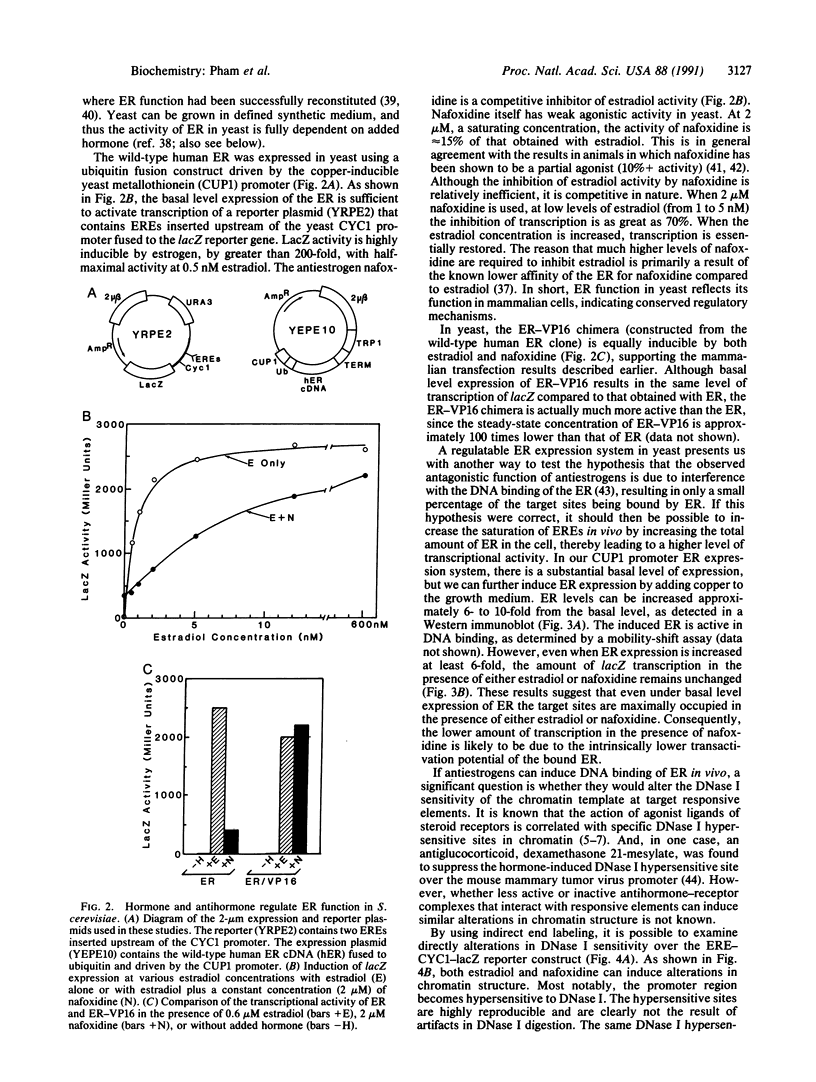
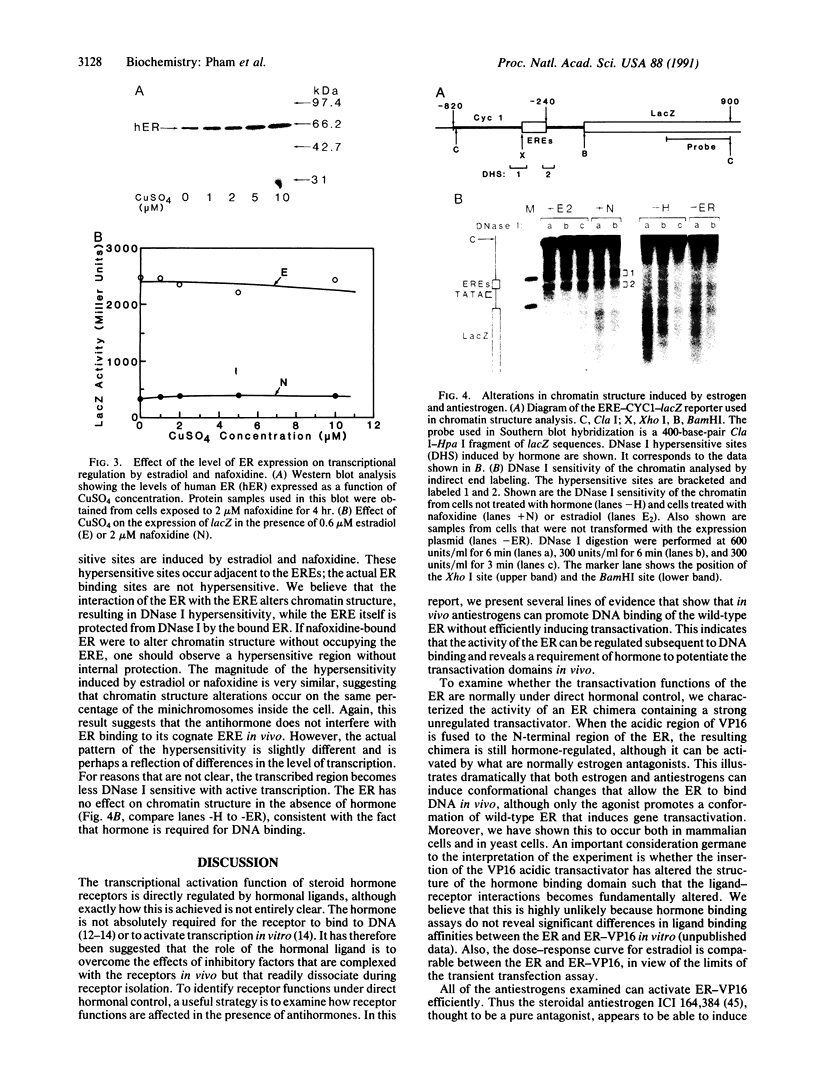
We describe in this report experiments in vivo that demonstrate that antiestrogens promote DNA binding of the estrogen receptor without efficiently inducing transcription. When the receptor is modified to carry a foreign unregulated transactivation domain, transcription can be induced efficiently by both estrogen and antiestrogens. Under apparent saturation conditions, antihormone-receptor complexes binding to responsive enhancer elements elicit only a low level of transcription. In addition, we show that both estrogen and an antiestrogen, nafoxidine, effect very similar alterations in chromatin structure at a responsive promoter. These results indicate that in vivo steroid receptor action can be regulated subsequent to the DNA binding step, by regulating interactions with the target transcriptional machinery. In this regard, antihormones can function by establishing receptor-DNA complexes that are transcriptionally nonproductive.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almer A., Hörz W. Nuclease hypersensitive regions with adjacent positioned nucleosomes mark the gene boundaries of the PHO5/PHO3 locus in yeast. EMBO J. 1986 Oct;5(10):2681–2687. doi: 10.1002/j.1460-2075.1986.tb04551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi M. K., Elliston J. F., Tsai S. Y., Edwards D. P., Tsai M. J., O'Malley B. W. Steroid hormone-dependent interaction of human progesterone receptor with its target enhancer element. Mol Endocrinol. 1988 Dec;2(12):1221–1229. doi: 10.1210/mend-2-12-1221. [DOI] [PubMed] [Google Scholar]
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Antihormone-steroid hormonal activity, heat-shock protein hsp 90 and receptors. Horm Res. 1987;28(2-4):181–195. doi: 10.1159/000180943. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Gloss B., Schmid W., Strähle U., Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986 Dec 18;324(6098):686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990 Sep;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliston J. F., Fawell S. E., Klein-Hitpass L., Tsai S. Y., Tsai M. J., Parker M. G., O'Malley B. W. Mechanism of estrogen receptor-dependent transcription in a cell-free system. Mol Cell Biol. 1990 Dec;10(12):6607–6612. doi: 10.1128/mcb.10.12.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliston J. F., Tsai S. Y., O'Malley B. W., Tsai M. J. Superactive estrogen receptors. Potent activators of gene expression. J Biol Chem. 1990 Jul 15;265(20):11517–11521. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Ragot T., Bailly A., Atger M., Misrahi M., Perricaudet M., Milgrom E. Receptors bound to antiprogestin from abortive complexes with hormone responsive elements. Nature. 1988 Dec 15;336(6200):695–698. doi: 10.1038/336695a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Tsai S. Y., Greene G. L., Clark J. H., Tsai M. J., O'Malley B. W. Specific binding of estrogen receptor to the estrogen response element. Mol Cell Biol. 1989 Jan;9(1):43–49. doi: 10.1128/mcb.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel D., Caplan A. J., Lee M. S., Adams C. C., Fishel B. R., Gross D. S., Garrard W. T. Basal-level expression of the yeast HSP82 gene requires a heat shock regulatory element. Mol Cell Biol. 1989 Nov;9(11):4789–4798. doi: 10.1128/mcb.9.11.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Pike J. W., Drutz D. J., Butt T. R., O'Malley B. W. Reconstitution of the vitamin D-responsive osteocalcin transcription unit in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Aug;9(8):3517–3523. doi: 10.1128/mcb.9.8.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Moudgil V. K., Hurd C. Transformation of calf uterine progesterone receptor: analysis of the process when receptor is bound to progesterone and RU38486. Biochemistry. 1987 Aug 11;26(16):4993–5001. doi: 10.1021/bi00390a017. [DOI] [PubMed] [Google Scholar]
- O'Malley B. The steroid receptor superfamily: more excitement predicted for the future. Mol Endocrinol. 1990 Mar;4(3):363–369. doi: 10.1210/mend-4-3-363. [DOI] [PubMed] [Google Scholar]
- Richard-Foy H., Sistare F. D., Riegel A. T., Simons S. S., Jr, Hager G. L. Mechanism of dexamethasone 21-mesylate antiglucocorticoid action: II. Receptor-antiglucocorticoid complexes do not interact productively with mouse mammary tumor virus long terminal repeat chromatin. Mol Endocrinol. 1987 Sep;1(9):659–665. doi: 10.1210/mend-1-9-659. [DOI] [PubMed] [Google Scholar]
- Rochefort H., Borgna J. L. Differences between oestrogen receptor activation by oestrogen and antioestrogen. Nature. 1981 Jul 16;292(5820):257–259. doi: 10.1038/292257a0. [DOI] [PubMed] [Google Scholar]
- Rochefort H. Nonsteroidal antiestrogens are estrogen-receptor-targeted growth inhibitors that can act in the absence of estrogens. Horm Res. 1987;28(2-4):196–201. doi: 10.1159/000180944. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Carson M. A., Weigel N. L., O'Malley B. W., Schrader W. T. Hormone-induced changes in the in vitro DNA-binding activity of the chicken progesterone receptor. Mol Endocrinol. 1989 Feb;3(2):356–362. doi: 10.1210/mend-3-2-356. [DOI] [PubMed] [Google Scholar]
- Segnitz B., Gehring U. Mechanism of action of a steroidal antiglucocorticoid in lymphoid cells. J Biol Chem. 1990 Feb 15;265(5):2789–2796. [PubMed] [Google Scholar]
- Simpson D. M., Elliston J. F., Katzenellenbogen J. A. Desmethylnafoxidine aziridine: an electrophilic affinity label for the estrogen receptor with high efficiency and selectivity. J Steroid Biochem. 1987 Sep;28(3):233–245. doi: 10.1016/0022-4731(87)91014-4. [DOI] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Tora L., Mullick A., Metzger D., Ponglikitmongkol M., Park I., Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 1989 Jul;8(7):1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Wilson A. P., Nicholson R. I., Davies P., Wakeling A. E. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30(1-6):263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- White J. H., Metzger D., Chambon P. Expression and function of the human estrogen receptor in yeast. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):819–828. doi: 10.1101/sqb.1988.053.01.093. [DOI] [PubMed] [Google Scholar]
- Willmann T., Beato M. Steroid-free glucocorticoid receptor binds specifically to mouse mammary tumour virus DNA. Nature. 1986 Dec 18;324(6098):688–691. doi: 10.1038/324688a0. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., Weatherill P. J., Nicholson R. I., Davies P., Wakeling A. E. A comparative study of the interaction of oestradiol and the steroidal pure antioestrogen, ICI 164,384, with the molybdate-stabilized oestrogen receptor. J Steroid Biochem. 1990 Mar;35(3-4):421–428. doi: 10.1016/0022-4731(90)90250-v. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]