Abstract
Objectives:
This study aimed to evaluate the effects of chlorhexidine mouthrinses on color stability of nanofilled and micro-hybrid resin-based composites.
Materials and Methods:
In this in-vitro study, 160 disc-shaped specimens (7x2mm) were fabricated of Filtek Z250 and Filtek Z350XT Enamel (A2 shade). The samples of each group were randomly divided into eight subgroups (n=10). The specimens were incubated in artificial saliva at 37°C for 24 hours. The baseline color values (L*, a*, b*) of each specimen were measured according to CIE LAB system using a reflection spectrophotometer. After baseline color measurements, the control samples were immersed in saliva and the test groups were immersed in Kin (Cosmodent), Vi-One (Rozhin), Epimax (Emad), Hexodine (Donyaye Behdasht), Chlorhexidine (Shahrdaru), Najo (Najo) and Behsa (Behsa) mouthrinses once a day for two minutes. The specimens were then immersed again in saliva. This process was repeated for two weeks. Color measurements were made on days seven and 14. Two-way and one-way ANOVA and Tukey's post hoc test, t-test and paired t-test were used to analyze data at a significance level of 0.05.
Results:
All specimens displayed color change after immersion in the mouthrinses. Significant interactions were found between the effects of materials and mouthrinses on color change.
Conclusions:
All composite resins tested showed acceptable color change after immersion in different mouthrinses. Filtek Z350XT showed less color change than Filtek Z250. Mouthrinses containing alcohol (Behsa and Najo) and citric acid (Vi-One) caused greater discoloration of composites.
Keywords: Chlorhexidine, Color, Composite Resins, Mouthwashes
INTRODUCTION
Recently, the demand for aesthetic restorative materials such as composite resins has greatly increased [1]. Resin composites are the most commonly used aesthetic restorative materials due to their universal usage, resemblance to tooth structure in color and mechanical properties, requiring minimal removal of tooth structure and ease of chair side use [2]. Today, the common problem of aesthetic dentistry is discoloration and color mismatch of tooth-colored restorations after consumption of chromogens [3]. The color change of composite resins depends on intrinsic and extrinsic factors; intrinsic factors involve changes in the filler, matrix and matrix/filler interface [4]; extrinsic factors involve absorption or adsorption of dyes from external sources, such as coffee, tea, nicotine and mouthrinses like chlorhexidine [2].
In the recent years, prescription of mouthrinses for the control of caries and periodontal disease has become more common [5]. In mentally retarded cases or after periodontal surgeries, plaque control with mechanical methods is not properly possible [6]; thus, chemical methods like the use of chlorhexidine mouthrinse as an antiseptic solution are required [7]. In comparison to common antibiotic therapies, antiseptics contain chemical materials, which represent better antibacterial action [8]. Chlorhexidine is a well-known biguanide compound with effective anti-plaque action [5] and chromogenic potential [9] causing brown staining of teeth, tongue, silicate and resin restorations [10]. Various staining mechanisms have been described for chlorhexidine including [11] degradation of chlorhexidine to release parachloraniline, non-enzymatic browning reactions (the Maillard reactions), protein denaturation by chlorhexidine with metal sulfide formation and precipitation of anionic dietary chromogens by cationic antiseptics. Researchers studied the effect of chlorhexidine and other chromogenic mouthrinses on different restorations. Lamba et al, [12] reported that immersion of composite, glass ionomer cement and compomer in mouthrinses can significantly change the color of these materials. Celik et al, [13] investigated the effect of three chlorhexidine mouthrinses on composites and reported that all composites showed acceptable discoloration.
In the recent years, considerable improvements have occurred in formulation of composite materials, mainly by use of nanotechnology. Nanofilled composites are new materials containing inorganic nanofillers scattered in the resin matrix [14] and have improved mechanical strength, optical properties, wear resistance [15], elasticity modulus and color stability [16]. Microhybrid composites are dominant in dental markets [17], thus, in this study, we compared the color stability of new efficient composites with each other.
Discoloration can be detected visually or by using instrumental techniques. Instrumental techniques decrease subjective interpretation as compared with the visual methods. In comparison of these two methods, spectrophotometer is a more accurate method to detect color changes of dental restorations [18]. A spectrophotometer uses CIE system, which is based on human perception and was developed by the Commission Intèrnationale de l’Eclairage in 1978 [19]. Color change is shown with ΔE in this system, which is total color change based on L*, a* and b* values. The L* value refers to lightness and a* and b* values refer to chromaticity coordinates. L* ranges from 0 (black) to 100 (white). Positive a* values show a shift towards red and negative values show a shift towards green; positive b* values show a shift towards yellow and negative values show a shift towards blue [20]; when ΔE is less than or equal to 1 unit, it is not visible to the human eye; when ΔE is between 1 and 3 units, the color change is visually perceptible to the experienced examiner; when ΔE is greater than or equal to 3.3 units, the color change is clinically unacceptable [21]. Discoloration of aesthetic restorations is problematic, wasting time and cost for replacement of restorations due to dissatisfaction of patients [22]. Chromogenic potential of chlorhexidine mouthrinses has been confirmed [23]. But prescription of chlorhexidine mouthrinses for a minimum of two weeks after periodontal surgeries is a common practice, which may cause discoloration of aesthetic composite restorations of patients. The aim of this study was to evaluate the effects of chlorhexidine mouthrinses available in the Iranian market on color stability of microhybrid and nanofilled composites.
MATERIALS AND METHODS
In this in-vitro study, two composite resins and seven types of chlorhexidine mouthrinses were used. Artificial saliva (Hypozalix Spray, Biocodex, France) was used as the control group. A pH meter (Labtron, Tehran, Iran) was used to determine the pH of mouthrinses. The details of materials used in this study are presented in Tables 1 and 2.
Table 1:
Mouthrinses used in this study
| Mouthrinses | Composition | Color | pH | Company |
|---|---|---|---|---|
| Kin | 0.12% chlorhexidine digluconate + aqua + sorbitol + glycerin + peg40 hydrogenated caster oil + aroma + sodium methyl paraben + citric acid + methyl salicylate + sodium saccharine + 0.05gr sodium fluoride + menthol + eugenol + d-limonene + cinnamal + cl 1420 | Pink | 6 | Cosmodent, Barcelona, Spain |
| Vi-One | 0.2% chlorhexidine digluconate + 0.04% thymol + 2% xylitol + sorbitol + glycerin+ Poloxamer 407+ peg 40 hydrogenated caster oil + deionized water + citric acid + flavor + menthol | White | 7.2 | Rozhin Company, Tabriz, Iran |
| Epimax | 0.12% chlorhexidine digluconate + sorbitol + propylene glycol + tetra sodium pyrophosphate + citric acid + polysorbate 20 + polysorbate 60 + sorbic acid + menthol + 0.05gr sodium fluoride + sodium saccharine + dye + deionized water | Brown | 4.3 | Emad pharmacy Lab., Esfahan, Iran |
| Hexodine | 0.2% chlorhexidine gluconate + aqua + glycerin + mint flavor + preservative + Cl.42090 | Blue | 5.3 | Donyaye Behdasht Lab., Tehran, Iran |
| Chlorhexidine | 0.2% chlorhexidine gluconate + glycerin + methyl paraben + propylparaben + flavor C.l 16035 | Pink | 5.1 | Shahrdaru Lab., Tehran, Iran |
| Najo | 0.2% chlorhexidine gluconate + 10% ethanol | Pink | 5.1 | Najo Pharmacy, Lab., Tehran, Iran |
| Behsa | 0.2% chlorhexidine gluconate + 11.6% ethanol | Pink | 6 | Behsa Pharmacy Lab., Arak, Iran |
Table 2:
Composites used in this study (Shade: A2)
| Brand name | Classification | Resin matrix | Filler (type and size) | wt% | Lot. number | Manufacturer |
|---|---|---|---|---|---|---|
| Filtek Z250 | Microhybrid | Bis-GMA1 Bis-EMA2 UDMA3 |
0.01–3.50 micrometer, zirconia/silica | 78.00 | N144264 | 3M ESPE, St. Paul, MN, USA |
| Filtek Z350 XT | Nanofilled | Bis-GMA Bis-EMA UDMA TEGDMA4 PEGDMA5 |
Non-aggregated 20 nm silica filler, non-aggregated 4–11 nm zirconia filler Aggregated zirconia/silica cluster filler comprised of 20 nm silica and 4 to 11 nm zirconia particles | 78.50 | N479908 | 3M ESPE, St. Paul, MN, USA |
Bisphenol A-glycidyl methacrylate
Bisphenol A ethoxylated dimethacrylate
Urethane dimethacrylate
Triethylene glycol dimethacrylate
Polyethylene glycol dimethacrylate
Preparation of samples:
One-hundred and sixty disc-shaped specimens, 80 from each composite, were made in prefabricated celluloid molds with dimensions of 7x2 mm. Composites were placed in molds and compressed with a 1mm thick glass slab for 30 seconds to obtain a uniformly smooth surface [24]. Each specimen was light-cured with Valo light curing unit (Ultradent, South Jordan, USA) with a light intensity of 800 mW/cm2 for 20 seconds on both sides [13]. A radiometer (Kerr, Demetron, Orange, CA, USA) was used to check the light intensity [13].
The bottoms of specimens were coded from one to 10 to facilitate the color measurement of each specimen. The upper surface of each specimen was ground with 600, 800, 1000, and 2000-grit silicon carbide papers (Matador, Cologne, Germany) successively [25] under running water.
All specimens were placed at the bottom of a container in such a way that they were completely dipped in the solution. After polishing, the specimens were immersed in artificial saliva at 37°C for 24 hours in an incubator in a closed container, allowing post-polymerization.
Baseline color measurement:
Each specimen was then blotted dry with a filter paper and then subjected to color measurement by placing the probe tip perpendicular to the specimen surface. The initial color values (L*, a*, b*) were measured using VITA Easy shade Advance® (VITA Zahnfabrik, Bad Säckingen, Germany) against a white background before insertion of samples in the solutions. The spectrophotometer was calibrated after color measurement of every three samples by placing the probe tip against the calibration block. The color measurement of each specimen was repeated three times.
Immersion of specimens in chlorhexidine mouthrinses:
The specimens were immersed in mouthrinses once a day for two minutes (n=10), which was equivalent to two rinses with mouthwash per day for one minute. After two minutes of immersion in the mouthrinses, the specimens were immersed in artificial saliva in an incubator at 37°C. Control groups were stored only in artificial saliva during two weeks. Artificial saliva was changed every day.
This process was repeated for two weeks to simulate the use of chlorhexidine mouthrinses for two weeks, which is commonly prescribed after dental treatments like periodontal surgeries.
Second and third color measurements:
After one and two weeks of immersion, color measurements were repeated with the same spectrophotometer, and these measurements were made under the same conditions and in the same manner described for initial color measurement.
The total color change (ΔE*) was calculated using the following formula:
The collected data were statistically analyzed with SPSS version 22 using two-way ANOVA to evaluate the interaction effects of the material type and mouthrinse on color change. One-way ANOVA, Tukey's post hoc test, t-test and paired t-test were used to analyze data at a significance level of 0.05.
RESULTS
Two-way ANOVA revealed that the interaction effects of composite type and mouthrinses on color change were statistically significant (P=0.049).
The mean values and standard deviations of ΔL, Δa and Δb color parameters for each group of composite resin after seven and 14 days of immersion in chlorhexidine mouthrinses are shown in Table 3.
Table 3:
The mean ΔL, Δa and Δb values and standard deviation of Filtek Z250 compared to Filtek Z350XT in different solutions after seven and 14 days
| 7 days | 14 days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Filtek Z250 | Filtek Z350 | Filtek Z250 | Filtek Z350 | |||||||||
| ΔL | Δa | Δb | ΔL | Δa | Δb | ΔL | Δa | Δb | ΔL | Δa | Δb | |
| Control | −0.59± 0.53 | 0.14± 0.08 | 1.01± 0.43 | 0.49± 0.24 | 0.15± 0.10 | 0.16± 0.32 | 0.11± 0.65 | −0.06± 0.11 | 0.85± 0.48 | 0.64± 0.39 | 0.27± .009 | −0.07± 0.35 |
| Kin | −0.49± 0.52 | 0.15± 0.25 | 1.46± 0.89 | 0.73± 0.95 | 0.36± 0.12 | −0.26± 0.58 | −0.29± 0.66 | 0.4± 0.46 | 1.34± 0.95 | 1.24± 0.09 | 0.59± 0.19 | −0.22± 0.59 |
| Vi-One | −0.87± 2.58 | 0.12± 0.27 | 1.83± 1.13 | 0.94± 0.83 | 0.27± 0.09 | −0.50± 0.33 | 0.34± 2.61 | −0.003± 0.2 | 1.71± 1.15 | 1.31± 0.92 | 0.42± 0.93 | −0.65± 0.32 |
| Epimax | −1.62± 0.74 | 0.40± 0.21 | 0.85± 0.33 | −0.51± 0.33 | 0.33± 0.09 | −0.58± 0.39 | −1.8± 0.66 | 0.54± 0.33 | 1.09± 0.56 | −0.7± 0.29 | 0.48± 0.08 | −0.73± 0.43 |
| Hexodine | −0.52± 0.29 | −0.35± 0.41 | 1.09± 1.18 | 0.15± 0.53 | −0.07± 0.10 | −1.02± 0.56 | 0.1± 0.45 | −0.69± 0.42 | 2.01± 1.4 | 0.39± 0.57 | −0.14± 0.15 | −1.26± 0.59 |
| Chlorhexidine | −0.55± 0.65 | 0.96± 0.56 | 2.08± 1.27 | −0.35± 0.39 | 0.21± 0.17 | −1.05± 0.4 | −0.6± 0.91 | 0.08± 0.48 | 1.95± 1.25 | −1.66± 0.4 | 0.37± 0.26 | −1.12± 0.5 |
| Najo | 0.16± 0.80 | 0.48± 1.64 | 1.97± 1.30 | 0.01± 0.3 | 0.20± 0.09 | −0.71± 0.33 | −0.59± 0.84 | 0.45± 1.68 | 2.09± 1.24 | 0.39± 0.34 | 0.36± 0.13 | −0.6± 0.37 |
| Behsa | −0.82± 0.34 | 0.99± 0.25 | 1.32± 0.39 | −0.17± 0.47 | 0.93± 0.13 | −1.35± 0.78 | −0.59± 0.84 | 1.44± 0.4 | 1.36± 0.68 | −2.06± 0.58 | 1.44± 0.27 | −1.67± 0.89 |
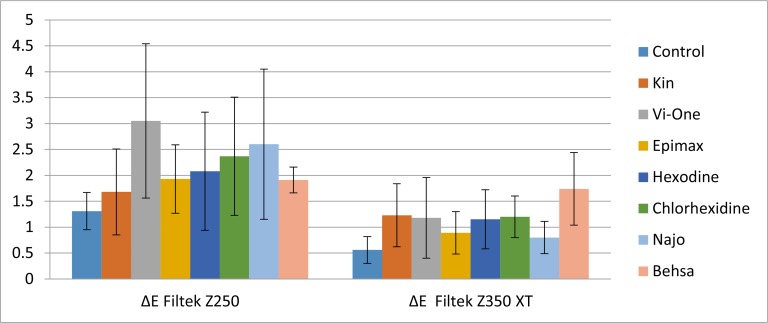
The mean values and standard deviations of color change (ΔE) for each group of composite resin after seven days of immersion in chlorhexidine mouthrinses are shown in Table 4. All groups showed acceptable color change after one week (ΔE<3.3; Fig. 1).
Table 4:
The mean ΔE values and standard deviation (SD) of Filtek Z250 compared to Filtek Z350XT in different solutions after seven and 14 days
| Groups | 7 days | 14 days | ||
|---|---|---|---|---|
| ΔE±SD Filtek Z250 | ΔE±SD Filtek Z350 | ΔE±SD Filtek Z250 | ΔE±SD Filtek Z350 | |
| Control | 1.31±0.36* A a | 0.56±0.26* A b | 1.05±0.51 A a | 0.83±0.28 A a |
| Kin | 1.68±0.83 AB a | 1.23±0.61* AB a | 1.66±0.88 A a | 1.62±0.66 BD a |
| Vi-One | 3.05±1.49 B a | 1.18±0.78* AB b | 2.43±2.23 A a | 1.61±0.80 BD a |
| Epimax | 1.93±0.66* AB a | 0.89±0.41* A b | 2.26±0.65 A a | 1.16±0.80 AD b |
| Hexodine | 2.08±1.14 AB a | 1.15±0.57* AB b | 2.18±1.44 A a | 1.45±0.56 AD a |
| Chlorhexidine | 2.37±1.14 AB a | 1.20±0.40 AB b | 2.31±1.15 A a | 1.29±0.44 AD b |
| Najo | 2.60±1.45 AB a | 0.80±0.31 A b | 2.65±1.46 A a | 0.93±0.14 AD b |
| Behsa | 1.91±0.25 AB a | 1.74±0.70* B a | 2.26±0.67 A a | 2.34±0.78 B a |
| P-value | 0.007 | <0.001 | 0.09 | <0.001 |
Statistically significant difference between the two time points in each group and composite at significance level of 0.05.
-The similar superscripted uppercase letters in the same column show no significant difference between groups at each time point (P>0.05)
-The similar superscripted lowercase letters in the same row show no significant difference between two composites at each time point (P>0.05)
Fig. 1:
Column chart showing the mean ΔE1–7 values and standard deviation of Filtek Z250 compared to Filtek Z350XT in different solutions
Filtek Z250 in Vi-one mouthrinse showed maximum discoloration (ΔE=3.05±1.49) after seven days, which had a statistically significant difference with the control group (P=0.004) but it did not show significant differences with other groups.
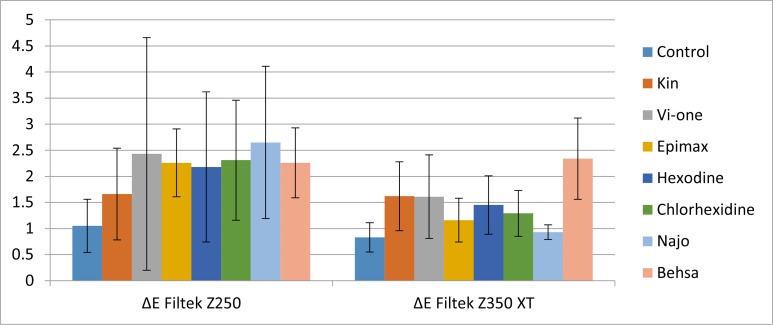
Filtek Z350XT in Behsa mouthrinse showed maximum discoloration (ΔE=1.74±0.7) after seven days, which had a statistically significant difference with the control (P=0.000), Najo (P=0.004) and Epimax (P=0.014) mouthrinses. The ΔE values after 14 days revealed that all groups had acceptable color change (ΔE<3.3; Fig. 2). The mean values and standard deviations of color change (ΔE) of each group of composite resin after 14 days of immersion in chlorhexidine mouthrinses are shown in Table 4.
Fig. 2:
Column chart showing the mean ΔE1–14 values and standard deviation of Filtek Z250 compared to Filtek Z350XT in different solutions
Filtek Z250 in Najo mouthrinse (ΔE=2.65±1.46) and after that in Vi-One (ΔE=2.43±2.23) showed maximum discoloration; although it did not have significant differences with other groups (P>0.05). Filtek Z350 XT in Behsa showed maximum discoloration (ΔE=2.34±0.78), which had significant differences with other groups (P<0.05). The control group showed significant differences with Kin and Vi-One.
Comparison of discoloration between ΔE1–7 and ΔE1–14 is shown in Table 3. The ΔE values in Filtek Z350XT after 14 days in comparison to seven days showed higher discoloration in all groups and the discoloration was significant in all groups except for chlorhexidine and Najo.
The ΔE values in Filtek Z250 after 14 days in comparison to seven days showed higher discoloration in Epimax, Hexodine, Najo and Behsa groups and lower discoloration in other groups, and the discoloration was only significant in the control and Epimax groups.
DISCUSSION
The present study evaluated the effects of seven commercially available chlorhexidine mouthrinses in the Iranian market on color stability of two types of resin-based composites. The results revealed that the color change of composites after seven and 14 days of immersion in mouthrinses was clinically acceptable (ΔE<3.3). With regard to color parameters (L*a*b*), the results showed that ΔL values were negative, which indicated that the specimens became darker in all groups. In a* axis, the specimens’ color shifted towards red in all groups, and in b* axis, the color of microhybrid composite shifted towards yellow and that of nanofilled composite shifted towards blue. In addition, Filtek Z250 (microhybrid) showed less color change than Filtek Z350XT (nanofilled). After the first and second weeks, the color change (ΔE value) of Filtek Z250 with larger filler particles was greater than that of Filtek Z350XT in all groups. Filtek Z250 contains filler particles with an average size of 0.6μm; while Filtek Z350XT contains filler particles ranging from 5 to 20nm [26]. Larger particles create a rough surface while polishing and smaller particles create a smooth surface; thus, composites with larger fillers are more susceptible to discoloration [27].
In addition, composites with larger filler particles are more susceptible to water sorption by the polymer network and the effect of coloring agents on the quality of the bond between the matrix and filler can cause discoloration [28]. Water sorption may cause micro-cracks by expanding and laminating the resin component and hydrolyzing the silane and decrease the functional life of composite resins; thus, micro-crack formation and interfacial gaps between the filler and matrix allow soluble dyes of mouthrinses to penetrate and cause discoloration [29]; these reasons can explain the greater color stability of Filtek Z350XT. Our findings are in accordance with those of Al-Qaisi and Alrahman [30] and Festuccia et al [31].
After seven days of immersion in mouthrinses, Vi-One mouthrinse showed greater discoloration after alcohol-based mouthrinses. Vi-One contains citric acid. Weak acids like citric acid have a softening effect on polymer matrix and decrease the microhardness of composites in addition to alcohol [32]. Kin, Epimax, and Vi-One mouthrinses have citric acid in their composition but in this study, Vi-One showed greater discoloration. The manufacturers of Kin and Epimax claim that these mouthrinses have color preservers in their composition that inhibit discoloration of teeth and composites [33]; therefore, the greater discoloration of Vi-one may be due to the absence of color preserver in its formulation. After 14 days of immersion in mouthrinses, microhybrid composite in Najo with 10% alcohol (ΔE=2.65) and nanofilled composite in Behsa with 11.6% alcohol (ΔE=2.34) showed greater discoloration. Alcohol concentration in mouthrinses varies from 0 to 27%, which is comparable to the alcohol percentage of beer (4%) and wine (12%).
Ethanol is a bipolar molecule, which enhances the dissolution of hydrophobic and hydrophilic components. Alcohol in mouthrinses does not have any other therapeutic effect except as a solvent. Due to this reason, in the clinical setting, alcohol-free mouthrinses have been proven to have the same effect as alcohol-based mouthrinses with fewer side effects [34]. Our findings are similar to those of Villalta et al, [35] Asmussen [36], and Stober et al, [37] who reported that alcohol had a softening effect on composites, so the chromaticity of composites increases in alcohol-based solutions. Comparison of discoloration between ΔE1–7 and ΔE1–14 revealed that Filtek Z350XT had significantly greater discoloration after 14 days in comparison to seven days in all groups except for two (Chlorhexidine and Najo); it means that all specimens showed a significant progressive increase in color change with increasing number of rinses in all chlorhexidine mouthrinses except for two groups, and Filtek Z250 showed a significant discoloration only in two groups (control and Epimax) after 14 days in comparison to seven days; this result may be due to the higher potential of nanofilled composites for discoloration as mentioned above and showed that increasing the frequency and duration of rinses can affect the color stability of nanofilled composites more than microhybrid composites. In this study, despite the low pH of Epimax (pH=4.3), it did not show greater discoloration than other groups. We did not find a correlation between pH and discoloration of composites, which is in accord with the findings of Diab et al, [38] who reported that the pH of test solutions did not have an effect on discoloration of restorative materials.
In clinical situations, different factors may affect the color stability of restorative materials such as the presence of saliva, salivary pellicle, and the effect of different foods and beverages, which are difficult to simulate in an in-vitro setting [39]. This result may be due to the absence of these chromogenic materials, which could intensify the chromogenic potential of chlorhexidine mouthwashes [40]. Therefore, further experiments are needed to better simulate the clinical conditions.
CONCLUSION
The results of the present study indicated that composites showed an acceptable color change after two weeks of immersion in chlorhexidine mouthrinses. Mouthrinses, which contained alcohol and citric acid showed greater discoloration; thus, it is better to limit their prescription. Nanofilled composite showed better color stability than microhybrid composite.
REFERENCES
- 1-.Catelan A, Briso AL, Sundfeld RH, Dos Santos PH. Effect of artificial aging on the roughness and microhardness of sealed composites. J Esthet Restor Dent. 2010. October;22(5):324–30. [DOI] [PubMed] [Google Scholar]
- 2-.Dietschi D, Campanile G, Holz J, Meyer JM. Comparison of the color stability of ten new-generation composites: an in vitro study. Dent Mater. 1994. November;10(6):353–62. [DOI] [PubMed] [Google Scholar]
- 3-.Furuse AY, Gordon K, Rodrigues FP, Silikas N, Watts DC. Colour-stability and gloss-retention of silorane and dimethacrylate composites with accelerated aging. J Dent. 2008. November;36(11):945–52. [DOI] [PubMed] [Google Scholar]
- 4-.Mundim FM, Garcia Lda F, Cruvinel DR, Lima FA, Bachmann L, Pires-de-Souza Fde C. Color stability, opacity and degree of conversion of pre-heated composites. J Dent. 2011. July;39 Suppl 1:e25–9. [DOI] [PubMed] [Google Scholar]
- 5-.Lee YK, Powers JM. Combined effects of staining substances on resin composites before and after surface sealant application. J Mater Sci Mater Med. 2007. May;18(5):685–91. [DOI] [PubMed] [Google Scholar]
- 6-.Wennström JL, Heijl L, Dahlén G, Gröndahl K. Periodic subgingival antimicrobial irrigation of periodontal pockets (I). Clinical observations. J clin periodontol. 1987. October;14(9):541–50. [DOI] [PubMed] [Google Scholar]
- 7-.Walker MP, Ries D, Kula K, Ellis M, Fricke B. Mechanical properties and surface characterization of beta titanium and stainless steel orthodontic wire following topical fluoride treatment. Angle Orthod. 2007. March;77(2):342–8. [DOI] [PubMed] [Google Scholar]
- 8-.Aulitto L, Ramaglia L, Ciaglia RN, Sbordone L. The use of antiseptic agents for the chemical control of dental plaque. Minerva Stomatol. 1991. Jan-Feb;40(1–2):29–35. [PubMed] [Google Scholar]
- 9-.Bagis B, Baltacioglu E, Oezcan M, Ustaomer S. Evaluation of chlorhexidine gluconate mouthrinse-induced staining using a digital colorimeter: an in vivo study. Quintessence Int. 2011. March;42(3):213–23. [PubMed] [Google Scholar]
- 10-.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988. September;15(8):488–98. [DOI] [PubMed] [Google Scholar]
- 11-.Watts A, Addy M. Tooth discolouration and staining: A review of the literature. Br Dent J. 2001. March 24;190(6):309–16. [DOI] [PubMed] [Google Scholar]
- 12-.Lamba B, Lamba A, Ponnappa KC. Effect of mouth rinses on the color of three tooth-colored restorative materials. Int J Stomatol occlusion Med. 2012. September;5(3):104–9. [Google Scholar]
- 13-.Celik C, Yuzugullu B, Erkut S, Yamanel K. Effects of mouth rinses on color stability of resin composites. Eur J Dent. 2008. October;2(4):247–53. [PMC free article] [PubMed] [Google Scholar]
- 14-.Ure D, Harris J. Nanotechnology in dentistry: reduction to practice. Dent Update. 2003. Jan-Feb;30(1):10–5. [DOI] [PubMed] [Google Scholar]
- 15-.Al-Shalan TA. In vitro staining of nanocomposites exposed to a cola beverage. PODJ. 2009. June;29(1):79–84. [Google Scholar]
- 16-.Rong MZ, Zhang MQ, Pan SL, Friedrich K. Interfacial effects in polypropylene–silica nanocomposites. J Appl Polym Sci. 2004. March;92(3):1771–81. [Google Scholar]
- 17-.O'Brien AJ. The therapeutic relationship: historical development and contemporary significance. J Psychiatr Ment Health Nurs. April 2001;8(2):129–37. [DOI] [PubMed] [Google Scholar]
- 18-.Barutcigil Ç, Yıldız M. Intrinsic and extrinsic discoloration of dimethacrylate and silorane based composites. J Dent. 2012. July;40 Suppl 1:e57–63. [DOI] [PubMed] [Google Scholar]
- 19-.Okubo SR, Kanawati A, Richards MW, Childressd S. Evaluation of visual and instrument shade matching. J Prosthet Dent. 1998. December;80(6):642–8. [DOI] [PubMed] [Google Scholar]
- 20-.Lee SH, Lee YK. Effect of thermocycling on optical parameters of resin composites by the brand and shade. Am J Dent. 2008. December;21(6):361–7. [PubMed] [Google Scholar]
- 21-.Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991. May;22(5):377–86. [PubMed] [Google Scholar]
- 22-.Doray PG1, Wang X, Powers JM, Burgess JO. Accelerated aging affects color stability of provisional restorative materials. J Prosthodont. 1997. September;6(3):183–8. [DOI] [PubMed] [Google Scholar]
- 23-.Carpenter GH1, Pramanik R, Proctor GB. An in vitro model of chlorhexidine-induced tooth staining. J Periodontal Res. 2005. June;40(3):225–30. [DOI] [PubMed] [Google Scholar]
- 24-.Awliya WY, Al-Alwani DJ, Gashmer ES, Al-Mandil HB. The effect of commonly used types of coffee on surface microhardness and color stability of resin-based composite restorations. Saudi Dent J 2010. October;22(4):177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25-.Malek Afzali B, Ghasemi A, Mirani A, Abdolazimi Z, Akbarzade Baghban A, Kharazifard MJ. Effect of Ingested Liquids on Color Change of Composite Resins. J Dent (Tehran). 2015. August;12(8):577–84. [PMC free article] [PubMed] [Google Scholar]
- 26-.Vichi A, Ferrari M, Davidson CL. Color and opacity variations in three different resin-based composite products after water aging. Dent Mater. 2004. July;20(6):530–4. [DOI] [PubMed] [Google Scholar]
- 27-.Bagheri R, Burrow MF, Tyas M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J Dent. 2005. May;33(5):389–98. [DOI] [PubMed] [Google Scholar]
- 28-.Manabe A1, Kato Y, Finger WJ, Kanehira M, Komatsu M. Discoloration of coating resins exposed to staining solutions in vitro. Dent Mater J. 2009. May;28(3):338–43. [DOI] [PubMed] [Google Scholar]
- 29-.von Fraunhofer JA, Kelley JI, DePaola LG, Meiller TF. The effect of a mouth rinse containing essential oils on dental restorative materials. Gen Dent. 2006. Nov-Dec;54(6):403–7. [PubMed] [Google Scholar]
- 30-.Al-Qaisi SD, Alrahman MS. Evaluating the Effect of One Alcoholic and Two Alcoholic-free Mouthwashes on the Color Stability and Surface Roughness of Two Resinbased composites (In vitro Comparative Study). IJSR. 2015. October;4(10):254–7. [Google Scholar]
- 31-.Festuccia MS, Garcia Lda F, Cruvinel DR, Pires-De-Souza Fde C. Color stability, surface roughness and microhardness of composites submitted to mouthrinsing action. J Appl Oral Sci. 2012. Mar-Apr;20(2):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32-.Yap AU, Tan BW, Tay LC, Chang KM, Loy TK, Mok BY. Effect of mouthrinses on microhardness and wear of composite and compomer restoratives. Oper Dent. 2003. Nov-Dec;28(6):740–6. [PubMed] [Google Scholar]
- 33-.Jmali A. Academic Center for Community Oral Health Products Ghazvin: Mouth and dental center; 2013. [updated 2013 Aug 10; cited 2015 Jan 10]. Available from: http://dental.roomfa.com/.
- 34-.Bahna P, Hanna HA, Dvorak T, Vaporciyan A, Chambers M, Raad I. Antiseptic effect of a novel alcohol-free mouthwash: a convenient prophylactic alternative for high-risk patients. Oral Oncol. 2007. February;43(2):159–64. [DOI] [PubMed] [Google Scholar]
- 35-.Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006. February;95(2):137–42. [DOI] [PubMed] [Google Scholar]
- 36-.Asmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res. 1984. June;92(3):257–61. [DOI] [PubMed] [Google Scholar]
- 37-.Stober T, Gilde H, Lenz P. Color stability of highly filled composite resin materials for facings. Dent Mater. 2001. January;17(1):87–94. [DOI] [PubMed] [Google Scholar]
- 38-.Diab M, Zaazou MH, Mubarak EH, Olaa MI. Effect of five commercial mouthrinses on the microhardness and color stability of two resin composite restorative materials. Aust J Basic Appl Sci. 2007;1(4):667–74. [Google Scholar]
- 39-.Lee SY, Huang HM, Lin CY, Shih YH. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J Oral Rehabil. 1998. August;25(8):575–88. [DOI] [PubMed] [Google Scholar]
- 40-.Moran JM. Home-use oral hygiene products: mouthrinses. Periodontol 2000. 2008;48:42–53. [DOI] [PubMed] [Google Scholar]