Abstract
Information about the association between cognitive functions, such as copying function, and sleep disturbances in patients with chronic obstructive pulmonary disease (COPD) is lacking. This cross-sectional observational study aimed to investigate the association between copying function and self-reported sleep quality disturbances and disease severity in an elderly COPD population.
Cognitive function performances, assessed using the Mini-Mental State Examination, were compared in 562 ambulatory COPD patients with and without sleep disturbances; assessed using the Established Populations for Epidemiologic Studies of the Elderly questionnaire; and stratified by Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades.
Sleep disturbances overall were not correlated with cognitive functioning. A trend was revealed towards worse design copying in patients with sleep disturbances overall. GOLD I patients with difficulties falling asleep and nocturnal awakenings had worse copying ability compared to GOLD I patients without these sleep disturbances. Copying ability was worse for GOLD III than GOLD I, orientation was worse for GOLD II than GOLD I and language was worse for GOLD II and III than GOLD I.
To conclude, sleep disturbances seem to be a weak correlate of cognitive functioning, and are not a marker of disease severity.
Short abstract
Sleep disturbances are a weak correlate of cognitive functioning in COPD http://ow.ly/gUhD301PvcQ
Introduction
In the general population, sleep deprivation has been demonstrated to negatively impact cognitive functions such as attention and working memory [1]. Both sleep disturbances [2] and cognitive impairment [3] are frequently found in patients with chronic obstructive pulmonary disease (COPD). However, knowledge concerning the relationship between sleep and cognition in COPD is scarce.
Bellia et al. [2] did not find a relationship between sleep disturbances and cognitive impairment in an elderly ambulatory mixed asthma–COPD population. Nevertheless, asthma patients accounted for ∼40% of the sample, and it is well known that cognitive impairment is rare in patients with asthma [4]. In contrast, Omachi et al. [5] found a relationship between verbal memory and sleep disturbances in patients with COPD. However, confounders potentially affecting cognitive functioning such as comorbid diseases, sensory impairment and physical functioning were not assessed.
In view of these conflicting results, we hypothesise that further insight into the sleep–cognition relationship in COPD might be obtained by focusing on a specific cognitive function, namely copying ability. The rationale for this hypothesis lies in the observation that the insula is involved in the neural circuits underlying the copying function, and limbic areas such as the amygdaloid complexes, hippocampal formation and anterior cingulate cortex are involved in sleep regulation [6]. Even if assessed in the context of a simple screening test such as the Mini-Mental State Examination (MMSE), copying ability has been proved to identify COPD patients at risk of improper use of inhalers [7], and to predict mortality in COPD [8]. Furthermore, it has been proved to have important classificatory and prognostic implications in the broader elderly population [8]. Thus, it carries information worthy of interest because of its practical application.
The present study purposed to verify whether and to what extent copying function and the remaining components of MMSE are correlated with self-reported sleep disturbances and with COPD severity in an elderly COPD population.
Methods
Study design
This observational follow-up study used data from the Salute Respiratoria nell'Anziano (Respiratory Health in the Elderly) study (SaRA), a multicentre study of various aspects of clinical and functional conditions and prognostic implications in older home-dwelling subjects with chronic respiratory and nonrespiratory conditions. A detailed description of recruitment criteria, studied population and diagnostic procedures is available elsewhere [9].
Study population
The SaRA study population consisted of 1970 participants aged ≥65 years, recruited in 24 geriatric institutions throughout Italy. For this study all patients with COPD were selected from all individuals included in the SaRA study. Patients were considered to have COPD if they had a pre-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio of <70% predicted and if there was no evidence of asthma. Patients gave their written consent to participate in the study. The SaRA study was approved by the ethical committee of the coordinating centre (#276/2012; University of Palermo, Palermo, Italy).
Outcome measures
The following outcomes were recorded: sociodemographic characteristics (age, sex, years of education); smoking habits (smoking status, pack-years); disease-specific health status (St George's Respiratory Questionnaire) [10]; physical functioning (a single 6-min walk test [11] and the Barthel index [12]); mood status (15-item version of the Geriatric Depression Scale (GDS) [13]); pre-bronchodilator spirometry (FVC, FVC % pred, FEV1, FEV1 % pred and FEV1/FVC); nocturnal symptoms and early morning symptoms using selected items of the International Union against Tuberculosis and Lung Disease (IUATLD) Bronchial Symptoms Questionnaire [14]; and comorbid diseases (International Classification of Diseases (ICD)-9) [15].
Sleep quality disturbances were evaluated by the index of disturbed sleep from the Established Populations for Epidemiologic Studies of the Elderly questionnaire (EPESE) [16] and defined as report of 0=never, 1=rarely (3–4 days per month), 2=sometimes (1–2 days per week), 3=often (≥3 days per week), 4=always, in response to the following questions: 1) trouble falling asleep, 2) frequent awakenings at night, 3) waking up too early in the morning and not being able to fall asleep again and 4) not feeling rested in the morning. A total score ranging from 0 (completely undisturbed sleep) to 16 points (most severely disturbed sleep) was then calculated. Scores on the EPESE domains were dichotomised with a threshold value for each item of 2 [2].
Cognitive functioning was assessed using the MMSE [17], which explores global cognitive functioning (total score 0–30 points) and five specific cognitive functions: orientation (0–10 points), memory (0–6 points), attention and calculation (0–5 points), language (0–8 points) and design copying (0–1 point). In order to be able to compare mean scores per cognitive function, a percentage correct score per MMSE cognitive function was calculated by dividing the patient's cognitive function score with the total possible points for the items that comprise a specific cognitive function. For dichotomous items, the percentage of patients who failed was calculated. Individual performances on the MMSE total scores were adjusted for age and level of education according to a random sample of 906 persons from the Italian population (online supplementary table S2) [18].
Statistics
Statistical analysis was performed using the SPSS statistical software package (version 21.0; SPSS Inc., Chicago, IL, USA). Probability values of p<0.05 were considered to be statistically significant. In descriptive analyses, data are presented as mean±sd or number of data items and percentage of the total number of items. Using Chi-squared tests for categorical variables and ANOVA or Kruskal–Wallis tests as appropriate for continuous variables, patient characteristics were compared between COPD patients across GOLD grades (GOLD I: FEV1 ≥80%; GOLD II: FEV1 ≥50% and <80%; GOLD III: FEV1 ≥30% and <50%; GOLD IV: FEV1 <30%) [19]. If p<0.05, pairwise comparisons were conducted. Chi-squared test or Pearson's r was used to establish whether there was a relationship between the presence of nocturnal symptoms and sleep disturbances and between sleep disturbances and cognitive functioning. An overall outcome for EPESE was defined by dichotomising the total score on the basis of a threshold value of 8 points [2]. MMSE total score was dichotomised as follows: scores ≤24 points indicate cognitive impairment and scores >24 points indicate no cognitive impairment [17]. Bar charts were used to summarise and present cognitive performance after stratification for GOLD grade or specific sleep disturbances. Cognitive functions were compared between patients with and without specific sleep disturbances, using independent sample t-tests or the Mann–Whitney U-test. To assess the degree to which the effect of sleep disturbances on cognitive functioning changes across GOLD grades, linear regression analysis was used with an interaction term between sleep disturbances and GOLD grade.
Results
General characteristics of participants
In total, 562 ambulatory patients (77% male) with COPD were included. Of these, 194 (34.5%) patients were classified as GOLD grade I, 232 (41.3%) patients as GOLD grade II, 103 (18.3%) patients as GOLD grade III and 33 (5.9%) patients as GOLD grade IV. Across GOLD grades, patients differed in age, sex, lung function, smoking habits, body mass index, depressive symptoms, disease-specific health status, physical functioning and comorbid diseases including myocardial infarction and congestive heart failure. (table 1). Sleep disturbances did not differ across GOLD grades (table 2). Significant associations were found between nocturnal symptoms and sleep disturbances, but not between early morning symptoms and sleep disturbances in the total COPD group. After stratification by GOLD grade, nocturnal symptoms were correlated with sleep disturbances only in GOLD grades I and III (online supplementary table S1).
TABLE 1.
Patient characteristics of the study population
| Total COPD group | GOLD I | GOLD II | GOLD III | GOLD IV | p-value# | |
| Subjects n | 562 | 194 | 232 | 103 | 33 | |
| Sociodemographic characteristics | ||||||
| Age years | 73.9±5.9 | 74.4±5.9¶ | 74.5± 6.2¶ | 71.9±5.0 | 72.9±5.8 | 0.001 |
| Male | 433 (77.0) | 143 (73.7)¶ | 172 (74.1)¶ | 89 (86.4) | 29 (87.9) | 0.022 |
| Education years | 6.6±5.1 | 7.2±4.0 | 6.1±3.9 | 6.0±3.9 | 7.8±14.3 | 0.056 |
| Spirometry | ||||||
| FEV1/FVC | 57.2±11.5 | 65.1±4.1¶,+,§ | 59.2±7.6¶,§ | 44.6±10.3§ | 36.5±10.8 | <0.001 |
| FEV1 % pred | 69.1±24.7 | 95.6±12.8¶,+,§ | 65.9±8.9¶,§ | 40.0±60.0§ | 25.7±2.9 | <0.001 |
| Smoking habits | ||||||
| Smokers | 97 (17.3) | 27 (13.9) | 42 (18.1) | 22 (21.4) | 6 (18.2) | 0.005 |
| Former smokers | 341 (60.7) | 112 (57.7) | 134 (57.8) | 72 (69.9) | 23 (69.7) | |
| Never-smokers | 124 (22.1) | 55 (28.4) | 56 (24.1) | 9 (8.7) | 4 (12.2) | |
| Pack-years | 45.8±33.8 | 34.8±25.9¶,+ | 50.1±35.9 | 52.7±1.8 | 50.4±35.7 | <0.001 |
| Clinical characteristics | ||||||
| BMI kg·m−2 | 25.8±3.9 | 26.1±3.5§ | 26.1±4.0§ | 25.2±4.5 | 24.3±3.3 | 0.023 |
| GDS points | 3.5±3.2 | 2.8±3.0¶,+,§ | 3.7±3.2 | 4.1±3.4 | 5.0±3.4 | <0.001 |
| GDS >5 points | 144 (25.6) | 34 (17.5)¶,+,§ | 63 (27.2) | 35 (34.0) | 12 (36.4) | 0.005 |
| Visual impairment | 51 (9.1) | 16 (8.2) | 28 (12.1) | 4 (3.9) | 3 (9.1) | 0.109 |
| Hearing impairment | 42 (7.5) | 12 (6.2) | 2 (9.5) | 8 (7.8) | 0 (0.0) | 0.212 |
| Disease-specific health status | ||||||
| SGRQ symptom score | 47.2±24.8 | 30.0± 21.9¶,+,§ | 43.6±23.0¶,§ | 61.9±19.6 | 62.4±20.1 | <0.001 |
| SGRQ activity score | 48.4±27.5 | 28.5±24.5¶,+,§ | 45.3±26.3¶,§ | 61.2±20.4 | 73.3±17.0 | <0.001 |
| SGRQ impact score | 26.3±22.4 | 13.4±16.8¶,+,§ | 21.7±19.8¶ | 37.0±19.5§ | 49.7±24.3 | <0.001 |
| SGRQ total score | 37.1±22.1 | 21.2±18.1¶,+,§ | 33.1±19.8¶,§ | 49.2±17.3 | 59.0±18.5 | <0.001 |
| Physical functioning | ||||||
| 6MWT m | 317.4±120.2 | 341.0±123.8§ | 322.0±111.1¶,§ | 280.2±124.1 | 255.6±100.6 | <0.001 |
| 6MWT % pred | 24.6±35.7 | 23.3±36.8 | 24.4±36.7 | 26.1±33.3 | 29.6± 30.4 | 0.797 |
| Barthel index | 93.1±9.1 | 94.7±8.9§ | 93.1±9.3§ | 91.9±7.8 | 87.7±10.2 | <0.001 |
| Barthel index <80 | 40 (7.1) | 7 (3.6)§ | 16 (6.9)§ | 8 (7.8)§ | 9 (27.3) | <0.001 |
| Comorbid diseases | ||||||
| Myocardial infarction | 48 (8.5) | 66 (5.7)¶ | 31 (13.4)¶ | 4 (3.9) | 4 (12.1) | 0.003 |
| Congestive heart failure | 47 (8.4) | 10 (5.2)¶ | 30 (12.9)¶ | 3 (2.9) | 4 (12.1) | 0.004 |
| Peripheral vascular disease | 34 (6.0) | 14 (7.2) | 16 (6.9) | 4 (3.9) | 0 (0.0) | 0.292 |
| Cerebrovascular disease or ischaemic stroke | 22 (3.9) | 7 (3.6) | 11 (4.7) | 4 (3.9) | 0 (0.0) | 0.612 |
| Diabetes mellitus | 64 (11.4) | 18 (9.3) | 31 (13.4) | 9 (8.7) | 6 (18.2) | 0.264 |
| Solid or malignant tumours | 30 (5.3) | 10 (5.2) | 17 (7.3) | 2 (1.9) | 1 (3.0) | 0.210 |
| Parkinson's disease | 9 (1.6) | 3 (1.5) | 4 (1.7) | 2 (1.9) | 0 (0.0) | 0.888 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. Missing variables were present for pack-years (24.2%) and St George's Respiratory Questionnaire (SGRQ) (34.5%). COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; BMI: body mass index; GDS: Geriatric Depression Scale; 6MWT: 6-min walk test. #: comparisons between patients with COPD in GOLD grades I–IV; ¶: p<0.05 versus GOLD III; +: p<0.05 versus GOLD II; §: p<0.05 versus GOLD IV.
TABLE 2.
Nocturnal symptoms, early morning symptoms, sleep disturbances and cognitive functioning in the study population
| COPD | GOLD I | GOLD II | GOLD III | GOLD IV | p-value# | |
| Subjects n | 562 | 194 | 232 | 103 | 33 | |
| Nocturnal symptoms | ||||||
| IUATLD shortness of breath | 100 (17.8) | 10 (5.2)¶,+,§ | 39 (16.8)+,§ | 31 (30.1)§ | 20 (60.6) | <0.001 |
| IUATLD coughing | 203 (36.1) | 39 (20.1)¶,+,§ | 80 (34.5)+,§ | 60 (58.3) | 24 (72.7) | <0.001 |
| Early morning symptoms | ||||||
| Coughing | 232 (41.3) | 35 (18.0)¶,+,§ | 91 (39.2)+,§ | 76 (73.8)§ | 30 (90.9) | <0.001 |
| Expectoration | 264 (47.0) | 37 (19.1)¶,+,§ | 112 (48.3)+,§ | 88 (85.4) | 27 (81.8) | <0.001 |
| Sleep disturbances | ||||||
| EPESE points | 4.2±4.4 | 4.5±4.5 | 4.1±4.4 | 3.7±4.1 | 5.1±4.6 | 0.257 |
| EPESE ≥8 | 175 (31.1) | 64 (33.0) | 70 (30.2) | 29 (28.2) | 12 (36.4) | 0.739 |
| EPESE difficulty falling asleep | 97 (17.3) | 38 (19.6) | 31 (13.4) | 23 (22.3) | 5 (15.2) | 0.160 |
| EPESE nocturnal awakenings | 171 (30.4) | 64 (33.0) | 68 (29.3) | 31 (30.1) | 8 (24.2) | 0.720 |
| EPESE morning tiredness | 38 (6.8) | 29 (14.9) | 38 (16.4) | 19 (18.4) | 8 (24.2) | 0.569 |
| EPESE early awakenings | 94 (16.7) | 14 (7.2) | 213 (5.6) | 9 (8.7) | 2 (6.1) | 0.748 |
| Cognitive functioning | ||||||
| Age and education adjusted MMSE | 28.4±3.7 | 28.9±2.9 | 28.4±4.0 | 27.7±4.6 | 28.6±3.1 | 0.064 |
| MMSE ≤24 | 142 (25.3) | 39 (20.1) | 68 (29.3) | 24 (23.3) | 11 (33.3) | 0.107 |
| MMSE orientation percentage correct | 0.93±0.1 | 0.96±0.09¶ | 0.92±0.16 | 0.92±0.15 | 0.93±0.14 | 0.014 |
| MMSE memory percentage correct | 0.86±0.2 | 0.87±0.17 | 0.86±0.18 | 0.83±0.20 | 0.85±0.18 | 0.228 |
| MMSE attention and calculation percentage correct | 0.76±0.3 | 0.78±0.31 | 0.75±0.34 | 0.72±0.36 | 0.79±0.30 | 0.462 |
| MMSE language percentage correct | 0.91±0.1 | 0.95±0.10¶,+ | 0.91±0.14 | 0.88±0.19 | 0.91±0.12 | 0.001 |
| MMSE design copying failed | 178 (33.3) | 48 (25.3)+ | 76 (34.5) | 41 (43.6) | 13 (43.3) | 0.001 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; IUATLD: International Union against Tuberculosis and Lung Disease Bronchial Symptoms questionnaire; EPESE: Established Populations for Epidemiologic Studies of the Elderly questionnaire; MMSE: Mini-Mental State Examination. #: comparisons between patients with COPD GOLD grade I–IV; ¶: p<0.05 versus GOLD II; +: p<0.05 versus GOLD III; §: p<0.05 versus GOLD IV.
Relationship between cognitive functioning and sleep disturbances
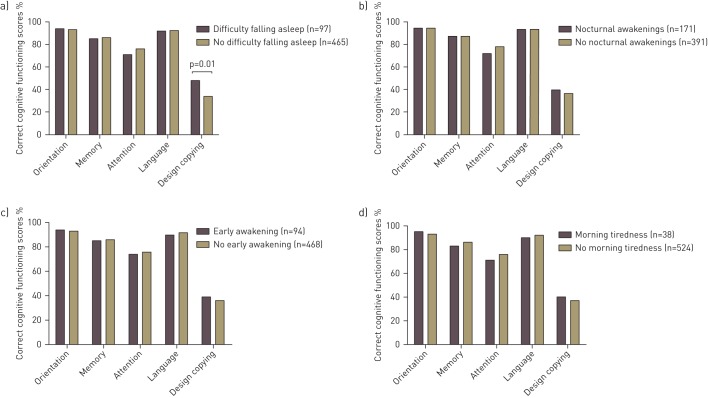
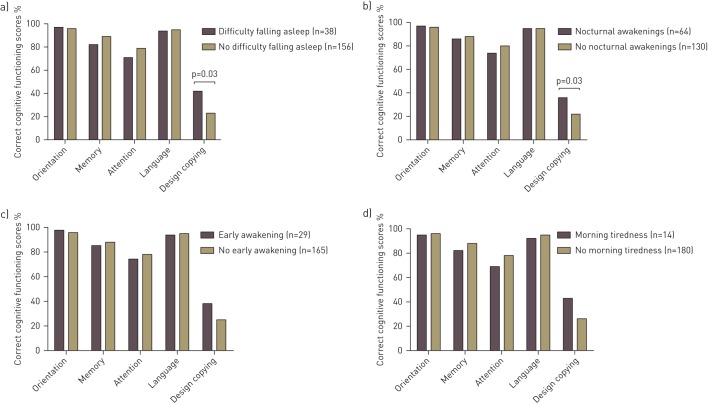
Copying ability and language were worse for GOLD III than GOLD I. Orientation and language were worse for GOLD II than GOLD I. However, the prevalence of global cognitive impairment and the adjusted MMSE score did not differ across GOLD grades (table 2). Sleep disturbances overall did not correlate with global cognitive functioning in the total COPD group (r=0.036, p=0.392), and this relationship was not influenced by COPD severity (interaction term GOLD grade×sleep disturbances in a linear regression model β=−0.257, p=0.797). Copying ability was comparable between patients with and without sleep disturbances overall in the total COPD group and across GOLD grades, except that a higher percentage of patients in GOLD I with sleep disturbances failed on copying function compared to those without sleep disturbances (41.5% versus 20.8%, p=0.008) (fig. 1). In the total COPD group, patients with difficulties falling asleep more often failed on the design copying compared to patients without difficulties falling asleep (fig. 2). Patients in GOLD I with difficulties falling asleep and nocturnal awakenings had worse copying ability than those without these specific sleep disturbances (fig. 3). For the remaining cognitive functions, percentage correct scores did not differ between patients with and without specific sleep disturbances (online supplementary figs S1–S3).
FIGURE 1.
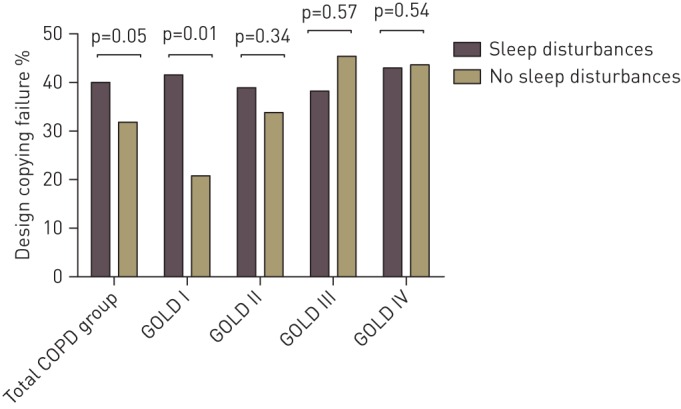
Design copying failure (percentage of patients) in the total chronic obstructive pulmonary disease (COPD) group with and without sleep disturbances and after stratification for Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade.
FIGURE 2.
Percentage correct cognitive functioning scores per cognitive function in patients with chronic obstructive pulmonary disease with and without a) difficulty falling asleep, b) nocturnal awakening, c) early awakening and d) morning tiredness. For the copying ability function, percentages of patient who failed are depicted. p>0.05, unless otherwise indicated.
FIGURE 3.
Percentage correct cognitive functioning scores per cognitive function in patients with chronic obstructive pulmonary disease Global Initiative for Chronic Obstructive Lung Disease grade I with and without a) difficulty falling asleep, b) nocturnal awakening, c) early awakening and d) morning tiredness. For the copying ability function, percentages of patient who failed are depicted. p>0.05, unless otherwise indicated.
Discussion
Key findings
The present study found that global cognitive functioning was not associated with self-reported sleep quality disturbances overall in an elderly COPD population. However, a trend was observed towards worse copying function in patients with sleep disturbances in the total group and in GOLD grade I. Moreover, higher level cognitive functions as reflected by the copying function, orientation and language decreased to some extent with increasing disease severity.
Cognitive functioning and sleep disturbances
Sleep disturbances were equally distributed across GOLD stages, and our a priori hypothesis that copying ability is associated with sleep disturbances in patients with COPD was confirmed for patients in GOLD I: patients with sleep disturbances overall had worse performance compared to those without sleep disturbances. A trend towards worse copying ability in the total COPD group with sleep disturbances overall compared to those without was also observed, suggesting that these patients are more prone to impairments in executive and visuospatial functions. In addition, GOLD I patients with difficulties falling asleep and nocturnal awakenings had worse copying ability. The fact that we disclosed an association between copying ability and sleep disturbances only in mild COPD suggests that factors different from those contributing to GOLD grading account for impairments of sleep and cognition. Indeed, MMSE and EPESE total scores were not correlated in the total COPD group.
Cognitive functioning and disease severity
A link between COPD severity and copying function, orientation and language was disclosed, but not on the remaining components of the MMSE. This information is important since higher level cognitive functions may have adverse effects on therapy [20]. Some large-scale studies did find a significant, albeit weak, association between cognitive functioning and lung function [21, 22]. It is possible that this relationship becomes evident after the onset of hypoxaemia or consequent hypoxia [23]. Hypoxaemia increases with disease severity, with >80% of patients with advanced disease using some form of oxygen therapy [24]. Since we included ambulatory patients with mostly mild-to-moderate COPD, hypoxia might not be highly prevalent in this study population. Yet, depression predates the onset of cognitive impairment [25], and depressive symptoms, measured using the GDS, were increased in GOLD III and IV compared to GOLD I and II. In addition, disease-specific health status, which has been shown to be associated with depressive symptoms [26], was worse for GOLD II–IV compared to patients in GOLD I. Moreover, a higher prevalence of myocardial infarction and congestive heart failure was found in GOLD II patients. Even though these comorbidities, as well as health status, are known to have a close association with both cognitive impairment and sleep disturbances [27], the prevalence of global cognitive impairment and sleep disturbances was comparable across all GOLD grades.
Until now, apart from the MMSE total score, no norm scores are available for specific MMSE cognitive function scores. Moreover, the copy function item of the MMSE is a pass or fail item and the clinical significance of its findings have to be further investigated. Nevertheless, the finding of a decrease in copying ability from GOLD I to III seems biologically plausible, because defective design copying qualified as a marker of COPD severity and a prognostic marker [8, 28]. Moreover, in the general population, impairments in copying designs have been found to identify drivers more likely to cause a car accident or to adapt their driving by avoiding peak times and keeping to familiar areas [29, 30]. This is not surprising, since the the design copying function reflects both executive and visuospatial functions [8].
Nocturnal symptoms and sleep disturbances are suggested to be the effects of COPD-related symptoms [31], and recent literature shows that nocturnal symptoms have a distinctive distribution with regard to COPD severity [32]. In line with this study, we found that early morning and nocturnal symptoms, but not sleep disturbances, were associated with COPD severity. Therefore, sleep disturbances might reflect a common symptom of the disease which cannot be entirely captured by COPD severity. Indeed, sleep disturbances are reported frequently in this population and might influence cognitive functioning.
Methodological considerations
A strength of the study is that data from individual centres were collected prospectively by a coordinating centre and the study population thus reflects an unselected cohort of COPD patients. However, this study was cross-sectional in design, and therefore is limited to draw valid conclusions as to causality and the strength of the association between COPD severity, sleep disturbances and cognitive functioning. Another limitation is that no post-bronchodilator spirometry was included, which is a prerequisite for the definition of airflow limitation that is not fully reversible. Additionally, no data were available regarding medication use. Not only may the use of pulmonary medication (e.g. (oral) corticosteroids, β2-agonists, anticholinergics and theophylline) and nonpulmonary medication (e.g. anticonvulsants, analgesics, antidepressants, β-adrenergic blockers, diuretics and thyroid preparations) may promote sleep disturbances, but so may the discontinuation of these medications [33]. Moreover, no data of elderly patients without COPD were included and the prevalence of sleep disturbance and cognitive impairment in normal elderly patients should be investigated. Administration of the MMSE was not only performed in a patient subset selected for a specific intervention, but as part of routine clinical work-up. Indication bias therefore can be excluded. Yet, elderly ambulatory patients from geriatric institutions with only a small minority of COPD patients in GOLD IV who were not likely to have hypoxia were included, so conclusions should be generalised with some caution. Moreover, we have no information about obstructive sleep apnoea syndrome, which may be a confounder in the association between sleep disturbances and cognitive deficits [34]. Furthermore, cognitive assessment was based upon a screening instrument, which is less sensitive than a detailed neuropsychological testing battery. Finally, the IUATLD questionnaire does not provide grading of nocturnal symptoms, and qualitative characteristics of sleep disturbances were studied. Objective polysomnographic data should confirm our findings.
Conclusions
Sleep disturbances seem to be a weak correlate of cognitive performance in patients with COPD; nor are they a marker of severity of COPD. Longitudinal studies including hypoxaemic patients and using a detailed neuropsychological testing battery in combination with polysomnography are needed to confirm our results.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Support statement: This study was supported by a European Respiratory Society Fellowship and Boehringer Ingelheim, Italy. Funding information for this article has been deposited with the Open Funder Registry.
Conflict of interest: None declared.
References
- 1.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat 2007; 3: 553–567. [PMC free article] [PubMed] [Google Scholar]
- 2.Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep 2003; 26: 318–323. [DOI] [PubMed] [Google Scholar]
- 3.Cleutjens FA, Janssen DJ, Ponds RW, et al. Cognitive-pulmonary disease. Biomed Res Int 2014; 2014: 697825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray M, Sano M, Wisnivesky JP, et al. Asthma control and cognitive function in a cohort of elderly adults. J Am Geriatr Soc 2015; 63: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med 2012; 13: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 2000; 9: 207–231. [DOI] [PubMed] [Google Scholar]
- 7.Board M, Allen SC. A simple drawing test to identify patients who are unlikely to be able to learn to use an inhaler. Int J Clin Pract 2006; 60: 510–513. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli-Incalzi R, Corsonello A, Pedone C, et al. Drawing impairment predicts mortality in severe COPD. Chest 2006; 130: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 9.Bellia V, Pistelli R, Catalano F, et al. Quality control of spirometry in the elderly. The SA.R.A. study. SAlute Respiration nell'Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med 2000; 161: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 10.Meguro M, Barley EA, Spencer S, et al. Development and validation of an improved, COPD-specific version of the St. George Respiratory questionnaire. Chest 2007; 132: 456–463. [DOI] [PubMed] [Google Scholar]
- 11.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 13.Sheikh VI, Yesavage VA. The short form of the Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: a Guide to Assessment and Intervention. New York, Haworth Press, 1986; pp. 165–174. [Google Scholar]
- 14.Burney PG, Laitinen LA, Perdrizet S, et al. Validity and repeatability of the IUATLD (1984) Bronchial Symptoms Questionnaire: an international comparison. Eur Respir J 1989; 2: 940–945. [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 16.Cornoni H, Brock D, Ostfeld A. Established Populations for the Epidemiologic Study of the Elderly Resource Data Book. Bethesda, National Institutes of Health. 1986; 86–2441. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 18.Measso G, Cavarzeran F, Zappalà G, et al. The mini-mental state examination: normative study of an Italian random sample. Dev Neuropsychol 1993; 9: 77–85. [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Pocket Guide to COPD Diagnosis, Management, and Prevention. GOLD, 2015. [Google Scholar]
- 20.Becker BW, Thames AD, Woo E, et al. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav 2011; 15: 1888–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleutjens FA, Spruit MA, Ponds RW, et al. Cognitive functioning in obstructive lung disease: results from the United Kingdom biobank. J Am Med Dir Assoc 2014; 15: 214–219. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord 2006; 21: 300–308. [DOI] [PubMed] [Google Scholar]
- 23.Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 2010; 5: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelicic M, Bosma H, Ponds RW, et al. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS). Int J Geriatr Psychiatry 2002; 17: 73–77. [DOI] [PubMed] [Google Scholar]
- 26.Incalzi RA, Bellia V, Catalano F, et al. Evaluation of health outcomes in elderly patients with asthma and COPD using disease-specific and generic instruments: the Salute Respiratoria nell'Anziano (Sa.R.A.) Study. Chest 2001; 120: 734–742. [DOI] [PubMed] [Google Scholar]
- 27.Garcia S, Alosco ML, Spitznagel MB, et al. Poor sleep quality and reduced cognitive function in persons with heart failure. Int J Cardiol 2012; 156: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonelli Incalzi R, Corsonello A, Trojano L, et al. Heart rate variability and drawing impairment in hypoxemic COPD. Brain Cogn 2009; 70: 163–170. [DOI] [PubMed] [Google Scholar]
- 29.Gallo JJ, Rebok GW, Lesikar SE. The driving habits of adults aged 60 years and older. J Am Geriatr Soc 1999; 47: 335–341. [DOI] [PubMed] [Google Scholar]
- 30.Johansson K, Bronge L, Lundberg C, et al. Can a physician recognize an older driver with increased crash risk potential? J Am Geriatr Soc 1996; 44: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 31.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011; 37: 264–272. [DOI] [PubMed] [Google Scholar]
- 32.Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res 2014; 15: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stege G, Vos PJ, van den Elshout FJ, et al. Sleep, hypnotics and chronic obstructive pulmonary disease. Respir Med 2008; 102: 801–814. [DOI] [PubMed] [Google Scholar]
- 34.Bédard MA, Montplaisir J, Malo J, et al. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP). J Clin Exp Neuropsychol 1993; 15: 330–341. [DOI] [PubMed] [Google Scholar]