Abstract
Aminoglycoside antibiotics cause death of sensory hair cells. Research over the past decade has identified several key players in the intracellular cascade. However, the role of the extracellular environment in aminoglycoside ototoxicity has received comparatively little attention. The present study uses the zebrafish lateral line to demonstrate that extracellular calcium and magnesium ions modulate hair cell death from neomycin and gentamicin in vivo, with high levels of either divalent cation providing significant protection. Imaging experiments with fluorescently tagged gentamicin show that drug uptake is reduced under high calcium conditions. Treating fish with the hair cell transduction blocker amiloride also reduces aminoglycoside uptake, preventing the toxicity, and experiments with variable calcium and amiloride concentrations suggest complementary effects between the two protectants. Elevated magnesium, in contrast, does not appear to significantly attenuate drug uptake, suggesting that the two divalent cations may protect hair cells from aminoglycoside damage through different mechanisms. These results provide additional evidence for calcium- and transduction-dependent aminoglycoside uptake. Divalent cations provided differential protection from neomycin and gentamicin, with high cation concentrations almost completely protecting hair cells from neomycin and acute gentamicin toxicity, but offering reduced protection from continuous (6 hr) gentamicin exposure. These experiments lend further support to the hypothesis that aminoglycoside toxicity occurs via multiple pathways in a both a drug and time course-specific manner.
Keywords: Hair cell, Ototoxicity, Aminoglycoside, Lateral line, Calcium, Magnesium
1. Introduction
Aminoglycosides are a clinically important group of antibiotics despite their unwanted ototoxic and nephrotoxic side effects. These drugs are cost-effective broad-spectrum antibiotics that are effective in treating gram-negative bacterial infections. Several lines of morphological and molecular evidence show that aminoglycosides act directly on the inner ear by killing sensory hair cells through stimulation of programmed cell death (apoptotic-like) pathways (Li et al., 1995; Lang and Liu, 1997; Nakagawa et al., 1998; Forge and Li, 2000; Cunningham et al., 2002; Hirose et al., 2004; Jiang et al., 2006). In vitro and in vivo studies in birds and mammals have uncovered some of the key molecular players in these apoptotic cascades (reviewed in Van de Water et al., 2004; Cheng et al., 2005). Aminoglycoside-induced cell death is thought to act through the so-called “intrinsic mitochondrial cell death pathway” although multiple overlapping death and protective factors are probably involved including oxidative stress, JNK signaling, and heat shock proteins (Hirose et al., 1997; Pirvola et al., 2000; Sha and Schacht, 2000; Dehne et al., 2002; Wang et al., 2003; Cunningham et al., 2004; Cunningham and Brandon, 2006; Sugahara et al., 2006; Taleb et al., 2008). Mitochondrial swelling and release of cytochrome c into the cytoplasm, hallmarks of classical apoptosis, are early signs of aminoglycoside ototoxicity (Hirose et al., 1999; Mangiardi et al., 2004; Matsui et al., 2004; Owens et al., 2007). Later events include activation of key caspases, particularly caspases-3 and -9 (Cunningham et al., 2002; Matsui et al., 2002; Cheng et al., 2003; Mangiardi et al., 2004). Some in vivo studies, however, downplay the role of caspases and suggest that aminoglycosides can trigger hair cell death in the absence of caspase activation (Jiang et al., 2006).
Despite the growing body of literature on intracellular cell death pathways and possible avenues of hair cell protection, comparatively little attention has been paid to the role of the extracellular environment on aminoglycoside ototoxicity. In vitro experiments in neonatal mouse cochlear cultures demonstrate that high levels of calcium or magnesium protect hair cells from neomycin damage (Richardson and Russell, 1991), suggesting that the external ionic environment may play a role in ototoxic responses. The present study examines the role of the external ionic environment in hair cell death and protection in the zebrafish (Danio rerio) lateral line.
Our group has recently established the larval zebrafish lateral line as an in vivo preparation to study the modulation of aminoglycoside toxicity (Harris et al., 2003; Murakami et al., 2003; Santos et al., 2006; Owens et al., 2007, 2008). The lateral line is a collection of mechanosensory structures called neuromasts, each containing several hair cells and associated supporting cells. The neuromasts are arrayed in a stereotyped pattern along the head and body of the animal (Metcalf et al., 1985; Coombs et al., 1989; Raible and Kruse, 2000), and are used to detect near-field, low frequency water movement important for many behaviors such as predator avoidance, prey detection, and orientation within the water column (Dijkgraaf, 1963; Montgomery and MacDonald, 1987; Montgomery et al., 1997; Coombs et al., 2001; New et al., 2001). Lateral line hair cells share many structural and functional similarities with vertebrate inner ear hair cells, including sensitivity to aminoglycoside- and cisplatin-induced cell death (Song et al., 1995; Williams and Holder, 2000; Harris et al., 2003; Murakami et al., 2003; Ton and Parng, 2005; Ou et al., 2007; Owens et al., 2007). Due to its external location, the relative ease of genetic and pharmacological manipulations and visualization, the lateral line provides a powerful model system for in vivo studies of hair cell toxicity (Harris et al., 2003; Ton and Parng, 2005; Hernández et al., 2006; Ou et al., 2007; Chiu et al., 2008; Owens et al., 2007, 2008).
In the present study, we examined the role of extracellular divalent cations in aminoglycoside uptake and aminoglycoside-induced hair cell death in the zebrafish lateral line. We found that increasing extracellular calcium or magnesium protected hair cells from neomycin in a dose-dependent manner while low concentrations of these ions facilitated hair cell death. We found similar ionic protection of hair cells from acute gentamicin damage, but diminished protection from continuous 6-hour gentamicin exposure. Experiments with the transduction channel blocker amiloride suggest that both aminoglycosides are taken up by similar transduction-dependent mechanisms. A separate set of experiments using fluorescently labeled gentamicin shows that high calcium, and to a lesser extent high magnesium, protects hair cells by preventing drug entry. These findings indicate that calcium plays a similar role in vivo in the zebrafish lateral line as it does in mammalian cochlear cultures, highlighting the similarities between these preparations and adding strength to the use of zebrafish as a rational model for studies of hair cell death. The present study also provides additional evidence for the hypothesis that neomycin and gentamicin damage lateral line hair cells by only partially overlapping mechanisms. Finally, these results highlight the necessity of carefully controlling the external ionic environment during ototoxicity studies and in considering differing ionic conditions when comparing findings between studies.
2. Materials and Methods
2.1 Animals
Adult stocks are maintained by the UW Zebrafish Facility according to standard protocols (Westerfield, 2000). Embryos were obtained from group matings of *AB wildtype adults and raised at 28.5 °C in E2 embryo medium (EM, see below) at a density of 50 larvae per 10 cm diameter Petri dish. Larvae were fed paramecia or rotifers and dry plant food daily starting at 4 days post-fertilization (dpf). All experiments were performed using larvae aged 5–6 dpf. The University of Washington Animal Care and Use Committee approved all animal procedures.
2.2 Embryo medium composition
Baseline E2 embryo medium contained 994 μM MgSO4, 150 μM KH2PO4, 42 μM Na2HPO4, 986 μM CaCl2, 503 μM KCl, 11.4 mM NaCl, and 714 μM NaHCO3, with the pH adjusted to 7.2. Ca2+ (as CaCl2) or Mg2+ (as MgSO4) concentrations were adjusted as indicated for each experiment while all other ion concentrations were held constant. Osmolality was not substantially different between solutions (110–113 mOsm/kg).
2.3 Aminoglycoside treatment
Neomycin (10 mg/ml stock) or gentamicin (50 mg/ml stock) were obtained from Sigma (St. Louis, MO) and were diluted in the appropriate embryo medium (see below) to final concentrations of 50, 100, 200, or 400 μM. Larvae (8–16 fish per treatment group) were distributed into transfer baskets (Harris et al., 2003) and all treatments were performed in six-well tissue culture plates. Two aminoglycoside exposure paradigms were used, based on prior observations that in the zebrafish lateral line, neomycin and gentamicin produce damage with differing time courses (Owens et al., submitted). For acute exposure paradigms, fish were incubated in the appropriate concentration of neomycin or gentamicin for 30 minutes, rinsed four times, and allowed to recover for one hour prior to hair cell assessment. For continuous exposure experiments, fish were incubated for six hours in neomycin or gentamicin and hair cell damage was examined immediately after the exposure period. Mock-treated controls, included in each experiment, were handled identically, including all rinses, except that the aminoglycoside was omitted. All rinses were performed in embryo medium with the same ionic composition as that used for drug treatment. For experiments with amiloride, fish were incubated in variable concentrations of amiloride for 15 minutes prior to addition of neomycin or gentamicin, then treated with aminoglycosides as described above. Amiloride treatment continued throughout each experiment. Fish were then rinsed twice before assessment of damage.
2.4 Vital dye labeling
Hair cells were labeled either pre-or post-treatment by two distinct labeling paradigms. For experiments where hair cells were labeled prior to drug treatment, free swimming larvae were immersed in 3 μM YO-PRO1 (Invitrogen, Eugene, OR) for 20 minutes, rinsed 3X in EM, immersed in 3 μM FM 1–43 FX ((n-(3-triethylammoniumpropyl)-4-(dibutylamino)-styryl) pyridinium dibromide; Invitrogen) for 45 seconds, then rinsed three times in EM. Standard EM was used for pre-labeling experiments to eliminate the possibility of differential dye uptake due to varying cation concentrations. This combination of nuclear (YO-PRO1) and cytoplasmic (FM 1–43) label allows for clear visualization of multiple hair cell compartments and using this labeling scheme both dyes are specific for hair cells within the lateral line (Santos et al., 2006; Owens et al., 2008). The mitochondrial dye DASPEI ((2-{4-(dimethylamino) styryl}-N-ethylpyridinium iodide; Invitrogen) was used for post-treatment hair cell labeling (Harris et al., 2003; Murakami et al., 2003; Owens et al., 2007, 2008). DASPEI was added to the final post-aminoglycoside rinse (for acute exposure) or directly to the drug treatment (for continuous exposure) at a final concentration of 0.005% and fish were incubated for 15 minutes, then rinsed twice in fresh EM.
2.5 Imaging and quantification
All imaging was performed in vivo on larvae anesthetized with 0.001% MS-222 (3-aminobenzoic acid ethyl ester methanesulfonate; Sigma). For direct counts of YO-PRO1/FM 1-43-labeled hair cells, anesthetized fish were mounted between bridged cover slips and visualized on a Zeiss Axioplan 2ie epifluorescence microscope. Labeled hair cells in each of four neuromasts (SO1, SO2, IO1, IO2; Raible and Kruse, 2000) were counted (8–12 fish per treatment group). Hair cell numbers were normalized to mock-treated controls for each individual neuromast and are reported as % survival based on this normalization. Images were collected with Slidebook software v. 4 (Olympus, Center Valley, PA).
DASPEI-labeled, anesthetized fish were placed in depression slides and observed with a Leica MZFLIII stereomicroscope equipped for epifluorescence. Ten neuromasts on each larva (8–16 fish/treatment) were each scored on a scale from 0 (no staining) to 2 (normal staining), resulting in a score from 0 to 20 for each fish (Harris et al, 2003; Owens et al., 2007, 2008). Average scores for each group were normalized to mock-treated controls. All hair cell quantification data (direct counts or DASPEI scores) are presented as the mean ± one standard deviation in all figures.
2.6 Uptake studies
Texas Red-conjugated gentamicin (GTTR) was prepared as described (Steyger et al., 2003; Dai et al., 2006). Hair cells were first prelabeled in YO-PRO1 diluted in standard EM. Larvae were exposed to 45 μM GTTR diluted in the appropriate embryo medium (see results) for 10 minutes at room temperature, anaesthetized in MS-222, and immobilized either between bridged coverslips or in a drop of 1.5% low-melt agarose. For uptake experiments involving amiloride, fish were incubated in amiloride (amiloride hydrochloride hydrate, Sigma; dissolved in 100% DMSO) for 15 minutes prior to the addition of GTTR. Fish were imaged on an Olympus FV-1000 confocal microscope with Fluoview software. All images were collected using identical laser power and software settings. Confocal stacks were transformed into brightest point projections with ImageJ (v. 1.40g). While YO-PRO1 fluoresces green, images were pseudocolored blue in ImageJ to increase contrast with Texas Red.
2.7. Statistics
Statistical analyses were performed using Graphpad Prism v. 5. Analysis of variance (ANOVA) was used to assess dose-response curves with Bonferroni-corrected t-tests used for post-hoc comparisons between individual data points. For analyses comparing only like data (direct hair cell counts or DASPEI scores), the raw data was used for all analyses. For comparisons between the two scoring measures, normalized scores were used in order to allow direct comparison between data sets. In the case of direct hair cell counts, raw counts were first normalized to the average control value for each individual neuromast because different neuromasts normally contain different numbers of hair cells. This generated four normalized values for each fish (one per neuromast), which were then averaged to arrive at one normalized hair cell survival score for each animal. Results are presented as means ± 1 SD.
3. Results
3.1 Minimal calcium requirement
Lateral line hair cells require a minimum level of calcium in the external medium. As shown in Fig. 1, bathing fish for 90 minutes in medium containing no added calcium (nominal zero) resulted in a significant loss of hair cells as compared to control fish held in standard E2 medium (unpaired t-test, p ≤ 0.01). Nuclear condensation, a hallmark feature of cell death, was evident in fish incubated in nominal zero calcium but not in fish held in E2 medium (Fig. 1A). Magnesium could not substitute for calcium (data not shown), demonstrating that hair cells specifically require extracellular calcium for survival.
Fig. 1.

Neuromast hair cells have a minimal calcium requirement. Fish were incubated for 90 minutes in embryo medium with no added calcium or in embryo medium with moderate (900 μM) calcium (standard embryo medium). (A) SO2 neuromast from a 5 dpf fish incubated in medium with no added calcium (nominal Ca2+-free; left) or in normal embryo medium (right). Hair cell nuclei are labeled with YO-PRO1 (blue) and hair cell cytoplasm with FM 1-43 (red). Arrows in the left panel point to condensed nuclei. Scale bar = 5 μm and applies to both panels. (B) Hair cells were counted in four neuromasts per fish and hair cell numbers were separately normalized to the number of hair cells in control neuromasts. The normalized average for all four neuromasts is shown here. N=9–10 fish/treatment, bars are mean ± 1 SD. Hair cell number is significantly different between the two conditions (unpaired t-test, p ≤ 0.01).
3.2 Divalent cations and aminoglycoside-induced hair cell death
Elevated external calcium protected hair cells from neomycin in a dose-dependent manner (Fig. 2). The highest calcium level tested (2100 μM) provided almost complete protection from acute 100 μM neomycin insult, as seen by both the retention in DASPEI labeling (Fig. 2A, B) and counts of surviving hair cells after 30 minutes of neomycin exposure and 1 hr of recovery (Fig. 2B). Hair cell loss decreased monotonically with successively increasing levels of external calcium concentrations, and both DASPEI scoring and direct hair cell counts showed the same pattern of hair cell loss. The two-way analysis of variance was significant (p<0.001) with differences in calcium concentration contributing most of the variance, while the method of data collection (DASPEI scoring or hair cell counts) made up only 4.6% of the total variation in hair cell survival. Previous reports from our laboratory have also validated the use of the DASPEI scoring method against hair cell counts (Harris et al., 2003; Santos et al., 2006). Experiments described below use DASPEI scoring.
Fig. 2.

Calcium protects hair cells from neomycin in a dose-dependent manner. (A) Fish were acutely exposed to 100 μM neomycin in embryo medium with variable Ca2+ concentration and hair cell survival was assessed with the vital dye DASPEI. The arrow indicates the MI1 neuromast in each image as a reference, while the arrowhead points to the olfactory epithelium, which serves as an internal labeling control. Numbers on each image indicate the Ca2+ concentration. The scale bar in the “control” image is 0.5 mm. (B) Mean hair cell survival following acute 100 μM neomycin exposure was assessed using DASPEI scoring (dashed line) from 10 neuromasts per fish or by direct counts of YO-PRO1-labeled hair cells (solid line) from four neuromasts per fish. Mock-treated controls are not shown but are represented by the 100% level. Increased calcium shows significant protection against neomycin at this dose (Two-way ANOVA, p<0.001) (C–D) Dose-response curves for acute (C) and continuous (D) neomycin exposure in embryo medium containing either 300, 900, 1500, or 2100 μM Ca2+. Mean hair cell survival significantly increases with increasing external Ca2+ for either exposure time course (p<0.001). The legend in panel C applies to both C and D. N=9–16 fish/treatment, error bars are ± 1 SD.
Calcium modulation of neomycin-induced hair cell damage across a range of neomycin concentrations is presented in Figure 2C and 2D. There is a robust relationship between calcium concentration and hair cell survival across all levels of aminoglycoside exposure, with significant main effects for both neomycin and calcium as well as the interaction term (p<0.001). This effect is consistent regardless of the drug exposure time course. While there is increasing hair cell death with increasing neomycin dose regardless of the external calcium environment, 2100 μM calcium still provided significant hair cell protection (p<0.001) from 400 μM neomycin, a dose sufficient to cause complete hair cell loss in medium containing ≤ 1 mM calcium. High (2100 μM) calcium provided equal protection from hair cells during acute or continuous exposure (2-way ANOVA, p=0.34). The combination of low (300 μM) calcium and high (400 μM) neomycin was toxic to fish in the continuous exposure experiment, suggesting that calcium may also play a generally protective role during aminoglycoside exposure in fish.
3.3 Calcium differentially protects against gentamicin exposure
Aminoglycoside antibiotics vary in their hair cell toxicity depending on the relative time of exposure (Owens et al., submitted). Acute gentamicin treatment (30 minutes exposure, 1 hr recovery) in standard E2 medium does not cause greater than 50% hair cell loss over a wide range of gentamicin concentrations (Owens et al., submitted). Similarly, acute gentamicin treatment in the present experiments did not result in greater than 50% hair cell loss, even under low (300 μM) calcium conditions (Fig. 3A, C). The nearly complete loss of DASPEI labeling in fish that were acutely treated with 100 μM gentamicin in “zero” calcium conditions (Fig. 3A) is most likely a compound effect of hair cell loss due to gentamicin combined with hair cell loss due to a lack of calcium (see Fig. 1). As shown in Figure 3C, high (2100 μM) calcium significantly protected hair cells from acute gentamicin exposure when moderate gentamicin concentrations were used (p<0.001), but this protection was not apparent when fish were treated with 400 μM gentamicin.
Fig. 3.

High calcium protects hair cells from acute gentamicin (A, C) exposure but not from continuous gentamicin (B, D). Hair cell survival was assessed with DASPEI scoring for all panels shown here. (A) Acute (30 min exposure, 1 hr recovery) and (B) continuous (6 hr exposure, no recovery) treatment with 100 μM gentamicin in medium with variable Ca2+ concentration. (C–D) Dose-response relationships for hair cells exposed to variable gentamicin in medium containing either 300 or 2100 μM Ca2+. (C) Increased calcium significantly protects against acute gentamicin exposure over a range of gentamicin concentrations (p<0.001), except at the highest dose tested. (D) High calcium significantly protects hair cells from continuous (6 hr) exposure to 50 or 100 μM gentamicin (p<0.001). The legend in (D) applies to panels C and D. N=9–13 fish/treatment, except for the “400 gentamicin, 300 calcium” group in panel D, as this combination appears toxic to fish. Here, n=5. For all panels error bars are ±1 SD.
In contrast to neomycin exposure, continuous (6 hr) gentamicin treatment caused additional hair cell damage beyond that seen with acute exposure at all calcium concentrations tested (Fig. 3B, D). Two-way analysis of variance shows that there is a significant main effect of calcium (F1,1 =147.3, p<0.001), but this effect is relatively small, accounting for only 3.23% of the variance, compared to the main effect of gentamicin concentration (86.31%). This effect of calcium may be due primarily to a residual protective effect from the acute phase of treatment, or calcium may play a moderately protective role throughout all phases of gentamicin-induced hair cell damage.
Magnesium modulates hair cell death in response to aminoglycoside treatment in a similar manner to calcium (Fig. 4). In these experiments only low (300 μM) and high (2100 μM) magnesium concentrations were used. High magnesium protected hair cells from both neomycin (Fig. 4A) and acute gentamicin (Fig. 4B) exposure, but again, this protection was attenuated with continuous gentamicin treatment (Fig. 4C). Both main effects of drug and magnesium concentration, as well as the interaction term, are significant for all experiments shown in Figure 4 (p<0.001). As with calcium, high magnesium does not protect hair cells from 400 μM gentamicin under either exposure paradigm. The experiments shown here were conducted by varying the concentration of MgSO4, but neomycin exposure experiments using MgCl2 yielded results that were not significantly different from those with MgSO4 (unpaired t-tests, p>0.05, Supplemental Fig. S1), demonstrating that it is the Mg2+ and not the anion concentration that is responsible for the protective effect.
Fig. 4.

External magnesium concentration affects aminoglycoside-induced hair cell death. (A) Acute neomycin exposure in medium containing low (300 μM) or high (2100 μM) Mg2+. (B–C) Dose response relationship for acute gentamicin (B) and continuous gentamicin (C) exposure in low or high Mg2+. In all cases there is a significant effect of both aminoglycoside concentration and magnesium concentration on hair cell survival (two-way ANOVA, p<0.0001). The legend in (A) applies to all panels. N=9–13 fish/treatment, error bars are ± 1 SD.
For the experiments described above, fish were treated in the designated embryo medium during all phases of the experiment. In order to test when divalent cations are exerting their effect, fish were pretreated in either low (300 μM) or high (2100 μM) calcium medium for 30 minutes, then acutely exposed to neomycin in the opposite calcium medium (Fig. 5). Pretreatment in high calcium medium followed by 200 μM neomycin exposure in low calcium medium resulted in a significant decrease in hair cell loss compared to the same experiment run completely in low calcium (t-test, p=0.02), but there was still considerably more hair cell loss than in fish exposed to neomycin under high calcium conditions. This marginal protection obtained from pretreatment is most likely due to a partial block of the transduction channel during pretreatment (see below) that was not completely washed out before neomycin exposure.
Fig. 5.

Pretreatment in reciprocal embryo medium does not alter the pattern of hair cell loss following neomycin exposure. Gray bars indicate fish that were pretreated for 1 hr in 300 μM calcium, black bars are fish that were pretreated in 2100 μM calcium. The calcium concentration during neomycin exposure and recovery is indicated on the x-axis. There is a significant difference in hair cell survival between the groups treated with neomycin in 300 μM calcium (t-test, p=0.02) but not between the groups treated in 2100 μM calcium (p=0.64). N=9–11 fish/treatment, error bars are ± 1 SD.
In all of the above experiments, only one divalent cation was varied while the other was held at moderate levels (~1 mM). It is therefore possible that lowering both cations simultaneously, or raising one while keeping the other low, would yield different results. We therefore examined the role of simultaneous manipulation of both divalent cations on hair cell death following acute exposure to 100 μM neomycin. The degree of hair cell loss differs between experiments when either calcium or magnesium is varied as compared to when both cations are varied together (One-way ANOVA, p<0.0001; Suppl. Fig S2). However, lowering both calcium and magnesium simultaneously does not further facilitate hair cell loss as compared to experiments run in low calcium or low magnesium medium (Suppl. Fig S2A). Similarly, lowering one cation while keeping the other high does not result in increased hair cell loss relative to experiments where one cation is high and the other is kept at moderate levels (Suppl. Fig S2B).
3.4 Calcium modulates aminoglycoside uptake
Previous studies have implicated calcium in hair cell transduction channel opening in other vertebrates (reviewed in Eatock, 2000) and in gentamicin uptake in cultured kidney cells (Myrdal and Steyger, 2005). In addition, changes in external calcium alter the kinetics of the aminoglycoside dihydrostreptomycin entry through the transduction channel (Marcotti et al., 2005). To investigate possible mechanisms for the altered hair cell sensitivity under changes in divalent cation concentrations, we directly visualized aminoglycoside uptake in vivo using Texas-Red labeled gentamicin (GTTR; Steyger et al., 2003). As exemplified in Figure 6, uptake of gentamicin is antagonized by calcium. On the other hand, we observed only slight differences in GTTR fluorescence between hair cells incubated in medium containing differing concentrations of magnesium; GTTR enters hair cells under either of these ionic conditions (Fig. 6C, D).
Fig. 6.

GTTR uptake is substantially antagonized by external calcium and to a lesser degree by magnesium. Fish were incubated for 10 minutes in GTTR diluted in (A) 300 μM calcium, (B) 2100 μM calcium, (C) 300 μM magnesium, or (D) 2100 μM magnesium. (E) Negative control neuromast from a fish incubated with Texas Red only. GTTR fluorescence is red; nuclei are counter-stained blue with YO-PRO1. GTTR fluorescence is greatly reduced in the presence of high calcium but there is little difference in fluorescence between the low and high magnesium conditions. The arrow in A indicates a fragmented hair cell. The scale bar in E is 5 μm and applies to all panels.
Aminoglycosides are hypothesized to enter hair cells in a transduction-dependent manner, possibly through the open pore of the mechanotransduction channel (Gale et al., 2001; Steyger et al., 2003; Marcotti et al., 2005). We therefore used the transduction channel blocker amiloride to further examine the role of calcium in aminoglycoside uptake. Amiloride is a well-validated inhibitor of many ion channels, including the hair cell transduction channel (Jørgensen and Ohmori, 1988; Tang et al., 1988; Garcia et al., 1990; Manev er al., 1990; Rüsch et al., 1994), and 1 mM amiloride inhibits uptake of the transduction marker FM 1-43 in the zebrafish lateral line (Seiler and Nicolson, 1999). Similar to what we observed with high calcium, 1 mM amiloride prevents both short-term GTTR uptake and hair cell death following neomycin or gentamicin exposure, suggesting similar transduction-dependent uptake of both aminoglycosides (Fig. 7).
Fig. 7.

Amiloride blocks drug uptake and the resulting loss of hair cells. (A) Amiloride attenuates short-term (10 min) GTTR uptake (right) as compared to GTTR uptake without amiloride (left). Hair cell nuclei are labeled with YO-PRO1 (blue), scale bar = 5 μm and applies to both panels. (B) 1 mM amiloride prevents hair cell loss from acute neomycin or gentamicin exposure (unpaired t-tests, p>0.05 for amiloride + aminoglycoside as compared to mock-treated controls). Black bars = no amiloride, gray bars include 1 mM amiloride. Controls show that amiloride alone does not alter DASPEI scores (unpaired t-test, p>0.05). Error bars in (B) are ± 1 S.D. All experiments shown here were conducted in standard EM (~1 mM Ca2+).
Given that either calcium or amiloride can inhibit GTTR uptake and subsequent hair cell death, we then examined the complementary interaction between the two. We first established that a moderate concentration of amiloride (50 μM) reduces GTTR uptake under either low (300 μM) or moderate (1500 μM) calcium conditions (Fig. 8A). Titration of amiloride and calcium against one another shows additive effects, indicating that both substances may operate on a similar uptake mechanism (Fig. 8B).
Fig. 8.
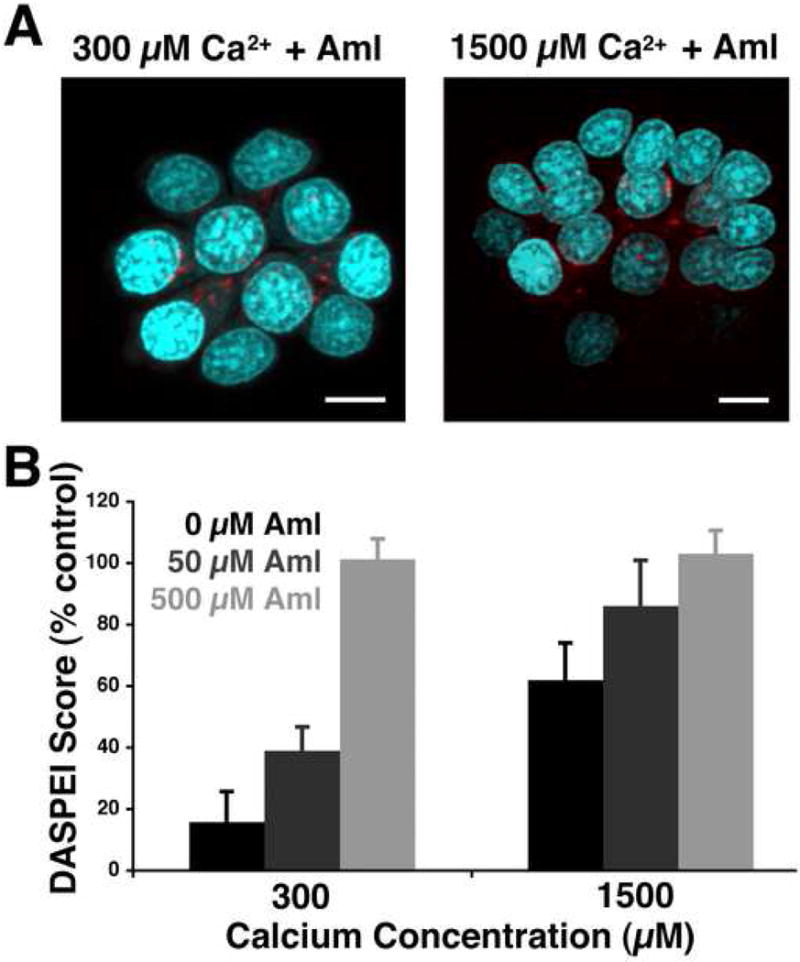
Interplay of amiloride and calcium in aminoglycoside uptake and hair cell death. (A) 50 μM amiloride reduces GTTR uptake under low calcium (300 μM) conditions (left) but does not completely prevent GTTR uptake even under higher (1500 μM) calcium conditions (right). Hair cell nuclei are labeled with YO-PRO1 (blue), scale bars are 5 μm. (B) Amiloride increases hair cell survival proportionally regardless of calcium concentration. Black bars represent the effect of calcium only on hair cell loss for two concentrations of calcium (300 or 1500 μM). Dark gray bars depict hair cell survival in the indicated calcium concentration with 50 μM amiloride added, while light gray bars represent hair cell survival with 500 μM amiloride. Error bars are ± 1 S.D.
4. Discussion
4.1 Divalent cations modulate hair cell death
We have shown that extracellular calcium and magnesium ions modulate aminoglycoside-induced hair cell death in the zebrafish lateral line, with high concentrations offering protection and low concentrations facilitating hair cell loss. These data are in agreement with in vitro studies in the mammalian cochlea (Richardson and Russell, 1991) and suggest that divalent cations act similarly on all vertebrate hair cells. Richardson and Russell (1991) found that removal of both calcium and magnesium from the culture medium also caused hair bundle damage. Our data suggest that it was probably the exclusion of calcium that caused the hair cell damage in their preparation. Combined, these results indicate that a minimal extracellular calcium requirement is a general feature of vertebrate hair cells.
How removal of extracellular calcium triggers hair cell death remains unresolved. It is well established that increasing intracellular free calcium is an important component of cell death in many cell types, including neurons, and that this increase often results from release of mitochondrial and endoplasmic reticulum calcium stores (reviewed in McConkey and Orrenius, 1997; Smaili et al., 2000; Arundine and Tymianski, 2003). In addition, previous studies have found that removing extracellular calcium causes cell death in multiple cell types including astrocytes, neuroblastoma cells, and cardiac myocytes (Chiesa et al., 1998; Zhu et al., 2000), possibly by triggering calcium release from mitochondria (Zhu et al., 2000). This process may also occur in hair cells following calcium removal. Future studies will examine changes in intracellular calcium storage and release during hair cell incubation in calcium-free medium.
The studies presented here conclusively demonstrate the modulatory effects of divalent cations on aminoglycoside-induced hair cell death in vivo. Prior studies in zebrafish and other fishes provide additional evidence for this phenomenon. For example, Kaus (1992) showed that extracellular calcium altered the effects of neomycin on a behavioral assay of putative lateral line function in killifish (Aplocheilus lineatus). An additional study, while not designed to address the influence of extracellular ions, further supports our finding that divalent cation concentration alters zebrafish lateral line hair cell death in response to aminoglycosides (Ton and Parng, 2005). This study found that low concentrations of neomycin or gentamicin (5 to 10 μM) elicited significant damage, and examination of the embryo medium used by this group reveals that their experiments were performed under low calcium and low magnesium conditions (300 μM CaCl2, 100 μM MgSO4). Low doses of aminoglycoside antibiotic were also reported to cause damage of zebrafish hair cells by Williams and Holder (2000), but the concentrations of divalent cations used in their media was not reported.
4.2 Calcium antagonizes transduction-dependent drug uptake
Extracellular calcium regulates mechanotransduction adaptation (Corey and Hudspeth, 1983; Crawford et al., 1991; Ricci and Fettiplace, 1998; Ricci et al., 1998). Adaptation is hypothesized to occur by two mechanisms, termed fast and slow adaptation, and both mechanisms may be regulated by calcium (Wu et al., 1999; Holt and Corey, 2000). During fast adaptation this regulation is thought to occur directly, by calcium entering through the open transduction channel and binding to an intracellular portion of the channel or nearby element, thereby inducing channel closure (Crawford et al., 1991; Cheung and Corey, 2006; Beurg et al., 2008). Transduction is dependent on tip links; extracellular filaments that connect stereocilia and are hypothesized to act as part of the gating spring apparatus that regulates opening of the transduction channel (reviewed in Ricci et al., 2006). The working hypothesis for slow adaptation posits that a molecular motor, most likely myosin 1c, is connected in series to the tip links and that during prolonged stimulation myosin 1c slides down individual stereocilia, resetting the transduction channel in a closed state (reviewed in Gillespie and Cyr, 2004). The interaction of myosin 1c with the actin core of the stereocilium is indirectly regulated by calcium through a series of calmodulin binding sites in the neck region of the myosin protein (Cyr et al., 2002).
Several studies implicate the transduction channel as one site of aminoglycoside uptake in hair cells (Gale et al., 2001; Steyger et al., 2003; Marcotti et al., 2005). Tip link breakage by calcium chelation appears to inhibit mechanotransduction and protect hair cells from aminoglycoside damage (Assad et al., 1991; Zhao et al., 1996; Gale et al., 2001). The shaker-1 mouse and mariner zebrafish mutant, both transduction-deficient myosin 7a mutants, show resistance to aminoglycoside ototoxicity, further supporting a link between mechanotransduction and aminoglycoside sensitivity (Richardson et al., 1997, 1999; Seiler and Nicolson, 1999; Kros et al. 2001). Extracellular calcium alters interactions of aminoglycosides with the transduction channel and changes their entry into the channel (Marcotti et al., 2005). Our findings provide additional support for the hypothesis that aminoglycoside uptake is dependent on hair cell transduction and that calcium can regulate the channel properties by modulating adaptation or in some other fashion. However, due to the time courses examined in the present study we cannot differentiate the contributions of fast vs. slow adaptation on aminoglycoside exclusion from hair cells.
While we think it likely that calcium reduces GTTR uptake by regulating transduction channel adaptation, we cannot rule out a direct extracellular interaction between calcium and gentamicin that would reduce drug entry. It is also possible that calcium antagonizes GTTR uptake by a charge screening mechanism, whereby a change in the external ionic environment would alter transduction channel gating via charge effects rather than protein binding. Given that the transduction channel is mechanically gated, however, we consider this scenario unlikely.
Experiments on isolated tectorial membrane preparations from mouse and chick inner ear have demonstrated that the thickness of this gelatinous structure may be modulated by extracellular calcium concentration, with high calcium (2000 μM) causing a slight shrinkage of the tectorial membrane (~ 1% in mouse, Shah et al., 1995; up to 16% in chick, Freeman et al., 1994). Hair cells in the lateral line are overlaid by a gelatinous cupula analogous to the tectorial membrane of the inner ear. While the response of the cupula to changing ionic concentrations is unknown, it is possible that our high calcium medium leads to shrinkage of the cupula. Cupular contraction could inhibit aminoglycoside access to the hair bundle, thereby reducing drug entry and subsequent toxicity. However, cupular calcium concentration in the lateral line of Xenopus laevis is directly proportional to the external calcium concentration, suggesting that calcium passively diffuses through the cupula (McGlone et al., 1979). While we cannot rule out an indirect effect of calcium concentration on drug entry by modulating cupular mechanics, we consider it more likely that calcium directly antagonizes aminoglycoside uptake by hair cells by regulating mechanotransduction channel gating characteristics.
The protective effects of high calcium medium appear to stem primarily from reduction of drug uptake, but our data do not rule out an intracellular protective role for calcium. This is unlikely, however, as mechanical and noise damage is correlated with increasing intracellular calcium in the mammalian cochlea, suggesting that increases in intracellular calcium may be toxic to hair cells (Gale et al., 2004; Piazza et al., 2007). It is, of course, possible that intracellular calcium acts differently in zebrafish hair cells or that these cells have a different calcium threshold for cytotoxicity. More likely, calcium influx during mechanotransduction is highly regulated within the hair bundle, preventing a concomitant rise in intracellular calcium with potentially damaging results. Studies in amphibians and rodents have suggested that plasma membrane calcium-ATPase (PMCA) isoforms in hair bundles play a pivotal role in actively regulating local calcium homeostasis, effectively separating the hair bundle and soma into distinct calcium compartments (Street et al. 1998; Crouch and Schulte, 1995; Yamoah et al., 1998; Dumont et al., 2001; Ficarella et al. 2007). Future studies will look at the influence of extracellular calcium on intracellular calcium dynamics and regulation in lateral line hair cells.
The present study clearly demonstrates a protective role for high calcium in preventing aminoglycoside ototoxicity in the zebrafish lateral line. However, it is possible that excess calcium alone could also damage these cells. On the other hand, the range of calcium concentrations used in our study are within the natural range of calcium conditions found in worldwide waters (Hem, 1992), and therefore within the physiologically relevant range for lateral line hair cells. Furthermore, hair cells exposed to high calcium medium for up to 6 hrs appeared morphologically healthy and showed normal uptake of both the vital dye DASPEI and the transduction-dependent dye FM 1–43 (Coffin, unpublished data), suggesting that 2100 μM calcium is not harmful to these cells.
4.3 Magnesium and hair cell protection
The mechanism by which extracellular magnesium protects hair cells is unknown. Magnesium is known to block NMDA receptors, which are implicated in neuronal excitotoxicity in a variety of human diseases (Mayer et al. 1984; Nowak et al. 1984; Wenk 2006; Fan and Raymond 2007). In addition, a previous study suggested that there is an excitotoxic, NMDA-dependent component to aminoglycoside-induced hair cell death in the guinea pig cochlea (Basile et al. 1996). High magnesium has been shown to prevent noise-induced hearing loss in guinea pigs (Scheibe et al. 2001; Haupt et al. 2003; Sendowski et al. 2006), suggesting that excitotoxicity may play a role in different types of hair cell damage. It is therefore possible that aminoglycoside-induced death of lateral line hair cells also involves glutamate-driven excitotoxic responses. Detailed study of glutamate receptors in lateral line hair cells and pharmacological inhibition of these receptors will be necessary to test this hypothesis.
4.4 Differences between neomycin and gentamicin
We recently demonstrated that neomycin and gentamicin do not have the same mode of action in the zebrafish lateral line (Owens et al., submitted). High concentrations of neomycin (200 and 400 μM) kill virtually all hair cells within 90 minutes. Longer incubations in lower neomycin concentrations have little effect beyond what is seen in the first 90 minutes. In contrast, 90 minutes of exposure to gentamicin (within the broad range of concentrations tested) leads to a maximum of 40–50% hair cell loss, while longer gentamicin exposure (or longer recovery time after short exposure) causes greater hair cell death, even at relatively low concentrations (50–100 μM). These findings suggest that neomycin acts through a “fast” death mechanism, while gentamicin kills hair cells by both “fast” and “slow” processes.
The present study used both acute (90 minute) and continuous (6 hr) aminoglycoside exposure paradigms in order to understand the role of external divalent cations on both the “fast” and “slow” death mechanisms. High extracellular calcium is sufficient to protect hair cells from both acute and continuous neomycin exposure. High extracellular calcium also protects hair cells from acute gentamicin exposure but is less effective against continuous gentamicin treatment, suggesting that extracellular calcium provides effective protection during the acute phase of damage, and is either not protective, or less effective, toward inhibiting the longer process(es). On the other hand, amiloride blocks hair cell death during both short-term and long-term exposure to neomycin and gentamicin, suggesting that both proposed mechanisms of aminoglycoside-induced damage require mechanotransduction. We think that the most parsimonious conclusion from these data is that extracellular calcium blocks aminoglycoside entry, but must do so only reversibly, since enough gentamicin can enter over prolonged exposure to damage cells.
4.5 Conclusion
In conclusion, we have shown that extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Calcium appears to afford protection by inhibiting drug uptake, while magnesium may protect hair cells by other additional mechanisms. These results demonstrate the importance of the extracellular environment for hair cell survival and underscore the necessity of precise characterization of this extracellular environment. They also show that comparisons between studies must take into account the ionic conditions used for each experiment, the particular aminoglycoside under investigation, and the protocol of treatment. Finally, these experiments suggest that therapeutic perturbations of the ionic microenvironment could protect hair cells from aminoglycoside ototoxicity.
Supplementary Material
Supplemental Figure S1. The anion coupled to magnesium does not affect hair cell death following neomycin treatment. Fish were exposed to 100 μM neomycin in embryo medium where the magnesium concentration was varied with either MgSO4 (as in Fig. 4; gray) or with MgCl2 (black). There is no significant difference between the MgSO4 and MgCl2 groups (unpaired t-test, p>0.05). N=10–12 fish/treatment, error bars are ± 1 S.D.
Supplemental Figure S2. Hair cell death following acute exposure to 100 μM neomycin when both calcium and magnesium concentrations are manipulated simultaneously. (A) There is a significant difference between fish treated in medium with only one divalent cation lowered vs. medium with both calcium and magnesium set to low levels (One-way ANOVA, p<0.001). However, concurrently lowering both calcium and magnesium (middle treatment group) does not further facilitate hair cell death when compared to lowering only calcium or magnesium. (B) Hair cell death following neomycin exposure is attenuated as long as calcium or magnesium is maintained at high levels (2100 μM).
Acknowledgments
We thank J. Simon and J. Stone for experimental advice, G. MacDonald for microscopy assistance, and two anonymous reviewers for feedback. Support was provided by NIDCD grants DC000018, DC004661, DC009931 and DC005987, and the Virginia Merrill Bloedel Hearing Research Center.
Footnotes
Suggested reviewers:
Dr. Andrew Forge, University College London, a.forge@ucl.ac.uk, 020 7679 8983
Dr. Jonathan Gale, University College London, j.e.gale@ucl.ac.uk
Dr. Jason Meyers, Colgate University, 315-228-6468, jmeyers@mail.colgate.edu
Dr. Guy Richardson, University of Sussex, +44 1273 678717, C.P.Richardson@sussex.ac.uk
Dr. Peter Steyger, Oregon Health and Science University, 503-494-1062, steygerp@ohsu.edu
Literature Cited
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7(6):985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Basile AS, Huang JM, Xie C, Webster D, Berlin C, Skolnick P. N-methyl-D-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat Med. 1996;2(12):1338–1343. doi: 10.1038/nm1296-1338. [DOI] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Crawford A, Fettiplace R. The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophysics J. 2008;94:2639–2653. doi: 10.1529/biophysj.107.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Hair cell death in the avian basilar papilla: characterization of the in vitro model and caspase activation. J Assoc Res Otolaryngol. 2003;4(1):91–105. doi: 10.1007/s10162-002-3016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13(6):343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Cheung EL, Corey DP. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys J. 2006;90(1):124–139. doi: 10.1529/biophysj.105.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R, Angeretti N, Del Bo R, Lucca E, Munna E, Forloni G. Extracellular calcium deprivation in astrocytes: regulation of mRNA expression and apoptosis. J Neurochem. 1998;70:1474–1483. doi: 10.1046/j.1471-4159.1998.70041474.x. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9(2):178–90. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Braun CB, Donovan B. The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. J Exp Biol. 2001;204:337–348. doi: 10.1242/jeb.204.2.337. [DOI] [PubMed] [Google Scholar]
- Coombs S, Görner P, Münz H. The Mechanosensory Lateral Line: Neurobiology and Evolution. Springer-Verlag; NY: 1989. [Google Scholar]
- Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J Physiol. 1991;434:369–398. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch JJ, Schulte BA. Expression of plasma membrane Ca-ATPase in the adult and developing gerbil cochlea. Hear Res. 1995;92(1–2):112–119. doi: 10.1016/0378-5955(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Brandon CS. Heat shock inhibits both aminoglycoside- and cisplatin-induced sensory hair cell death. J Assoc Res Otolaryngol. 2006;7(3):299–307. doi: 10.1007/s10162-006-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22(19):8532–40. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60(1):89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Cyr JL, Dumont RA, Gillespie PG. Myosin-1c interacts with hair-cell receptors through its calmodulin-binding IQ domains. J Neurosci. 2002;22(7):2487–2495. doi: 10.1523/JNEUROSCI.22-07-02487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res. 2006;213:64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne N, Rauen U, de Groot H, Lautermann J. Involvement of the mitochondrial permeability transition in gentamicin ototoxicity. Hear Res. 2002;69(1–2):47–55. doi: 10.1016/s0378-5955(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. The functioning and significance of the lateral line organs. Biol Rev. 1963;38:51–105. doi: 10.1111/j.1469-185x.1963.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21(14):5066–5078. doi: 10.1523/JNEUROSCI.21-14-05066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Annu Rev Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ficarella R, Di Leva F, Ortolano S, Donaudy F, Petrillo M, Melchionda S, Lelli A, Domi T, Fedrizzi L, Lim D, Shull GE, Gasparini P, Brini M, Mammano F, Carafoli E. A functional study of plasma-membrane calcium-pump isoform 2 mutants causing digenic deafness. Proc Natl Acad Sci USA. 2007;104(5):1516–1521. doi: 10.1073/pnas.0609775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Freeman DM, Cotanche DA, Ehsani F, Weiss TF. The osmotic response of the isolated tectorial membrane of the chick to isosmotic solutions: Effect of Na+, K+, and Ca2+ concentration. Hear Res. 1994;79:197–215. doi: 10.1016/0378-5955(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21(18):7013–1025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14(6):526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Garcia ML, King VF, Shevell JL, Slaughter RS, Suarez-Kurz G, Winquist RJ, Kaczorowski GJ. Amiloride analogs inhibit L-type calcium channels and display calcium entry blocker activity. J Biol Chem. 1990;265(7):3763–3771. [PubMed] [Google Scholar]
- Gillespie PG, Cyr JL. Myosin-1c, the hair cell’s adaptation motor. Annu Rev Physiol. 2004;66:521–545. doi: 10.1146/annurev.physiol.66.032102.112842. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4(2):219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt H, Scheibe F, Mazurek B. Therapeutic efficacy of magnesium in acoustic trauma in the guinea pig. ORL J Otorhinolaryngol Relat Spec. 2003;65(3):134–139. doi: 10.1159/000072250. [DOI] [PubMed] [Google Scholar]
- Hem JD. Study and interpretation of the chemical characteristics of natural water. 3rd. US Govt Printing Office; Washington, DC: 1992. (USGS Water Supply Paper 2254). [Google Scholar]
- Hernández PP, Moreno V, Olivari FA, Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio) Hear Res. 2006;213(1–2):1–10. doi: 10.1016/j.heares.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Holt JR, Corey DP. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc Natl Acad Sci USA. 2000;97(22):11730–11735. doi: 10.1073/pnas.97.22.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997;104(1–2):1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Cunningham DE, Rubel EW. Electron microscopy of degenerative changes in the chick basilar papilla after gentamicin exposure. J Comp Neurol. 2004;470:164–180. doi: 10.1002/cne.11046. [DOI] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann NY Acad Sci. 1999;884:389–409. doi: 10.1111/j.1749-6632.1999.tb08657.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13(1):20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen F, Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J Physiol. 1988;403:577–588. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaus S. The influence of calcium on the ototoxicity of aminoglycosides. Acta Otolaryngol. 1992;112:83–87. doi: 10.3109/00016489209100787. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5(1):41–7. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Lang H, Liu C. Apoptosis and hair cell degeneration in the vestibular sensory epithelia of the guinea pig following a gentamicin insult. Hear Res. 1997;111(1–2):177–184. doi: 10.1016/s0378-5955(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Manev H, Bertolino M, DeErausquin G. Amiloride blocks glutamate-operated cationic channels and protects neurons in culture from glutamate-induced death. Neuropharmacology. 1990;29(12):1103–1110. doi: 10.1016/0028-3908(90)90033-n. [DOI] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano -electrical transducer channels. J Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Gale JE, Warchol ME. Critical signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J Neurobiol. 2004;61(2):250–266. doi: 10.1002/neu.20054. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22(4):1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. Biochem Biophys Res Comm. 1997;239:357–366. doi: 10.1006/bbrc.1997.7409. [DOI] [PubMed] [Google Scholar]
- McGlone FP, Russell IJ, Sand O. Measurement of calcium ion concentrations in the lateral line cupulae of Xenopus laevis. J Exp Biol. 1979;83:123–130. doi: 10.1242/jeb.83.1.123. [DOI] [PubMed] [Google Scholar]
- Metcalf WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, MacDonald JA. Sensory tuning of lateral line receptors in Antarctic fish to the movements of planktonic prey. Science. 1987;235:195–196. doi: 10.1126/science.235.4785.195. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. [Google Scholar]
- Murakami SL, Cunningham LL, Werner LA, Bauer E, Pujol R, Raible DW, Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186:47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res. 2005;204(1–2):170–182. doi: 10.1016/j.heares.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New JG, Fewkes LA, Khan SN. Strike feeding behavior in the muskellunge, Esox masquinongy: contributions of the lateral line and visual sensory systems. J Exp Biol. 2001;204:1207–1221. doi: 10.1242/jeb.204.6.1207. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamane H, Takayama M, Sunami K, Nakai Y. Time-dependent response of vestibular hair cells of guinea pigs following high-dose application of streptomycin. Acta Otolaryngol Suppl. 1998;538:32–35. doi: 10.1080/00016489850182701-1. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233(1–2):46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502(4):522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genetics. 2008;4(2):e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium. 2007;41(1):77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibition of c-Jun N-terminal kinase activation. J Neurosci. 2000;20(1):43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Phyiol. 1998;506:159–173. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Kachar B, Gale J, Van Netten SM. Mechano-electrical transduction: new insights into old ideas. J Membr Biol. 2006;209(2–3):71–88. doi: 10.1007/s00232-005-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci. 1998;18(20):8261–8277. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ. Cochlear cultures as a model system for studying aminoglycoside induced ototoxicity. 1991;53:293–311. doi: 10.1016/0378-5955(91)90062-e. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Fleming J, Brown SDM, Steel KS. Myosin VIIA is required for aminoglycoside accumulation in cochlear hair cells. J Neurosci. 1997;17(24):9506–9519. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Marcotti W, Becker D, Williams DS, Thorpe J, Fleming J, Brown SDM, Steel KP. A missense mutation in myosin VIIA prevents aminoglycoside accumulation in early postnatal cochlear hair cells. Ann NY Acad Sci. 1999;884:110–124. [PubMed] [Google Scholar]
- Rüsch A, Kros CJ, Richardson GP. Block by amiloride and its derivatives of mechano-electrical transduction in outer hair cells of mouse cochlear cultures. J Physiol. 1994;474(1):75–86. doi: 10.1113/jphysiol.1994.sp020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Mazurek B, König O. Therapeutic effect of magnesium on noise-induced hearing loss. Noise Health. 2001;3(11):79–84. [PubMed] [Google Scholar]
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- Sendowski I, Raffin F, Braillon-Cros A. Therapeutic efficacy of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Acta Otolaryngol. 2006;126(2):122–129. doi: 10.1080/00016480500312547. [DOI] [PubMed] [Google Scholar]
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hear Res. 2000;142(1–2):34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Shah DM, Freeman DM, Weiss TF. The osmotic response of the isolated, unfixed mouse tectorial membrane to isosmotic solutions: effect of Na+, K+, and Ca2+ concentration. Hear Res. 1995;87:187–207. doi: 10.1016/0378-5955(95)00089-m. [DOI] [PubMed] [Google Scholar]
- Smaili SS, Hsu YT, Youle RJ, Russell JT. Mitochondria in Ca2+ signaling and apoptosis. J Bioenerg Biomembranes. 2000;32(1):35–46. doi: 10.1023/a:1005508311495. [DOI] [PubMed] [Google Scholar]
- Song J, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hear Res. 1995;91(1–2):63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Peters SL, Rehling J, Hordichok A, Dai CF. Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol. 2003;4:565–578. doi: 10.1007/s10162-003-4002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street VA, McKnee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Rubel EW, Cunningham LL. JNK signaling in neomycin-induced vestibular hair cell death. Hear Res. 2006;221(1–2):128–35. doi: 10.1016/j.heares.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb M, Brandon CS, Lee FS, Lomax MI, Dillmann WH, Cunningham LL. Hsp70 Inhibits Aminoglycoside-Induced Hair Cell Death and is Necessary for the Protective Effect of Heat Shock. J Assoc Res Otolaryngol. 2008 doi: 10.1007/s10162-008-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988;249(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, Polak M, Malgrange B, Lefebvre PP, Staecker H, Balkany TJ. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurootol. 2004;25(4):627–632. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- Wang J, Van de Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23(24):8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer’s disease: potential targets for treatment. J Clin Psychiatry. 2006;67(Suppl 3):3–7. [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio rerio) 4th. University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J Neurophysiol. 1999;82:2171–2181. doi: 10.1152/jn.1999.82.5.2171. [DOI] [PubMed] [Google Scholar]
- Yamoah EN, Lumpkin EA, Dumont RA, Smith PJS, Hudspeth AJ, Gillespie PG. Plasma membranes Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J Neurosci. 1998;18(2):610–624. doi: 10.1523/JNEUROSCI.18-02-00610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA. 1996;93(26):15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LP, Yu XD, Ling S, Brown RA, Kuo TH. Mitochondrial Ca2+ homeostasis in the regulation of apoptotic and necrotic cell deaths. Cell Calcium. 2000;28(2):107–117. doi: 10.1054/ceca.2000.0138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. The anion coupled to magnesium does not affect hair cell death following neomycin treatment. Fish were exposed to 100 μM neomycin in embryo medium where the magnesium concentration was varied with either MgSO4 (as in Fig. 4; gray) or with MgCl2 (black). There is no significant difference between the MgSO4 and MgCl2 groups (unpaired t-test, p>0.05). N=10–12 fish/treatment, error bars are ± 1 S.D.
Supplemental Figure S2. Hair cell death following acute exposure to 100 μM neomycin when both calcium and magnesium concentrations are manipulated simultaneously. (A) There is a significant difference between fish treated in medium with only one divalent cation lowered vs. medium with both calcium and magnesium set to low levels (One-way ANOVA, p<0.001). However, concurrently lowering both calcium and magnesium (middle treatment group) does not further facilitate hair cell death when compared to lowering only calcium or magnesium. (B) Hair cell death following neomycin exposure is attenuated as long as calcium or magnesium is maintained at high levels (2100 μM).


